Diagnostics of educational achievements in physics. Features of preparing students for the USE and GIA. First law of thermodynamics
1. The table shows the density of gases at normal atmospheric pressure. At the same time, molecules of 1) nitrogen 2) hydrogen 3) xenon 4) chlorine have the highest root-mean-square velocity Gas Gas density, kg / m 3 nitrogen 1.25 xenon 5.9 hydrogen 0.09 chlorine 3.2 2. The table shows the density of gases at normal atmospheric pressure. At the same time, the molecules of 1) nitrogen 2) hydrogen 3) xenon 4) chlorine have the lowest root-mean-square velocity. Gas Gas density, kg / m 3 nitrogen 1.25 xenon 5.9 hydrogen 0.09 chlorine 3.2 1) Excreted 2) Absorbed 3) Neither emitted nor absorbed 4) can both be released and absorbed
4. Bodies A and B have different temperatures, lower than those of body C. Bodies A and B were brought into thermal contact with each other and waited for thermal equilibrium to be established. If after that body A is brought into thermal contact with body B, then body B 1) will receive heat 2) will give off heat 3) can both receive and give off heat 4) will immediately be in a state of thermal equilibrium with body A 5. Bodies A and B have different temperatures, greater than those of body C. Bodies A and B are brought into thermal contact with each other and wait for thermal equilibrium to be established. If after that body A is brought into thermal contact with body B, then body B 1) will receive heat 2) will give off heat 3) can both receive and give off heat 4) will immediately be in a state of thermal equilibrium with body A 6. During the melting of solid paraffin, energy is 1) released 2) absorbed 3) neither released nor absorbed 4) can both be released and absorbed

7. In the process of boiling water at normal pressure, its temperature 1) decreases 2) increases 3) does not change 4) the answer depends on the rate of heat supply to boiling water 8. If the liquid is in equilibrium with its saturated steam, then the rate of evaporation of the liquid 1) is greater than the rate of vapor condensation 2) is less than the rate of vapor condensation 3) is equal to the rate of vapor condensation 4) is zero 9. How does the internal energy of a substance change when it passes from a gaseous state to a liquid state at constant temperature and pressure? 1) decreases 2) increases 3) varies for different substances 4) remains constant

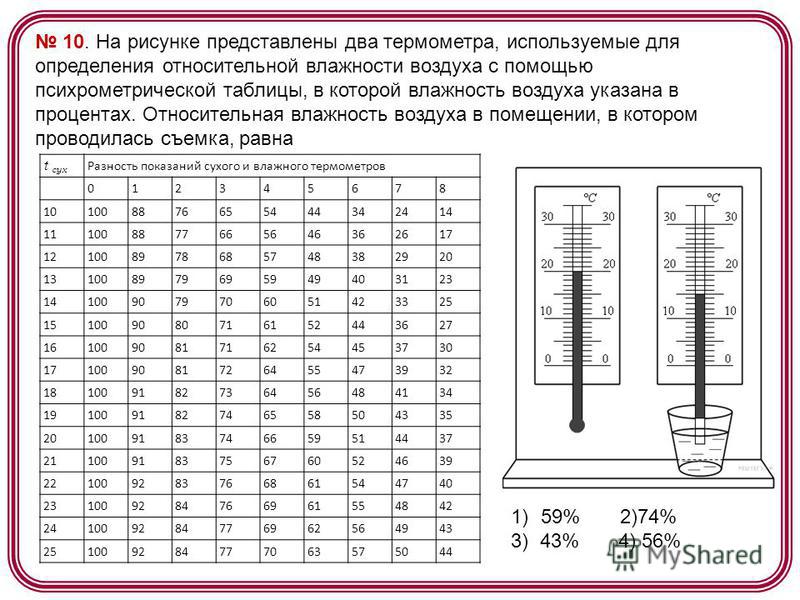
10. The figure shows two thermometers used to determine the relative humidity of the air using a psychrometric table, in which the humidity of the air is indicated as a percentage. The relative humidity of the air in the room in which the shooting was carried out is equal to t dry
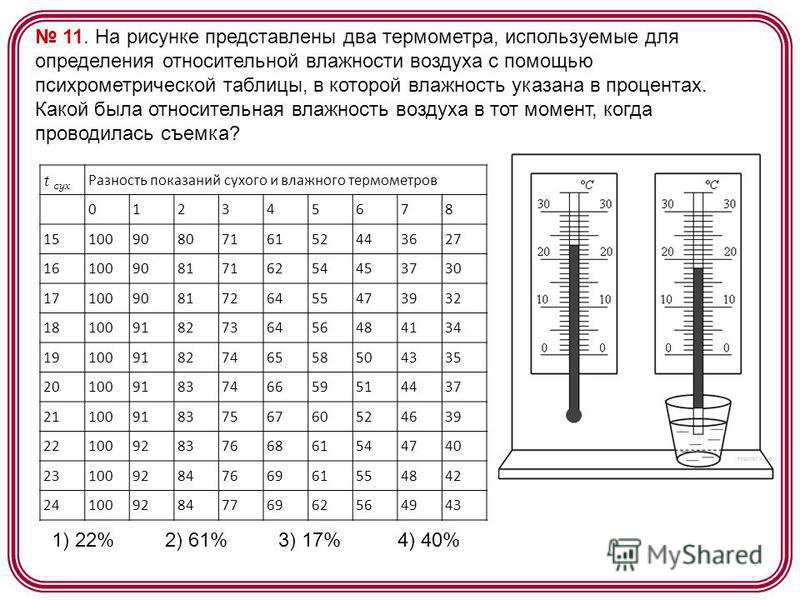
11. The figure shows two thermometers used to determine the relative humidity of the air using a psychrometric table, in which the humidity is indicated as a percentage. What was relative humidity air at the time the picture was taken? t dry Difference between dry and wet bulb readings) 22% 2) 61% 3) 17% 4) 40%
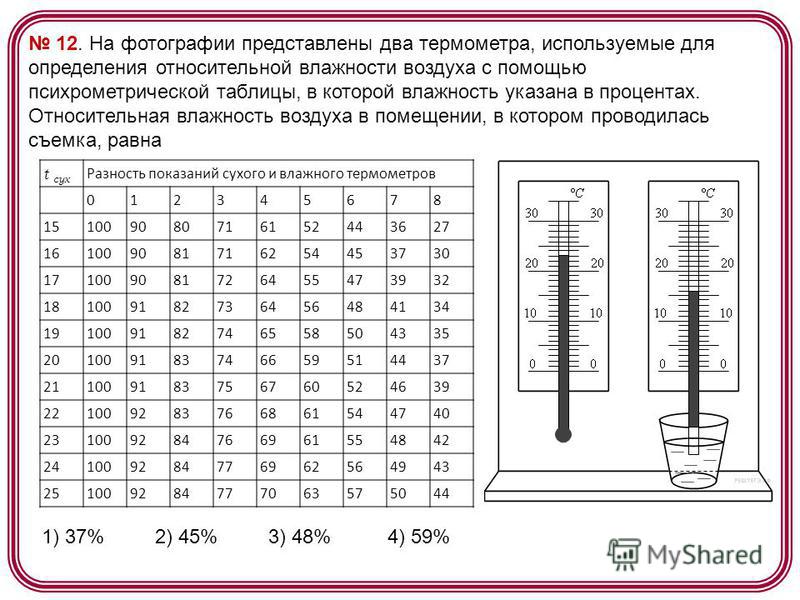
12. The photo shows two thermometers used to determine the relative humidity of the air using a psychrometric table, in which humidity is indicated in percent. The relative humidity of the air in the room in which the shooting was carried out is t dry
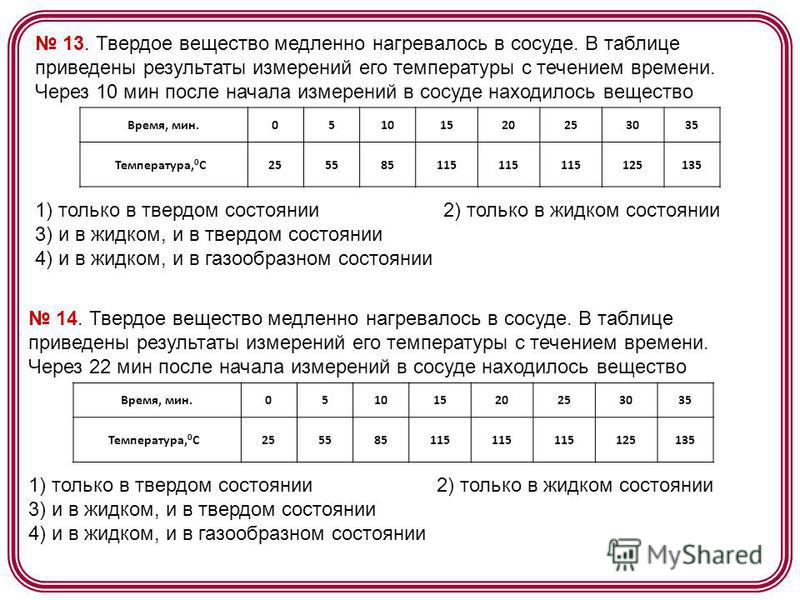
13. Solid heated slowly in the vessel. The table shows the results of measurements of its temperature over time. 10 min after the start of measurements, the vessel contained a substance Time, min Temperature, 0 C) only in the solid state 2) only in the liquid state 3) in both liquid and solid states 4) both in liquid and in gaseous state 14. A solid was slowly heated in a vessel. The table shows the results of measurements of its temperature over time. 22 min after the start of measurements, the vessel contained a substance Time, min Temperature, 0 C) only in the solid state 2) only in the liquid state 3) in both liquid and solid states 4) in both liquid and gaseous states
15. A solid was slowly heated in a vessel. The table shows the results of measurements of its temperature over time. 34 min after the start of measurements, the vessel contained the substance Time, min Temperature, 0 C) only in the solid state 2) only in the liquid state 3) in both liquid and solid states 4) in both liquid and gaseous states 16. The solid was slowly heated in the vessel. The table shows the results of measurements of its temperature over time. 6 min after the start of measurements, there was a substance in the vessel Time, min Temperature, 0 C) only in the solid state 2) only in the liquid state 3) in both the liquid and solid states 4) in both the liquid and gaseous states
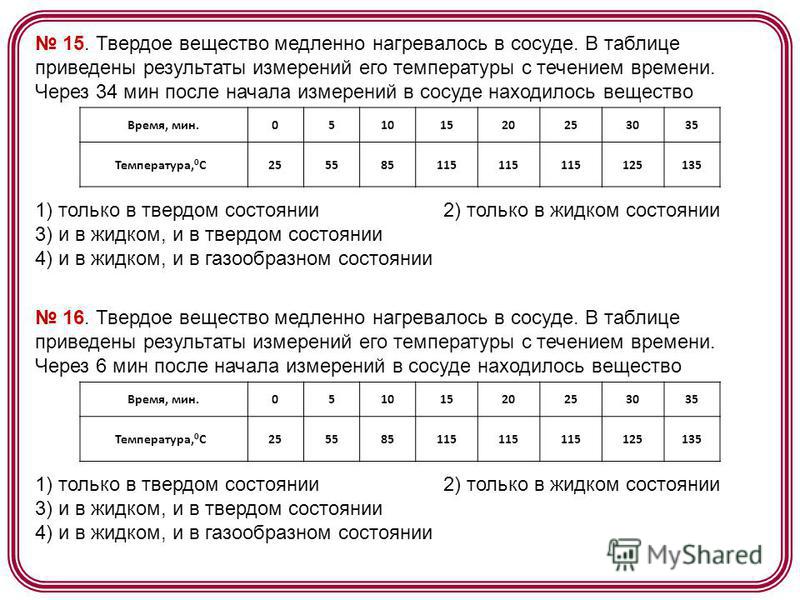
17. A solid was slowly heated in a vessel. The table shows the results of measurements of its temperature over time. 17 min after the start of measurements, the vessel contained the substance Time, min Temperature, 0 C) only in the solid state 2) only in the liquid state 3) in both the liquid and solid states 4) in both the liquid and gaseous states 18. Body A is in thermal equilibrium with body C, and body B is not in thermal equilibrium with body C. Find the correct statement. 1) the temperatures of bodies A and C are not the same 2) the temperatures of bodies A, C and B are the same 3) bodies A and B are in thermal equilibrium 4) the temperatures of bodies A and B are not the same 19. The vapor pressure in a room at a temperature of 5 0 C is 756 Pa. Pressure saturated steam at the same temperature is 880 Pa. Relative humidity is equal to (round up the answer to integers) 1) 1% 2) 60% 3) 86% 4) 100%
20. Heated steel bar A was brought into contact with a smaller cold steel bar B. In the process of establishing thermal equilibrium, bar A gave up the amount of heat Q. The system is in the calorimeter. Bar B 1) gave the amount of heat Q1 
23. Relative humidity is 42%, partial pressure steam at a temperature of 20 0 C early 980 Pa. Saturated vapor pressure at a given temperature is (round up the answer to integers) 1) 980 Pa 2) 2333 Pa 3) 1022 Pa 4) 412 Pa 24. The amount of water in the vessel decreases due to evaporation 1) only when boiling 2) only when heated 3 ) at any temperature, if the vapor in the air above the water surface is unsaturated 4) at any temperature, if the vapor in the air above the water surface is saturated 25. The relative humidity of the air in the cylinder under the piston is 60%. The air is isothermally compressed, reducing its volume by half. Relative humidity has become 1) 120% 2) 100% 3) 60% 4) 30%

