Project. Demo version of exam questions. in both liquid and solid states
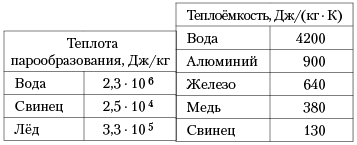
To cool a 200g lemonade, ice cubes were thrown into it at 0°C. The mass of each cube is 8 g. The initial temperature of the lemonade is 30°C. How many whole cubes must be thrown into the lemonade to bring the temperature to 15°C? The specific heat capacity of lemonade is the same as that of water.
Answer:
m=0.2, specific heat capacity of water= 4200J; according to the formula Q \u003d cm (t2-t1) Q \u003d 4200 * 0.2 * (30-15) \u003d 12.6 kJ; specific heat of ice melting = 3.3 * 10 in the fifth J / kg Q = m * 3.3 kJ / kg + m * 4.2 * (15 - 0); Q= m* 393kJ/kg; 12.6 \u003d m * 393; m = 31.2 approximately 32g; 32g/8g = 4(cubes)
Similar questions
- Please, help! Two boys simultaneously ran to meet each other along the sports track. length 100 m. They met after 10 s. The first boy ran at a speed of 4 m/s. How fast was the second boy running?
- II. Complete the sentences with the present perfect or past perfect 1. Who is that woman? I…… never……. before (see). 2. The house was dirty. She ………… it for weeks (not clean). 3. I didn't know who that old man was. I …………… never …………… him before (see). 4. We are hungry. We …………… yet (not have dinner). 5. The office is clean. She…………… just………… it (wash). 6. We weren "t hungry. We……………just……………lunch (have). 7. I ………….. my report(prepare), now I can have a rest. 8. The archaeologist found an ancient piece of jewelry. They …………. anything of that kind before (never see).
Seminar 10.
Heat balance equation.
Some 30kW plant is cooled by running water flowing through a special pipe with a diameter of 15mm. In the steady state, running water is heated by 15 0 C. Determine the speed of the water, assuming that the entire power of the installation is used to heat the water.
A bullet flying at a speed of 1000 m/s hits a melting ice floe. Bullet weight 10g. Assuming that 50% kinetic energy turns into heat and goes to melt the ice, find how much ice has melted. An iron ball with a radius of 1 cm, heated to 120 0 C, is placed on ice. To what depth will the ball plunge into the ice if the specific heat capacity of iron is 461J/kgK and the density is 7800kg/m3? Temperature environment 0 0 C.
In a snow melter with an efficiency of 25%, 2 tons were burned. firewood. What area can be freed from snow at a temperature of -5 0 C when burning such an amount of fuel, if the snow cover is 50 cm thick? The density of snow is 300 kg/m3, the specific heat of snow is 1.7 kJ/kgK, the specific heat of melting of snow is 0.335 MJ/kg, the heat of combustion of firewood is 12.57 MJ/kg.
The flask contained water at a temperature of 0 0 C. Having pumped out the air from the flask, they froze all the water by evaporating it. What part of the water evaporated at the same time, if there is no heat inflow from the outside?
For operation, the engine consumes 150 kg of coal in 1 hour. It is cooled by water, the temperature of which at the inlet is 12 0 C, and at the outlet 27 0 C. Determine the water flow in 1 s if 25% of the total amount of heat is spent on heating the water.
The figure shows a graph of temperature T of a substance versus time t. At the initial moment of time, the substance was in a crystalline state. Which of the points corresponds to the end of the melting process of the substance?
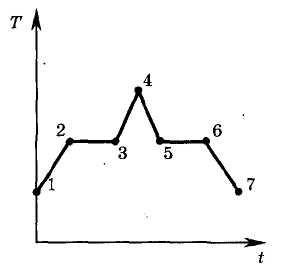
The graph shows the dependence of the temperature T of the substance on time t. At the initial moment of time, the substance was in a crystalline state. Which of the points corresponds to the end of the curing process?
To determine the specific heat of melting of ice, pieces of melting ice were thrown into a vessel with water weighing 300 g and a temperature of 20 ° C with continuous stirring. By the time when the ice stopped melting, the mass of water increased by 84 g. Determine the specific heat of ice melting from the experimental data. Express your answer in kJ/kg.
To cool a 200 g lemonade, ice cubes are thrown into it at 0°C. The mass of each cube is 8 g. The initial temperature of the lemonade is 30°C. How many whole cubes must be thrown into lemonade to bring the temperature to 15°C? Ignore heat loss. The specific heat capacity of lemonade is the same as that of water.
To cool a 200 g lemonade, ice cubes are thrown into it at 0°C. The mass of each cube is 8 g. What is the initial temperature (in Celsius) of lemonade if the temperature is 15 ° C after 4 cubes are thrown into it? The specific heat capacity of lemonade is equal to the specific heat capacity of water. Ignore heat loss. Round your answer to the nearest integer.
Test work on the topic "Thermodynamics