26. In the vessel under the piston is unsaturated steam. It can be converted to saturated by 1) increasing the temperature isobarically 2) adding another gas to the vessel 3) increasing the volume of steam 4) decreasing the volume of steam 27. The relative humidity in the room is 40%. What is the ratio of the concentration of n water molecules in the air of the room and the concentration of n n water molecules in saturated water vapor at the same temperature? 1) n is less than n n by 2.5 times 2) n is more than n n by 2.5 times 3) n is less than n n by 40% 4) n is greater than n n by 40% 28. The relative humidity of the air in the cylinder under the piston is fifty%. The air is isothermally compressed, reducing its volume by 3 times. Relative humidity became 1) 150% 2) 100% 3) 50% 4) 25%

29. The boiling point of water is determined mainly by 1) heater power 2) heater temperature 3) ambient air pressure 4) ambient temperature 30. There is a tall pot of water on a gas stove, covered with a lid. If the water from it is poured into a wide pan, whose bottom area is twice as large, and also covered with a lid, then the water will boil noticeably faster than if it had remained in a narrow one. This fact is explained by the fact that 1) the heating area increases and, consequently, the rate of water heating increases 2) the required saturation vapor pressure in the bubbles decreases by 2 times and, therefore, the water at the bottom must be heated to a lower temperature 3) the water surface area increases and, consequently, evaporation is more active 4) the depth of the water layer decreases by 2 times and, therefore, steam bubbles reach the surface faster

31. Ice at a temperature of 0 ° C was brought into a warm room. The temperature of the ice before it melts 1) will increase, since the ice receives heat from the environment, which means that its internal energy increases, and the temperature of the ice rises 2) will not change, because when melting, the ice receives heat from the environment, and then it gives it back 3) will not change, since all the energy received by ice at this time is spent on the destruction of the crystal lattice 4) will decrease, since during melting ice gives off a certain amount of heat to the environment 32. What is the relative humidity of air at a temperature of 20 0 C if the dew point is 12 0 C ? The pressure of saturated water vapor at 20 0 C is 2.33 kPa, and at 12 0 C 1.4 kPa. Express your answer as a percentage and round to the nearest whole number. 1) 60% 2) 50% 3) 40% 4) 75%

33. What will be the change in temperature of an ideal gas if during the process pV 2 = const its volume has decreased by 2 times? 1) will increase by 2 times 2) will decrease by 2 times 3) will not change 4) will increase by 4 times What volume will this gas occupy under normal conditions? Express your answer in cubic meters and round to the nearest hundredth. 1) 1.53 m 3 2) 1.89 m 3 3) 3.12 m 3 4) 1.19 m How much heat is needed to melt 2.5 tons of steel taken at the melting point? The specific heat of fusion of steel is 80 kJ/kg. Ignore heat loss. 1) 100 MJ 2) 200 MJ 3) 50 MJ 4) 150 MJ

36. The figure shows a cyclic process 12341 performed on ideal gas. It can be argued that 1) in section 12 the gas does no work 2) in section 41 the internal energy of the gas increases 3) in section 12 a certain amount of heat is imparted to the gas 4) in section 23 the gas does positive work V T Which graph corresponds to the isochoric heating of three oxygen gases , helium and carbon dioxide, having the same masses and occupying the same volumes? 1) 1 helium, 2 oxygen, 3 carbon dioxide 2) 1 carbon dioxide, 2 oxygen, 3 helium 3) 1 helium, 2 carbon dioxide, 3 oxygen 4) 1 oxygen, 2 helium, 3 carbon dioxide p T Three bars with different temperatures(50 °C, 70 °C, 10 °C) brought into contact. In the process of establishing thermal equilibrium, heat was transferred in the directions indicated by the arrows in the figure. The bar 1) A 2) B 3) C 4) A and B B A C had a temperature of 70 ° C
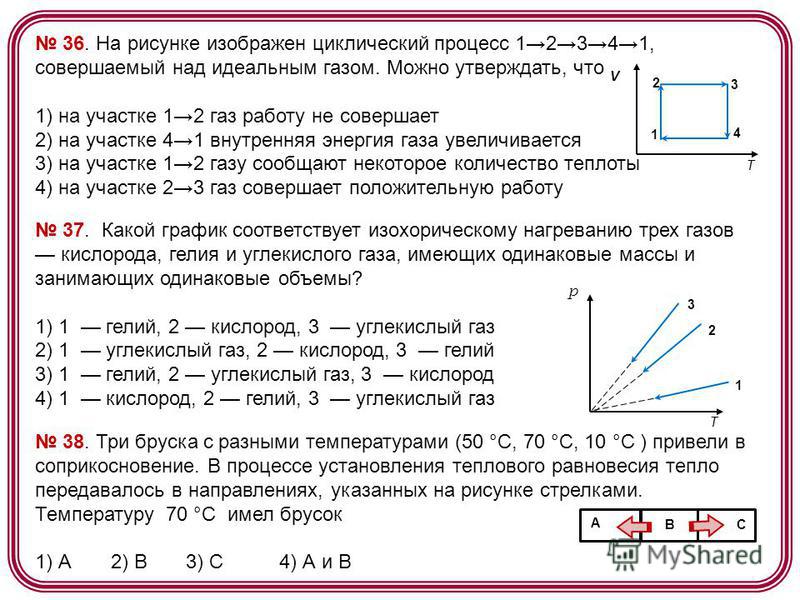
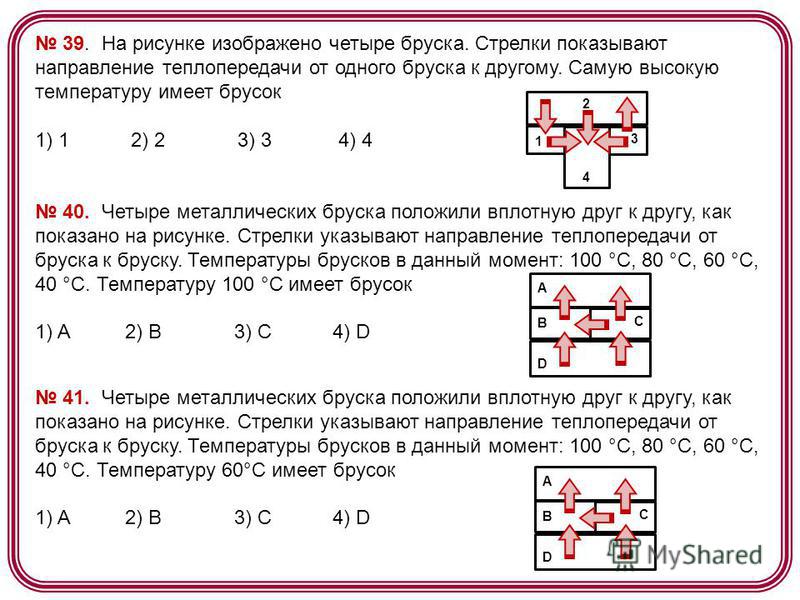
39. The figure shows four bars. The arrows show the direction of heat transfer from one bar to another. most high temperature has a bar 1) 1 2) 2 3) 3 4) Four metal bars are placed close to each other, as shown in the figure. The arrows indicate the direction of heat transfer from bar to bar. Bar temperatures in this moment: 100°C, 80°C, 60°C, 40°C. A bar 1) A 2) B 3) C 4) D B A C D 41 has a temperature of 100 ° C. Four metal bars were placed close to each other, as shown in the figure. The arrows indicate the direction of heat transfer from bar to bar. Bar temperatures at the moment: 100 °C, 80 °C, 60 °C, 40 °C. The bar has a temperature of 60°C 1) A 2) B 3) C 4) D B A C D
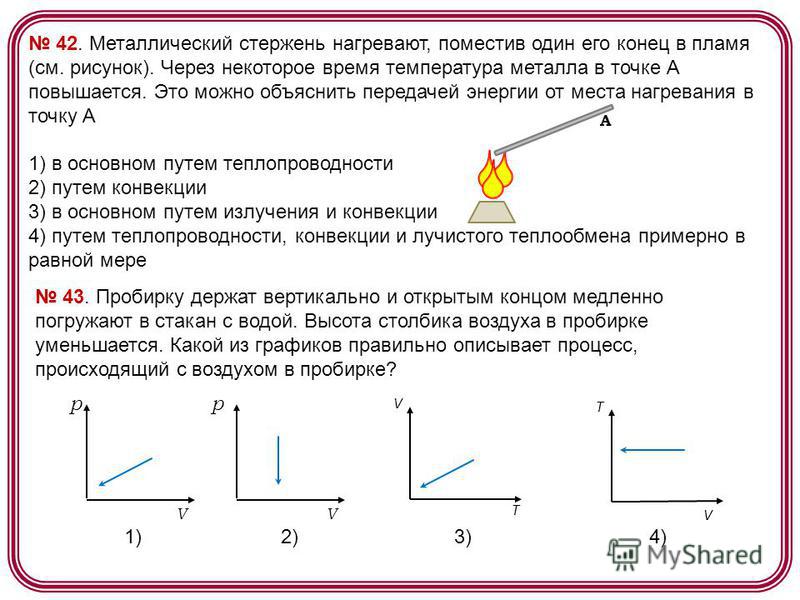
42. A metal rod is heated by placing one end of it in a flame (see figure). After some time, the temperature of the metal at point A rises. This can be explained by the transfer of energy from the place of heating to point A 1) mainly by heat conduction 2) by convection 3) mainly by radiation and convection 4) by heat conduction, convection and radiant heat exchange approximately equally A 43. The test tube is held vertically and open the end is slowly immersed in a glass of water. The height of the air column in the test tube decreases. Which of the graphs correctly describes the process that occurs with air in a test tube? 1) 2) 3) 4) p V p V V T V T
44. The specific heat of vaporization of water is 2, J / kg. This means that for the evaporation of 1) any mass of water at the boiling point, the amount of heat required is 2, J; 2) 1 kg of water at the boiling point requires an amount of heat equal to 2. J; equal to 10 6 J 4) 1 kg of water at any temperature requires an amount of heat 2, J / kg 45. A certain amount of aluminum was placed in the furnace. A diagram of aluminum temperature changes over time is shown in the figure. The furnace at a constant heating power transfers to aluminum 1 kJ of heat per minute. How much heat was required to melt aluminum, already heated to its melting temperature? 1) 5 kJ 2) 15 kJ 3) 20 kJ 4) 30 kJ t, min T
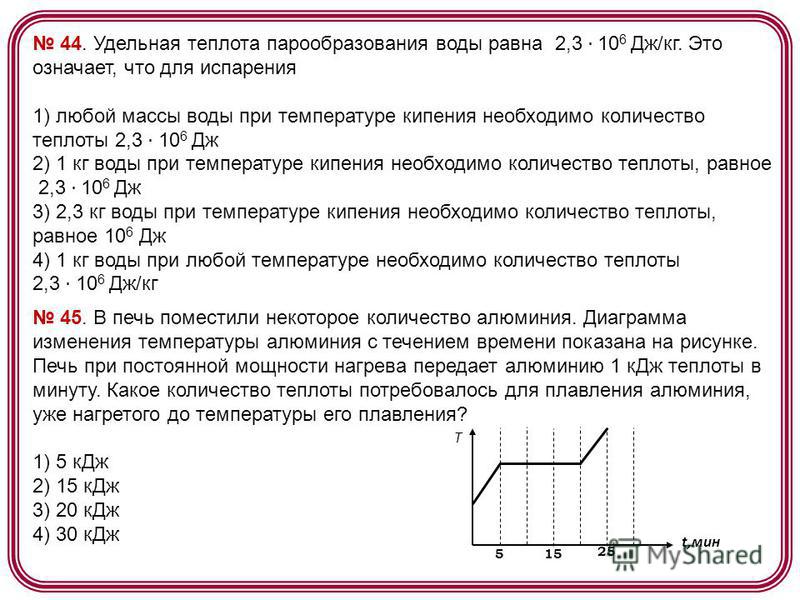
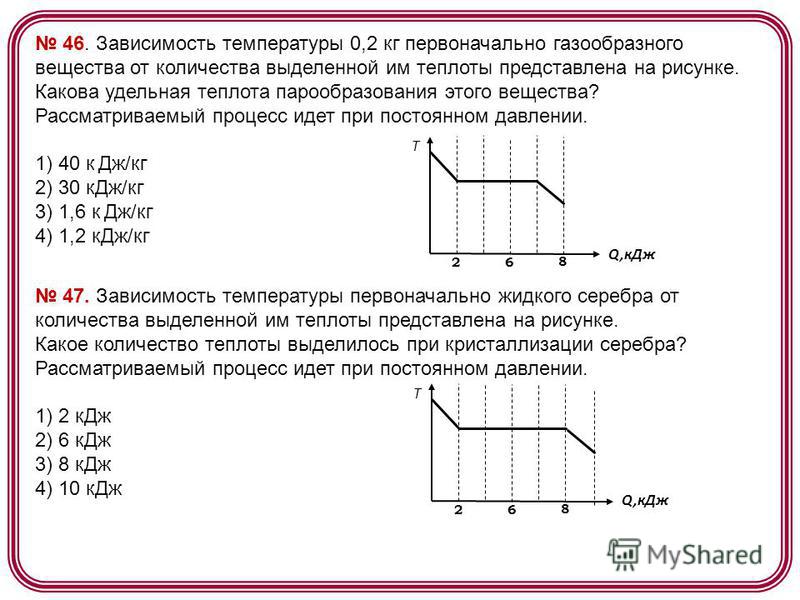
46. The dependence of the temperature of 0.2 kg of an initially gaseous substance on the amount of heat released by it is shown in the figure. What is specific heat vaporization of this substance? The process under consideration is constant pressure. 1) 40 kJ/kg 2) 30 kJ/kg 3) 1.6 kJ/kg 4) 1.2 kJ/kg Q,k J T . How much heat is released during the crystallization of silver? The process under consideration proceeds at constant pressure. 1) 2 kJ 2) 6 kJ 3) 8 kJ 4) 10 kJ Q,k J T 2 6 8
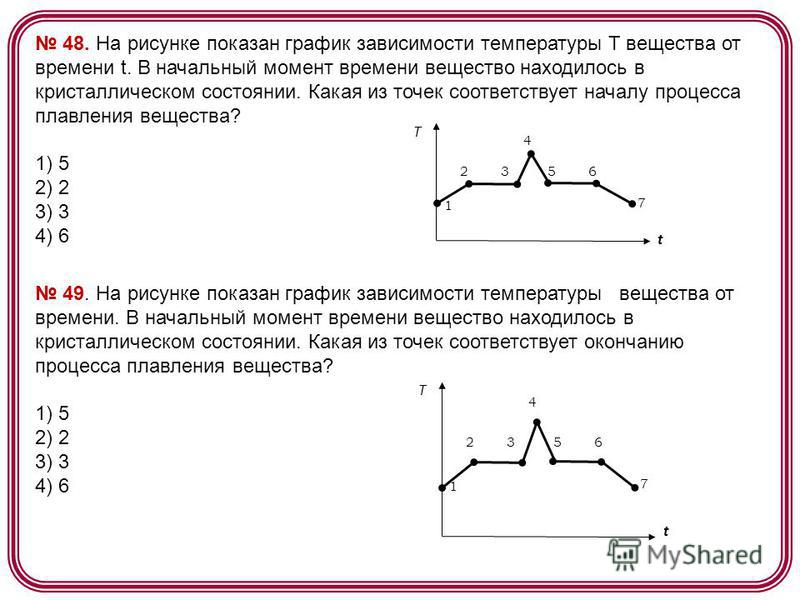
48. The figure shows a graph of the temperature T of a substance versus time t. At the initial moment of time, the substance was in a crystalline state. Which of the points corresponds to the beginning of the melting process of the substance? 1) 5 2) 2 3) 3 4) 6 t T The figure shows a graph of the temperature of a substance versus time. At the initial moment of time, the substance was in a crystalline state. Which of the points corresponds to the end of the melting process of the substance? 1) 5 2) 2 3) 3 4) 6 t T
50. crystalline substance using a heater uniformly heated from 0 to the moment Then the heater was turned off. The graph shows the dependence of the temperature T of the substance on time t. Which section corresponds to the process of heating a substance in a liquid state? 1) 5-6 2) 2-3 3) 3-4 4) 4-5 t T constant power R. At time t 0 the water was in a gaseous state. Which of the following expressions determines the specific heat of crystallization of water based on the results of this experiment? 1) P t 1 /mT 1 2) P t 2 /m 3) P t 2 /mT 2 4) P t 4 /m
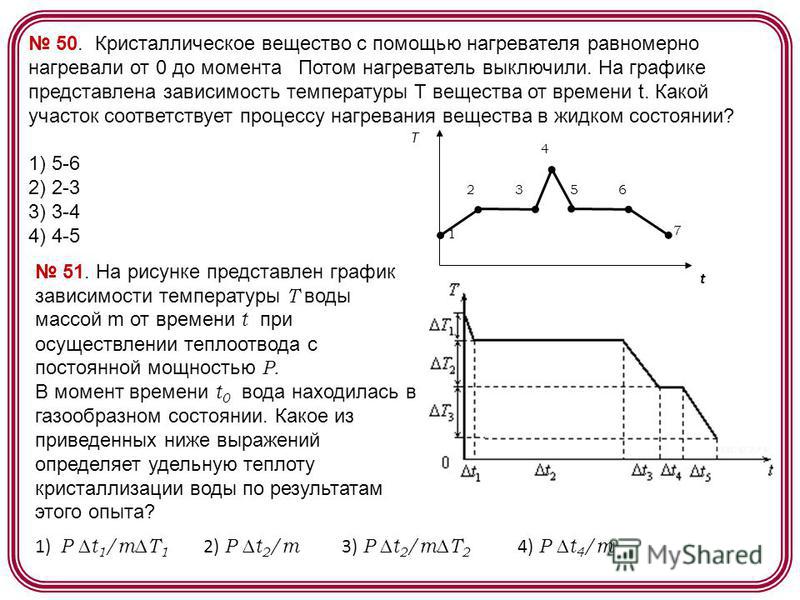
52. The amount of heat Q is transferred to the liquid at a constant temperature T. As a result, the liquid of mass m passes into a gaseous state. Which of the following expressions determines the specific heat of vaporization of this substance? 1) Q/m 2) Q/T 3) Q/(m ΔT) 4) Q. Δm. ΔT 53. When a substance of mass m and specific heat of solidification λ is transformed from liquid state into a solid at a constant temperature T, the amount of heat Q given off by the substance is 1) λmT 2) λm 3) λm / T 4) λT / m 54. When heat transfer is carried out at a constant temperature T, a substance of mass m is transformed from a liquid state into a gaseous state. Which of the following expressions determines the amount of heat Q transferred to the substance in this process, if the specific heat of vaporization of this substance is r? 1) r mT 2) r m 3) r m/T 4) r T/m

55. The figure shows a graph of the dependence of the temperature T of water with a mass m on time t during heat transfer with a constant power P. At time t 0, the water was in a solid state. During what time interval was the heating of ice, and in what interval was the heating of water vapor? 1) t 4 and t 5 2) t 1 and t 4 3) t 1 and t 5 4) t 3 and t time t 0 the water was in a gaseous state. Which of the following expressions determines the specific heat of condensation of water vapor based on the results of this experiment? 1) P t 5 /mT 2 2) P t 2 /m 3) P t 2 /mT 2 4) P t 4 /m
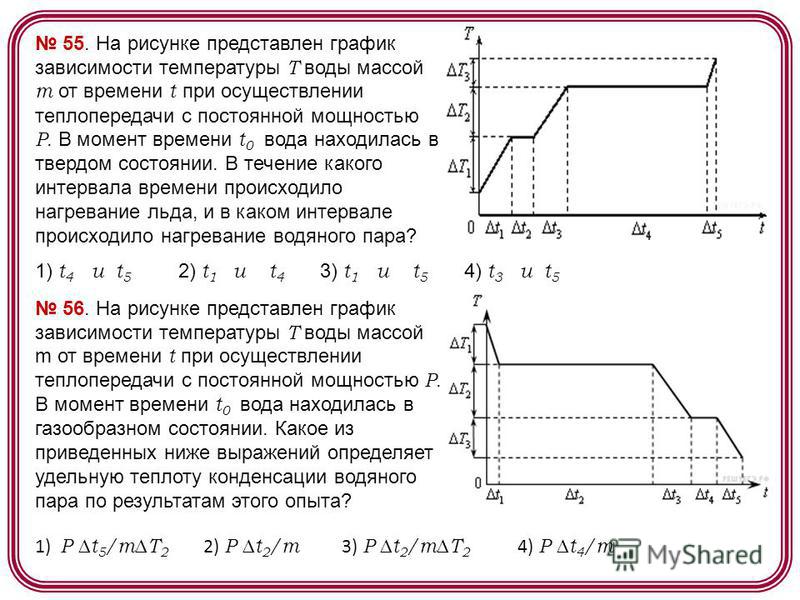
57. The figure shows a graph of the temperature T of water with mass m versus time t when heat transfer is carried out with a constant power P. At time t 0, water was in solid state. Which of the following expressions determines the specific heat of melting of ice based on the results of this experiment? 1) P t 1 /mT 1 2) P t 2 /m 3) P t 3 /mT 2 4) P t 4 /m 58. The figure shows three cases of the location of two copper bars. Heat transfer from one bar to another will be carried out 1) only in situation 3 2) only in situations 1 and 2 3) only in situations 2 and 3 4) in all three situations
Literature and Internet - resources: 1. The most complete edition of standard variants of tasks for the Unified State Examination: 2010: Physics / author-compilation A.V. Berkov, V.A. Gribov. - M .: AST: Astrel, The most complete edition of typical options for USE assignments: 2011: Physics / author-comp. A.V. Berkov, V.A. Gribov. - M .: AST: Astrel, The most complete edition of typical options for USE tasks: 2012: Physics / author-compilation A.V. Berkov, V.A. Gribov. - M .: AST: Astrel, The most complete edition of typical options for USE tasks: 2013: Physics / author-compilation A.V. Berkov, V.A. Gribov. - M .: AST: Astrel, Internet - portal "I will solve the Unified State Examination of the Russian Federation" - physics

1.
The tube is held vertically and the open end is slowly immersed in a glass of water. The height of the air column in the test tube decreases. Which of the graphs correctly describes the process that occurs with air in a test tube?
Solution:
The test tube is lowered into water slowly, which means that the air in the test tube has time to exchange heat with environment, the process is isothermal, the temperature is constant. Thus, the process that occurs with the air in the test tube correctly describes graph 4.
2.
The dependence of the temperature of 0.2 kg of an initially gaseous substance on the amount of heat released by it is shown in the figure. 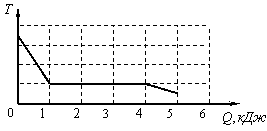
What is the specific heat of vaporization of this substance?
Solution:
During the condensation process, the temperature of the substance did not change. At the same time, as can be seen from the figure, in the process of condensation, it managed to release
Therefore, the specific heat of vaporization of this substance is equal to ![]()
3.
A metal rod is heated by placing one end of it in a flame (see figure). 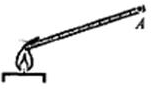
After some time, the temperature of the metal at the point BUT rises. This can be explained by the transfer of energy from the place of heating to the point BUT
1) Mainly by conduction
2) by convection
3) mainly by radiation and convection
4) by conduction, convection and radiant heat transfer approximately equally
Solution:
Thermal conductivity is the transfer of thermal energy by particles of a substance in the process of their thermal motion. Convection is the phenomenon of heat transfer in liquids or gases by mixing the substance itself. Thermal radiation is the emission of thermal energy by heated bodies. From this it is clear that in the situation described in the picture, the temperature of the metal at point A rises mainly due to thermal conductivity.
4.
The specific heat of vaporization of water is 2.3 10 6 J/kg. This means that for evaporation...
1) any mass of water at the boiling point requires the amount of heat 2.3 10 6 J
2) 1 kg of water at the boiling point requires the amount of heat 2.3 10 6 J
3) 2.3 kg of water at the boiling point requires an amount of heat of 10 6 J
4) 1 kg of water at any boiling point requires the amount of heat 2.3 10 6 J
Solution:
The specific heat of vaporization is a physical quantity that shows how much energy is needed to evaporate one kilogram of a substance taken at the boiling point. The value of this value 2.3 10 6 J/kg for water means that the amount of heat required to evaporate 1 kg of water at the boiling point is 2.3 10 6 J.
5.
10 min after the start of measurements, the vessel contained a substance...
2) only in liquid state
Answer:
Solution:
It can be seen from the table that 10 minutes after the start of measurements, the temperature of the contents of the vessel still continued to rise uniformly. Consequently, the melting process had not yet begun, and the vessel contained only the substance in the solid state.
6. The solid was heated in a vessel. The table shows the results of measurements of its temperature over time.
22 minutes after the start of measurements, the vessel contained a substance
1) only in solid state
2) only in liquid state
3) in both liquid and solid state
4) both in liquid and gaseous state Answer:
Solution:
The table shows that in the time interval between 15 minutes and 25 minutes the temperature of the contents of the vessel did not change. Therefore, at this time there was a process of melting. Thus, 22 minutes after the start of measurements, the vessel contained a substance in both solid and liquid states.
7. The solid was slowly heated in the vessel. The table shows the results of measurements of its temperature over time.
34 minutes after the start of measurements, the vessel contained a substance
1) only in solid state
2) only in liquid state
3) in both liquid and solid state
4) both in liquid and gaseous state Answer:
Solution:
The table shows that in the time interval between 15 minutes and 25 minutes, the temperature of the contents of the vessel did not change. Therefore, at this time there was a process of melting. After the end of melting, the temperature of the contents of the vessel continued to increase. Thus, 34 minutes after the start of measurements, the vessel contained only the substance in the liquid state.
8. The solid was slowly heated in the vessel. The table shows the results of measurements of its temperature over time.
6 minutes after the start of measurements, the vessel contained a substance
1) only in solid state
2) only in liquid state
3) both in liquid and in solid state and in liquid
4) and in the gaseous state Answer:
Solution:
It can be seen from the table that 6 minutes after the start of measurements, the temperature of the contents of the vessel still continued to rise uniformly. Consequently, the melting process had not yet begun, and the vessel contained only the substance in the solid state.
M. Yu. Demidova,
, FIPI, Moscow;
E. E. Kamzeeva,
FIPI, Moscow;
G. G. Nikiforov,
, ISMO RAO, FIPI, Moscow
Diagnostics of educational achievements in physics. Features of preparing students for the USE and GIA
Lecture 3. Unified state exam in physics for 11th grade students
1. Planning the examination work
The USE in Physics is an exam of choice for graduates. It is chosen mainly by those who are going to enter universities, where physics is one of the admission tests. Therefore, the federal component of the SES (complete) education in physics was chosen as the basis for constructing the codifier and the list of activities profile level. The current USE codifier is characterized by the following:
- the content elements correspond to the didactic units listed in the Compulsory minimum of the content of education of the State Educational Standard, the elements highlighted in the State Educational Standard in italics as not subject to final verification are not included in the codifier;
- to clarify and more detailed characteristics of didactic units, elements of the content from the part "Practical activity", as well as elements (concepts, phenomena, laws, theories, etc.) listed in the section "Requirements for the level of training of graduates" were used;
The list of activities is compiled on the basis of the operationalization of the requirements set out in the section of the State Educational Standard “Requirements for the level of training of graduates”. They can be classified into three:
– possession of the basic conceptual apparatus of the school physics course;
– solving problems of various types and levels of complexity;
- Possession of basic knowledge about methods scientific knowledge and development of experimental skills.
From a comparison of these types of activities with those listed in lecture 2, it can be seen that work with information of physical content is not submitted to the USE. Such tasks show, as a rule, the level of formation of various general educational skills in relation to the context. For the USE, which is designed primarily to differentiate graduates in terms of their readiness to enter universities, this type of activity is not fundamental. However, individual skills are still indirectly tested (working with graphs, diagrams, tables, transferring information from one type to another). As mentioned above, each task of the USE bank is characterized by the nature of the presentation of information, therefore, in each examination option, there are approximately the same number of graphs, diagrams, photographs of real experiments, etc. Tasks also play an important role here. high level difficulties that are formulated in the USE as fully as possible, without the “default” abbreviations adopted in various problem books.
KIM for the USE is a written work with assignments covering the main topics of the school course in physics and differing both in level of complexity and in form depending on the type of answer. The examination variant consists of three parts, each of which includes tasks of only one type. The first part is tasks with the choice of one correct answer, the second part is tasks with a short answer, the third part is tasks with a detailed answer. The division of work into parts is only technological in nature and is associated with recording answers in different form and in different forms.
The examination paper presents tasks of basic, advanced and high levels of complexity. Previously, the physics version was built on the principle of increasing the level of complexity of tasks from the first to the third part of the work: first, all the tasks of the basic level with a choice of answer went, then the advanced level (first with a choice of an answer, and then with a short answer), and all tasks of a high level of complexity were concentrated in the third part of the work. At present, this system has been preserved in relation to high-level tasks, while basic-level tasks are distributed between the first and second parts.
KIM includes assignments for all the main substantive sections of the physics course: mechanics, Molecular physics and thermodynamics, electrodynamics, elements of SRT and quantum physics. Moreover, each part of the work contains tasks for all four sections, which are sequentially distributed according to the thematic basis: from mechanics to quantum physics. The total number of tasks for each of the sections in the examination version corresponds to its content in school course physics and the total study time devoted to the study this section in accordance with the Exemplary Program in Physics of the profile level.
Each examination option is designed in such a way as to check not only a certain range of content elements, but also to control the level of formation of the selected range of skills. At the same time, equivalent options can be constructed based on both thematic affiliation and the need to verify certain types of activities.
In the first case, one line of tasks uses a series of similar tasks for the same content element, controlling the same skill. For example, the task line A2 can test the content element "acceleration", and the whole series is tasks that control the ability to apply a formula to calculate acceleration in different situations. This is how most of the lines of equivalent variants of one series are currently filled.
With another approach, the priority may be to check the formation of a particular skill. In this case, the entire series of tasks tests the same skill, but the content elements from different sections of the physics course are used. For example, a series of tasks A25 can check the ability to evaluate the compliance of the conclusions with the available experimental data, while experiments of different thematic affiliation can be used (friction force, Hooke's law, gas laws etc.).
Currently, when designing examination options, both approaches are used. The first is for assignments. A1–A23, B3–B5 and С2–С6, and the second - so far only for tasks A24, A25, B1, B2, C1. Thus, the priority so far is the construction of a variant based on the thematic affiliation of tasks, but gradually the emphasis is shifting towards checking certain types of activities.
Every year in September, regulatory documents are published for the development of KIMs of the Unified State Examination of the current school year. These include the codifier of controlled content elements, the specification of the examination USE work in physics and demo version.
AT codifier reflects all the content elements that are submitted for control this year. For example, in 2009 there is no section on astronomy in the codifier (although it is present in the GOS).
AT specifications a generalized plan is presented, on the basis of which several plans are formed for all variants of the current year. Analysis of the plan shows the probability of "meeting" in the variant with one or another of the content elements. For example, the law gravity(exercise A3) explicitly occurs five times less frequently than the law of conservation of momentum, which occurs in each variant (task A4). Therefore, a conscientious analysis of the generalized plan can significantly help in developing a strategy for preparing for the exam.
Demo version shows an exemplary implementation of the generalized plan. It can be used to judge the distribution of tasks by complexity, by type of activity, etc.
2.1. Features of tasks with a choice of answers. Everyone who comes to the exam receives two forms - No. 1 and No. 2. The first form indicates the correct answers to the tasks of the first and second parts of the work, i.e. for multiple choice and short answer questions. This form is checked by a computer, which compares the answers with the correct ones.
Multiple choice tasks cover a very wide range of questions, and both individual elements (for example, a formula or definition of a quantity) and the complex application of several elements at once (for example, when solving problems) can be checked. It should be noted that verification of the definitions or wording of laws is rarely carried out "directly". Due to the need to prepare a series of the same type of options, as a rule, they use the method of paraphrasing the definition on a specific example.
Example 1 The half-life of the nuclei of radon atoms is 3.9 s. It means that:
1) in 3.9 s, the atomic number of each nucleus will be halved;
2) half of the original large number of nuclei will decay in 3.9 s;
3) one nucleus decays every 3.9 s;
4) all initially available nuclei will decay in 7.8 s.
When preparing students for tasks with a choice of answers, it is necessary to pay their attention not only to the content, but also to the form of presenting the correct answer and distractors. There are questions in which distractors are formulated as partially correct answers (as a rule, in this case, the word “only” is placed before the answer). In this case, you must carefully read all the distractors and choose the most complete one.
Example 2 In a sample containing an isotope of neptunium, reactions of its transformation into uranium occur. In this case, the following types of radioactive radiation are recorded:
1) only α-particles;
2) only β-particles;
3) both α- and β-particles simultaneously;
4) only γ-particles.
Sometimes in simple tasks for checking certain formulas, the same numerical answers are used, expressed in different units or differing in order of magnitude.
Example 3 A boy of mass 50 kg jumps high. The force of gravity acting on it during the jump is approximately equal to:
1) 500 N; 2) 50 N; 3) 5 kN; 4) 0.
Therefore, in any, even the simplest tasks with an obvious answer, it is necessary to read all the answers to the end and not make a mistake with the choice.
As mentioned in the section on the typology of tasks of the USE bank, in exam options tasks with different forms of information presentation are used. The most common case is when the missing data needs to be obtained from the graph.
Example 4 The dependence of the temperature of 0.2 kg of an initially gaseous substance on the amount of heat released by it is shown in the figure. What is the specific heat of vaporization of this substance?
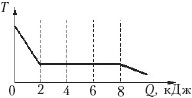
1) 40 kJ/kg;
2) 30 kJ/kg;
3) 1.6 kJ/kg;
4) 1.2 kJ/kg.
Tables are used much less often, from which students must also be able to extract the necessary data.
Example 5 In the study of the elastic properties of the spring, the student received the following table of the results of measuring the elastic force of the spring and its elongation.
X, cm |
The stiffness of the spring is:
1) 0.5 N/m; 2) 5 N/m; 3) 50 N/m; 4) 500 N/m.
Tasks based on photographs of real experiments deserve special attention here. The values \u200b\u200bnecessary for the answer must be obtained by correctly reading the readings of the instruments. Moreover, if in tasks with a choice of answers, as a rule, unambiguous photographs are used with digital instruments or arrows that clearly stand on any division, then in tasks with a detailed answer, readings are allowed, taking into account the reading error.
Example 6 The figure shows a photograph of the installation for studying the uniformly accelerated sliding of a carriage (1) weighing 0.1 kg along inclined plane set at an angle of 30° to the horizontal.
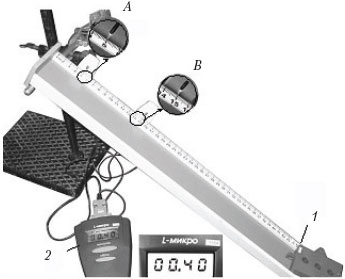
At the moment of the start of movement, the upper sensor ( BUT) turns on the stopwatch 2 , and when the carriage passes the lower sensor AT stopwatch turns off. The numbers on the ruler indicate the length in centimeters. Which expression describes the dependence of the carriage speed on time in SI units?
1) υ = 1,25t; 2) υ = 0,5t; 3) υ = 2,5t; 4) υ = 1,9t.
Particular attention in preparing for the exam should be paid to the last tasks with a choice of answers, which are aimed at testing methodological skills. Currently, the bank of such tasks diagnoses skills:
Distinguish between the use of various methods for studying physical objects (observation, experiment, measurement, description, modeling, hypothesis).
Example 7 The student lowered the electrodes into a vessel with a chemical solution and connected them to a current source. In her report, she wrote: “Bubbles appeared on one of the electrodes.” This statement is:
1) theoretical conclusion;
2) experimental fact;
3) the hypothesis of the experiment;
4) an explanation of the fact.
Suggest (choose) the procedure for conducting an experiment or observation, choose measuring instruments and equipment, depending on the purpose of the study.
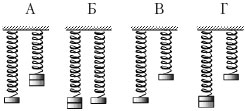 Example 8 It is necessary to experimentally check whether the period of oscillation of a spring pendulum depends on the mass of the load. What pair of pendulums should be used for such a check?
Example 8 It is necessary to experimentally check whether the period of oscillation of a spring pendulum depends on the mass of the load. What pair of pendulums should be used for such a check?
1) A or D; 2) only B;
3) only B; 4) A, B or D.
Analyze the procedure for conducting an observation or experiment, highlight errors in the course of setting up a study.
Example 9 The student suggested that electrical resistance piece of metal wire is directly proportional to its length. To test this hypothesis, he took pieces of wire made of aluminum and copper. The student marked the results of measuring the lengths of the segments and their resistances with dots on the graph of the dependence of the resistance on the length of the conductor. The measurement errors of length and resistance are 5 cm and 0.1 Ohm, respectively. What conclusion follows from the results of the experiment?
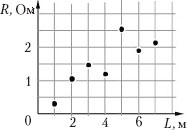 1)
Taking into account the measurement error, the experiment confirmed the correctness of the hypothesis;
1)
Taking into account the measurement error, the experiment confirmed the correctness of the hypothesis;
2) the order of setting up the experiment did not correspond to the hypothesis put forward;
3) measurement errors are so large that they did not allow testing the hypothesis;
4) most of the measurement results confirm the hypothesis, but when measuring the resistance of a piece of wire 5 m long, a gross error was made.
Build graphs based on the results of research (taking into account absolute measurement errors), find values based on the results of the experiment physical quantities(indirect measurements), evaluate the consistency of the conclusions with the available experimental data.
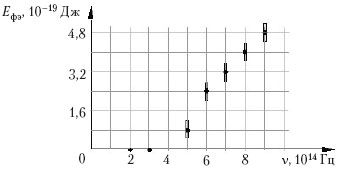 Example 10 When studying the phenomenon of the photoelectric effect, the dependence of the energy E PE of photoelectrons emitted from the illuminated plate on the frequency ν of the incident light. The measurement errors of the light frequency and photoelectron energy were 5 × 10 13 Hz and 4 × 10–19 J, respectively. The results of measurements, taking into account the error, are shown in the figure.
Example 10 When studying the phenomenon of the photoelectric effect, the dependence of the energy E PE of photoelectrons emitted from the illuminated plate on the frequency ν of the incident light. The measurement errors of the light frequency and photoelectron energy were 5 × 10 13 Hz and 4 × 10–19 J, respectively. The results of measurements, taking into account the error, are shown in the figure.
According to these measurements, Planck's constant is approximately equal to:
1) 2 10 –34 J s; 2) 5 10 –34 J s;
3) 7 10 –34 J s; 4) 9 10 –34 J s.
Compare the results of research given in the form of a verbal description, table or graph (transfer available data from one form of description to another), draw conclusions, explain the results of experiments and observations based on known physical phenomena, laws, theories.
Example 11. The figure shows a graph of the dependence of the coordinates of a bead, freely sliding along a horizontal spoke, on time. Based on the graph, we can say:
1) on site 1 the movement is uniform, and on the site 2 - uniformly accelerated;
2) the projection of the acceleration of the bead increases everywhere;
3) on site 2 the bead's acceleration projection is positive;
4) Location on 1 the bead rests, and on the site 2 - moves evenly.
I would like to draw attention to the fact that the formation of the above skills is possible only when used in teaching the subject laboratory work research nature. Only when performing this kind of work, which implies maximum independence of students' actions, is the entire chain of skills formed as a whole, in their interconnection. The use of test tasks (similar to those used in the USE) is possible only at the stage of diagnosing certain skills, but cannot be a tool for developing research skills.
2.2. Features of tasks with a short answer
The answers to the tasks of the second part are scanned and recognized by the computer. Therefore, it is necessary to strictly adhere to the rules for recording them: each character in a separate cell, including a comma and a minus sign.
In assignments IN 1 and IN 2 to establish a correspondence, the answer must be given in the form of a set of numbers. If we are talking about the nature of the change of certain physical quantities under the specified conditions, then the figures can be repeated. For example, it could be 331 or 121, etc.
Example 12 A block slides down an inclined plane without friction. What happens in this case with its speed, kinetic energy, reaction force of the inclined plane?
For each position of the first column, select the corresponding position of the second and write down the selected numbers in the table under the corresponding letters.
Transfer the resulting sequence of numbers to the answer sheet (without spaces or any symbols).
Tasks B3–B5 are computational problems to which you need to give the answer in the form of a number. It can be an integer (for example: -2650) or decimal(for example: 4.23 or 0.025). Units of physical quantities, as is usually customary in tasks, do not need to be written. They can be recognized by the computer as additional numbers and counted as an error.
When completing the test, the ability to do simple arithmetic calculations, represent numbers in standard form and perform mathematical transformations is essential, since more than half of the tasks require knowledge of mathematics. You can use a non-programmable calculator to facilitate more complex calculations during the exam. When preparing for the exam, you must choose a calculator that has not only all arithmetic operations, squaring and square root operations, but also calculation operations trigonometric functions(sine, cosine, tangent).
When carrying out calculations in tasks of all parts of the work, it is often necessary to use various physical constants. As a rule, their values are indicated not in the text of the task, but in special reference tables at the beginning of each option in approximations that minimize the complexity of calculations. For example, acceleration free fall g\u003d 10 m / s 2, not 9.8 m / s 2, Planck's constant h\u003d 6.6 10 -34 J s, and not the usual value of 6.63 10–34 J s, etc. All answers in the test are calculated taking these roundings into account.
In addition, in tasks with a short answer, there are requirements for recording the answer. For example: "Write down your answer in millinewtons." Or: “Multiply your answer by 10–19 and round to the nearest tenth.”
Example 13 In a cylinder with a volume of 16.6 m 3 there are 20 kg of nitrogen at a temperature of 300 K. What is the pressure of this gas? Express your answer in kilopascals and round to the nearest integer. ( Answer. 107.)
In these cases, you need to pay special attention to operations with numbers written in standard form, and to the rounding rules. In order to avoid unnecessary arithmetic difficulties and errors, it is necessary to provide training in the use of reference materials and in carrying out calculations with rounded values.
2.3. Features of tasks with a detailed answer
Tasks with a detailed answer are evaluated by two experts, taking into account the correctness and completeness of the answer. For each task for experts, detailed instructions are provided, which indicate what each point is set for - from zero to the maximum. In the examination version for the tasks of the third part, instructions are also offered, which provide general requirements for the design of answers. Currently, all six tasks with a detailed answer are estimated at 3 points maximum.
Two types of tasks are used: qualitative question and calculation tasks. Qualitative tasks allow you to test the ability to analyze physical phenomena, build logically sound reasoning, apply existing theoretical knowledge to explain phenomena from the surrounding life. As a rule, tasks are given here to explain the physical phenomena observed in the surrounding life, or to explain the experience illustrating the course of a particular physical phenomenon.
Criteria for evaluating qualitative tasks are based on the description of the complete correct solution. Such a decision must include the following elements:
- a correct indication of the observed physical phenomenon and the correct use in the explanation (if necessary) of the physical quantities and laws that characterize the course of the phenomenon;
- a logical chain of reasoning leading to the correct answer.
When teaching schoolchildren written detailed answers to qualitative problems, it is recommended to adhere to the following solution scheme: familiarization with the condition of the problem, a brief note of the condition, or creating a picture explaining the condition of the problem (as a rule, in the types of tasks listed above, the use of pictures when analyzing the condition is most effective). Analysis of the conditions of the problem. Isolation in the task of a chain of questions, on the basis of which a logical explanation is further built. The selection of physical phenomena and the physical quantities and laws that characterize them, which must be used when answering a compiled chain of questions. A record of a chain of reasoning, which is a consistent answer to the questions posed and includes indications of the selected physical phenomena, quantities and laws. The formulation of the conclusion representing the answer to the question of the problem.
All computational tasks are evaluated according to a single generalized scheme. The grading system does not depend on the method of solving the problem, it takes into account, if possible, the most typical mistakes or shortcomings made by students, and their impact on assessment is determined. A decision is considered complete and correct if it:
- the formulas expressing the physical laws, the application of which is necessary to solve the problem in the chosen way, are correctly written;
– the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed.
If the condition of the problem does not contain numerical data, then the requirements related to obtaining a numerical answer are removed. If in the task it is necessary to determine the initial data according to the graph, figure, table, and the examiner made a mistake, then the score is also reduced by 1 point. Care should be taken when rewriting the solution from the draft to the answer form, in no case should logically important steps in mathematical transformations be missed - for their absence they can reduce the score.
In CIMs there are a number of tasks, the solution of which requires the presence of a drawing. (For example, in geometric optics, where the figure explains the path of rays and the introduced designations of quantities.) In this case, the condition for the presence of a figure is introduced into the criterion for a complete correct solution, and its absence in the work leads to a decrease in the score by 1 point.
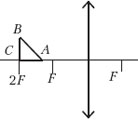 Example 14 Isosceles right triangle ABC with an area of 50 cm 2 is located in front of a thin converging lens so that its leg AC lies on the main optical axis of the lens. Lens focal length 50 cm. Top right angle C lies farther from the center of the lens than the apex acute angle A. Distance from lens center to point C equal to twice the focal length of the lens. Construct an image of a triangle and find the area of the resulting figure.
Example 14 Isosceles right triangle ABC with an area of 50 cm 2 is located in front of a thin converging lens so that its leg AC lies on the main optical axis of the lens. Lens focal length 50 cm. Top right angle C lies farther from the center of the lens than the apex acute angle A. Distance from lens center to point C equal to twice the focal length of the lens. Construct an image of a triangle and find the area of the resulting figure.
Sample possible solution (drawing required)
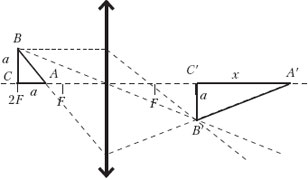
Leg length: AC=BC=a= = 10 cm.
Length x horizontal leg A′ C′ images are found by the lens formula: ![]() where is the length of the vertical leg B′ C′ of the image is equal to a, because for him d=f = 2F.
where is the length of the vertical leg B′ C′ of the image is equal to a, because for him d=f = 2F.
Image area:
Evaluation of tasks under which photographs of real experiments are given takes into account the need for correct recording of instrument readings. In this case, the condition of correct recording of readings is introduced into the decision criteria. If the readings of the instruments are recorded incorrectly and the deviation in the recording exceeds the division value of the instrument, then the expert has the right to reduce the score by 1 point.
At present, when solving tasks with a detailed answer, it is not required to record any comments about the laws or formulas used, to translate all the physical quantities specified in the condition of the problem into SI and to check the received answer “in general view» by the units of the quantities included in it. And if the decision contains laws or formulas that were not used later in the course of the decision, then errors in the records of these laws do not affect the assessment and are not grounds for lowering the assessment.
However, I would like to make two recommendations here. First: comments on the solution of the problem (about what processes are described in it, why certain laws were chosen for the solution, explaining the pictures) are not evaluated, but it is still desirable to write them. They testify to the understanding of physical processes and influence the attitude of experts to work.
“Given” to tasks is also better to write down, but in no case should you write down any consequences from the condition. For example, the problem states that isothermal process the pressure increased by 3 times, and the examiner immediately realized that the volume of gas would decrease by 3 times, and wrote this ratio in “Given”, and not in the solution through the Boyle–Mariotte law. Such an entry may be considered for the absence of one of the main equations and reduce the score.
The solution of the problem must be brought to the correct numerical answer and be sure to check it for compliance with common sense. In the Unified State Examination, tasks cannot be found in which cars move at the speed of airplanes, and bullets fly at the speed of a pedestrian, which is quite common in the works of graduates. Only if there is a correct numerical answer with the correct recording of units of physical quantities, you can get the maximum score for completing the tasks of the third part of the work.
3. Development of individual tactics for the examination test
When preparing for the exam, the student must clearly understand the level of his own claims and correlate them with his real capabilities. Using the USE training options, it is advisable to determine the time period for completing each part of the work, to develop an individual test execution tactic that would lead to the planned results.
Before each new type of tasks in the variant, instructions for formatting the answer are presented. Since the forms of tasks located in certain places in each variant,
exactly match the demo, it makes sense to study all these instructions in advance so that you don’t waste time on them during the exam.
You need to start working on the option from the first part of the work, because. multiple choice tasks are the most numerous and provide 50% of success. In addition, almost all tasks of the basic level are concentrated in this part of the work, i.e. the most simple, testing knowledge of basic physical phenomena, laws or formulas. Here it is advisable to first mark all the correct answers in the variant itself (circling them), and then transfer them to the answer sheet.
All tasks with a choice of answers are arranged in accordance with the thematic affiliation: first there are tasks in mechanics, then - in MKT and thermodynamics, then - in electrodynamics, and at the end - in quantum physics. And you need to remember that each thematic section is completed by tasks of an increased level of complexity: A7, A12, A19 and A23. Special attention should be paid to them, since sometimes behind the outwardly familiar and simple formulation there are questions that require a rather serious analysis of the physical situation.
Currently, the rules for checking the USE option do not have a system of penalties for an incorrectly completed test task. If some tasks of the first part of the work cannot be solved, you should not leave them unanswered. Using intuition and common sense, it is best to try to write down all the answers.
The second and third parts of the work together provide 50% of the maximum primary score, and tasks with a detailed answer turn out to be 3 times more “weighty”. The level of difficulty of these tasks varies significantly within one option. Here there can be both typical tasks with rather voluminous mathematical calculations, and tasks “with a twist”, in the solution of which it is necessary to clearly present the described physical processes or phenomena.
Unlike the first two parts of the work, where each task is evaluated only in the framework of "true" or "false", detailed tasks are checked by experts and evaluated on a three-point scale. With an incomplete solution or mistakes made, it is possible to get 1-2 points for the task. Therefore, if a graduate is not completely sure of the correctness of the decision, he still needs to be written down, because they are not scolded for mistakes yet, and the probability that experts will find a rational grain in these notes and appreciate it is great.
Questions for self-control
- In what parts of the USE work are tasks of an increased level of complexity?
- What elements does a complete correct solution of a qualitative problem and a calculation problem include?
Literature
- USE-2009. Physics. Federal Bank of Examination Materials/Ed.-comp. Demidova M.Yu., Nurminsky I.I. – M.: Eksmo, 2009.
- Results of the USE-2008 in physics and preparation for the USE-2009. - Physics-PS, 2009, No. , , .
- Demidova M.Yu., Nikiforov G.G. The main results of the USE-2007 in physics. - Physics-PS, 2008, No., .
- Demidova M.Yu., Gribov V.A., Nikiforov G.G. Recommendations for preparing for the USE-2008 in physics. - Physics-PS, 2008, No., .
- Demo Options on the official website of FIPI
In the analytical report Federal Institute pedagogical measurements, the results of the Unified State Examination (USE), which was held in 84 regions of the country in May-June 2008, are presented. The materials include a description of the features of the USE in 2008; brief description control measuring materials(KIM) used for the exam in 2008, and their difference from the KIM of previous years; analysis of the overall results of the exam, the performance of individual groups of tasks that differ in the content and skills being tested, as well as the examination work as a whole. Particular attention is paid to comparing the results of the unified state exam different years. Based on the analysis of the exam results, areas for improvement were identified educational process. Recommendations are given for improving the control measuring materials for the USE in each subject. The report is intended for a wide range of people: representatives of educational authorities at various levels; specialists of institutes for advanced training of teaching staff, developers of educational standards, authors of textbooks, developers of educational materials, specialists dealing with problems general education, as well as problems of assessing the quality of education. Materials may be useful to teachers and graduates educational institutions general secondary and vocational education.
The text below is automatically extracted from the original PDF document and is intended for preview purposes only.
Images (pictures, formulas, graphs) are missing.
Isoprocesses, application of the gas equation of state, understanding of graphs of isoprocesses in various coordinates. Below is an example of a task that 77% of test takers can complete. Example 5. The figure shows a graph of the state change V, 10–3 m3 of constant gas mass. In this process, the gas gave 3 an amount of heat equal to 3 kJ, as a result of which 2 its internal energy decreased by 2 1 1 1) 1.2 kJ 0 300 600 T, K 2) 1.8 kJ 3) 2.4 kJ 4 ) 3 kJ Answer: 4. The experimental foundations of the MKT are mastered at an average level of just over 65%. At the same time, it is obvious that there is a need to strengthen experimental support for this part of the section. This is clearly indicated by the results of answers to questions about the properties of diffusion and brownian motion. (For example, almost 20% of graduates believe that diffusion can only occur in gases and liquids). Of the basic concepts of thermodynamics (the amount of heat, work, internal energy), the highest level of assimilation is noted for the relationship Q = mc∆t. Depending on the method of presenting information, the average percentage of task completion ranges from 73% to 82%. The maximum success of performing direct calculations, calculation specific heat if there is a schedule, it reduces the percentage of completion by 10%. The exception here was a group of tasks for determining the specific heat of vaporization or melting according to the graph of the dependence of temperature on the amount of heat, only 45% of the examinees coped with them on average. An example of such a task is shown below. Example 6. T The dependence of the temperature of 0.2 kg of initially gaseous substance on the amount of heat released by it is shown in the figure. What is the specific heat of vaporization of this substance? 0 2 4 6 8 Q, kJ 1) 40 kJ/kg 2) 30 kJ/kg 3) 1.6 kJ/kg 4) 1.2 kJ/kg Answer: 2. 141 from the mechanical energy of the body and the state of its movement and is directly proportional to the absolute temperature, assimilated by 70% of students. The third component of the first law of thermodynamics, work, has been mastered somewhat worse. In 2008, to test this element at a basic level, fairly simple tasks were used, which essentially boiled down to the quantitative application of the relationship Q = ∆U + A for various processes. In general, compared with the previous year, the success of their implementation has increased. However, in most tasks, there was a fairly large percentage of choosing the wrong distractor, associated with shortcomings in mastering the concept of work in thermodynamics, as a process of changing internal energy. For example, in the task below, the erroneous first distractor selects almost 22% of test takers. Example 7. During the experiment, the internal energy of the gas decreased by 40 kJ, while he did the work of 35 kJ. Therefore, as a result of heat exchange, the gas gave off to the environment 1) 75 kJ 2) 40 kJ 3) 35 kJ 4) 5 kJ Answer: the state of a substance in the process of melting or crystallization (58%) and the calculation of the specific heat of melting or vaporization using graphs (45%). For the first of the above types of tasks, the most difficult was the question of adiabatic process. In it, 35% of students consider the process adiabatic, and 30% - isothermal. Example 8. In an air pump, the outlet was blocked and the air in the pump cylinder was quickly compressed. What process happens to the air in the pump cylinder? 1) isobaric 2) isochoric 3) isothermal 4) adiabatic Answer: 4. Obviously, to correct the situation, it is necessary to increase attention to the adiabatic process. Only when this process proceeds is the role of work as a means of changing internal energy most clearly and clearly visible. Usually, the adiabatic process is studied after isoprocesses, so students' attention to it is weakened. In addition, the experiment on adiabatic heating (fire flint) is still shown to schoolchildren, while the experiment on adiabatic expansion is demonstrated very rarely. 142 Electrodynamics In the section "Electrodynamics", each option contained 9 tasks with a choice of answers (7 of them at the basic level and 2 at the advanced level). At the basic level, the assimilation of the following elements was demonstrated: - Coulomb's law; - law of conservation of charge; - dependence of the capacitance of the capacitor on the area of the plates and the distance between them; - application of Ohm's law for the circuit section; - work calculation electric current; - the nature of the interaction of magnets; - voltage distribution in series connection of resistors; - dependence of the Ampere force on the current strength and the length of the conductor; - properties of the image in a flat mirror. Coulomb's law, the law of conservation of charge, the dependence of the electric capacitance of a capacitor on its size, the phenomenon of the interaction of magnets and simple calculations using Ohm's law for a circuit section are most successfully assimilated. So with assignments similar example 9, 73% of graduates manage on average. Example 9. The modulus of the force of interaction between two motionless point charged bodies is equal to F. What will be the modulus of this force if the charge of one body is increased by 3 times, and the second by 2 times? 1 1 1) 5F 2) F 3) 6F 4) F 5 6 Answer: 3. The following elements are much worse absorbed by students at the basic level: properties electric field capacitor and the dependence of its energy on voltage and capacitance (41%), calculation of electrical circuits (41%), refraction of light rays in a plane-parallel plate (50%), change in the diffraction pattern with a change in the color of the incident light (40%), electric current carriers in metals, electrolytes and semiconductors (49%). Here are examples of tasks that caused difficulties. Example 10. The first capacitor with a capacity of 3 C is connected to a current source with EMF ε, and the second one with a capacity of C is connected to a source with EMF 3ε. The ratio of the energy of the electric field of the second capacitor to the energy of the electric field of the first is 1 1) 1 2) 3) 3 4) 9 3 Answer: 3. 40% chose the correct answer, and 29% chose the first distractor. Perhaps the result obtained is due to the fact that usually in such problems it is said about the voltage across the capacitor, and not about source emf to which it is connected. In this case, the students found themselves in a changed situation and “did not recognize” the familiar formula. 143 The 2007 exam showed that there was an extremely low score (about 40%) for typical questions that offered circuits of five identical resistors connected in two parallel branches of two and three resistors, respectively, and it was necessary to determine the current or voltage on which - or from resistors. This year's results have confirmed the persistence of the problem. So, for example, only about 40% of the test-takers performed tasks on the application of the Joule-Lenz law using an electric circuit diagram, although at least 75% cope with direct calculations on the application of this formula. Even in the simplest schemes for calculating the total resistance, there is a clear lack of independence in analyzing the situation. As soon as the scheme loses its usual shape from the school problem book, students experience difficulties even with its qualitative analysis. This is evidenced by the result of performing tasks similar to example 11. Example 11. What will be the resistance of the circuit section (see figure) if the key K is closed? (Each of the resistors has a resistance R.) K 1) 2R 2) 0 3) 3R 4) R Answer: 2. Only 30% of students choose the correct answer, realizing that in this case there is a short circuit. To solve the problem, obviously, it is necessary not so much to increase the number of tasks to be solved, but to use tasks for different topologies of circuits, their transformation and design. Another striking example of the inability to act in a changed situation and follow common sense is the nature of the performance by graduates of a group of tasks for calculating power. An example of one of these tasks is shown below. Example 12. There is a fuse at the entrance to the electrical circuit of the apartment that opens the circuit at a current of 10 A. The voltage supplied to the circuit is 110 V. What is the maximum number of electric kettles, each with a power of 400 W, that can be simultaneously turned on in the apartment? 1) 2.7 2) 2 3) 3 4) 2.8 Answer: 2. Here, 43% of the tested understand that there can be only an integer number of dummies, but almost 40% more safely choose the first and fourth distractors, not paying attention to the meaninglessness of the answer. The fulfillment of a number of tasks of the basic level shows that the difficulties observed by students in mastering the phenomenon of light refraction, dispersion, interference and diffraction of light in 2007 persist. Thus, only 44% of the testees cope with high-quality tasks for light dispersion, and 48% of testees cope with the change in the diffraction pattern when the color of the incident light wave changes. Even 144 simple tasks, almost completely repeating the standard laboratory work (see example 13), are performed by only 52% of the test-takers. Example 13. The figure shows the path of a light beam through a glass prism. B A O D C The refractive index of glass n is determined by the ratio of the lengths of the segments CD AB OB OD 1) 2) 3) 4) AB CD OD OB Answer: 1. The results of these tasks demonstrate an underestimation in the practice of teaching the subject of a demonstration and laboratory experiment. Students do not remember the order of the colors in the spectrum, a direct demonstration of refraction in a prism is required instead of using a direct vision prism. As often as possible, the observation of phenomena should be transferred to a frontal experiment, which is much more effective in assimilating the properties of various phenomena. For optics, the entire spectrum of phenomena can be transferred to practical work students. This allows you to make L-micro sets: both “Wave Optics” and “Geometric Optics”. On the elevated level difficulties in the section "Electrodynamics" successfully completed tasks that control the following elements: - understanding of the phenomenon electromagnetic induction; - EMF of self-induction; - movement of charged particles in a magnetic field; - period and frequency of oscillations in the electromagnetic circuit (graph). The questions on the calculation of the strength of the electrostatic field of a system of two charges (42%), on the understanding of the properties of a stationary electric field (48% - see example 14) turned out to be difficult. The most difficult task was to understand how the focus of a lens placed in different environments would change. Only a group of strong students coped with such tasks, and the average percentage of completion was 35%. Example 14. A copper wire 20 cm long is included in an electrical circuit. At an electric field strength of 50 V / m, the current in the wire is 2 A. A voltage is applied to the ends of the wire 1) 10 V 2) 20 V 3) 40 V 4) 50 V Answer: 1. 145 The study of the electric field in the traditional method occurs only in electrostatics and in the study of electromagnetic waves. The results of these tasks show that the properties of the stationary homogeneous field in the conductors along which it goes D.C. not getting enough attention. Quantum physics On the topic "Elements of SRT" one line of tasks of the basic level tested the assimilation of the postulates of SRT. Unfortunately, for all series of variants, the results of implementation turned out to be extremely low: from 36 to 49%. Even the simplest tasks that test the principle of ISO equality are performed by no more than half of those tested. The principle of the constancy of the speed of light was tested using the following task: Example 15. Light from a stationary source falls υ perpendicular to the surface of a mirror that moves away from the source with a speed υ. What is the speed of reflected light in the inertial reference frame associated with the mirror? υ2 1) c–υ 2) c+υ 3) c 4) c 1− c2 Answer: 3. 47% of students indicate the correct answer here, and each of the distractors with numbers 1 and 2 is chosen by another 20%. These results mean that students not only do not learn the principle of the constancy of the speed of light, but also get confused in the application of Galileo's theorem on the addition of velocities. The results of completing assignments on the elements of SRT are a vivid example of the situation with the assimilation of the most general principles of physical science. Unfortunately, the results of the Unified State Examination of this year and previous years show that fundamental principles, laws, empirical patterns, and particular consequences, on average, are assimilated in the same way. This means that the principle of highlighting the main thing in the organization of the educational process is not implemented, a small number of fundamental laws, principles and ideas are dissolved in particulars, there is no clear definition of the status of the student being studied, the basic principles and patterns are studied at the thematic level and are not generalized as general physical. In the section "Quantum physics" in the first part of the work, five tasks were included, of which four were of a basic level and one was of an advanced level. Learned at a basic level the following content elements: - line spectra; - equations nuclear reactions, α- and β-decays; - half life; - the law of radioactive decay (determination of the half-life according to the schedule). Problematic were the tasks for determining the momentum of photons (59%) and the properties of the photoelectric effect (45%). 146 Here is one of the tasks on the use of the laws of conservation of charge and mass number - the only one in the entire set of such tasks, the success of which was 93%. Example 16. As a result of the fusion reaction of the deuterium nucleus with the X Z nucleus, a boron nucleus and a Y neutron are formed in accordance with the reaction: 1 H + X Z ⎯→ 10 B + 1 n. What are mass number 2 Y 5 0 X and charge Y (in units elementary charge ) of the nucleus that reacted with deuterium? 1) X = 11 2) X = 10 3) X = 9 4) X = 10 Y=5 Y=5 Y=4 Y=4 Answer: 3. The following elements were mastered at an increased level: equations of nuclear reactions; the red border of the photoelectric effect and the maximum kinetic energy of photoelectrons. As in the previous year, a series of tasks for understanding the laws of the photoelectric effect turned out to be extremely difficult. An example of such a task is shown below. Example 17. In experiments on the photoelectric effect, they took a metal plate with a work function of 3.5 eV and began to illuminate it with light with a frequency of 3⋅1015 Hz. Then the frequency of the light wave incident on the plate was increased by a factor of 2, leaving the intensity of the light beam unchanged. As a result, the maximum kinetic energy of photoelectrons 1) has not changed, because there will be no photoelectrons 2) increased by more than 2 times 3) increased by 2 times 4) increased by less than 2 times Answer: 2. The second distractor was chosen by 42%, the third distractor - by 31% of students. At the same time, tasks for the direct (numerical) application of the law of conservation of energy for the photoelectric effect, on which the Einstein equation is based, are performed by more than 70% of students. Methods of scientific knowledge The level of formation of methodological skills, as in the previous year, was determined using a series of tasks A30. A series of tasks were used to test the ability to select equipment when testing a formulated hypothesis, analyze the correctness of the experiment to test a particular hypothesis, and draw conclusions based on the results of the experimental graph. Tasks of the first type were used in the USE for the first time and all hypotheses were formulated on the basis of the material known and studied in the physics course. Let's take the following task as an example: 147 Example 18. Conductors are made of the same material. Which pair of conductors should be chosen in order to experimentally discover the dependence of the resistance of the wire on its diameter? 1) 2) 3) 4) Answer: 3. The success of this kind of tasks was quite high and averaged 70%. The only exception here was one task for the study of diverging lenses, which was completed by only 40% of the test subjects. On average, 72% of students coped with the analysis of graphs, although such a high result was most likely due to the need to interpret only graphs of mechanical movement. The tasks for analyzing the course of the experiment turned out to be more difficult, in which it was necessary to determine the error during its implementation. In general, about 65% of graduates coped with them. It should be noted that the system of tasks for testing methodological skills, created over two years, covers all substantive sections of the physics course and is aimed at diagnosing a fairly wide range of skills. The tasks used well differentiate students with different levels of training and make it possible to judge the formation of certain skills, regardless of the thematic affiliation of individual tasks. Therefore, it is advisable next year to increase the share of such tasks in the control measuring materials of the exam. 4.4.2. Analysis of the performance of tasks with a short answer (part 2) The second part of the examination paper contained three calculation tasks with a short answer in mechanics, MKT and thermodynamics, electrodynamics. As in 2007, problems in quantum physics were not included in this part of the work. A new moment was the appearance in this section in place of B1 of the task "for correspondence", in which it was required to answer the question about the behavior under given conditions of several physical quantities related to the same object or phenomenon. Regardless of its subject, at least 94% of the test-takers started to complete task B1. And this despite the novelty of the form of the problem. Perhaps the reason is that the task resembles multiple choice questions and does not require calculations, and is also estimated at two primary scores. Correctly answered two or all three questions, on average, 60% of those who performed these tasks. At the same time, tasks in mechanics showed the best results (change in the reaction force of the plane, speed and potential energy of the bar when it moves along an inclined plane), which, on average, were correctly completed by 74% of the tested. The tasks for changing physical quantities in the process of mechanical vibrations turned out to be the most difficult. An example of one of these tasks is shown below. 148 Example 19. A load of mass m, suspended from a spring, oscillates with period T and amplitude x0. What will happen to the period, oscillation frequency and maximum potential energy of the spring if the mass is reduced at a constant amplitude? For each position of the first column, select the corresponding position of the second and write down the selected numbers in the table under the corresponding letters. PHYSICAL VALUES OF THEIR CHANGES A) the period of oscillations 1) will increase B) the frequency of oscillations 2) will decrease C) the maximum potential energy springs 3) will not change A B C Transfer the resulting sequence of numbers to the answer sheet (without spaces or any symbols). Answer: 213. 97% of those tested started to solve this task, 54% answered all three questions correctly, and 14% of those who started solving answered any two questions. New assignments for compliance test knowledge of various formulas and laws of physics, but without using even elementary mathematical calculations. At the same time, the results of their performance turned out to be better than for similar tasks with a choice of answers, in which computational skills also have to be connected to construct distractors. Therefore, it can be recommended to expand the range of tasks for compliance in the examination options and use them as tasks of the basic level. An average of 82% of the test-takers started to perform calculation tasks B2-B4, and an average of 42% of those who solved them received the correct answer in them. (As in previous years, answers received, for example, by incorrect rounding or using values of physical constants different from those given in the table attached to the option, were counted as correct.) Table 4.11 shows the topics of all the tasks used and the average percentage of completion each series of tasks from total number tested. Table 4.11 Results of completing tasks with a short answer Designation Average % Checked content in the execution work Law of conservation of momentum 35 B2 Kinetic energy 20 Harmonic vibrations 28 Application of the Mendeleev-Clapeyron equation 49 B3 Application of the equation heat balance(heating and melting) 35 Coulomb's law 26 Ohm's law for a closed circuit 42 Movement of a charged particle in a uniform magnetic field. Force B4 17 Lorentz Law of electromagnetic induction 29 149 The simplest of the calculation tasks in the second part of the work were the tasks on the topics "Application of the Mendeleev-Clapeyron equation" and "Ohm's law for a closed circuit". Here is a typical example of a task that more than half of the test-takers (57% of the participants) cope with. Example 20. In a cylinder with a volume of 1.66 m3 there is 2 kg of gas at a pressure of 105 Pa and a temperature of 47°C. What is molar mass gas? Express your answer in g/mol. Answer: 32. A high percentage of those who solved this problem can be explained by the fact that in order to solve it, you just had to substitute the numbers given in the condition into a well-known ready-made formula and calculate the value of the resulting fraction. However, it is necessary to formulate a problem on the same topic with the same working formula a little less familiarly, as in Example 21 below, and only 25% of the examinees solve it correctly. Example 21. The atmosphere of Venus consists mainly of carbon dioxide with a molar mass МВ = 44⋅10–3 kg/mol, has a temperature (near the surface) of about 700 K and a pressure of 90 Earth atmospheres. For the Earth's atmosphere, the surface temperature is close to 300 K. What is the ratio of atmospheric densities near the surfaces of Venus and the Earth? Round your answer to the nearest integer. Answer: 59. The lowest results were shown when solving problems on the topic “Motion of a charged particle in a uniform magnetic field. Lorentz force. Here is an example of such a task. Example 22. The ion beam enters the mass spectrometer chamber through the hole at point A with a velocity υ = 3⋅104 m/s, directed B perpendicular to the AS wall. A uniform υ magnetic field is created in the chamber, the lines of the induction vector of which are perpendicular to the ion velocity vector. Moving in this field, the ions hit an A C target located at point C at a distance of 18 cm from point A (see figure). What is induction magnetic field B, if the ratio of the ion mass to its charge m = 6⋅10–7 kg/C? q Answer: 0.2. The problem is typical, when studying the Lorentz force, these problems are dealt with in large numbers. However, a formal increase in the number of equations and mathematical calculations leads to a sharp decrease in the results. The propensity for typical formulations has also added problems, where the radius of the circle is usually given, and here the AC distance is equal to the diameter. Result: only 70% of those tested started solving the problem, 29% of those who started solving got the correct answer, i.e. 20% of participants. Compared to last year, the average percentage of completion of calculation tasks with a short answer remained unchanged (31%), however, the scatter in statistical data slightly decreased when performing both individual series of tasks and across 150






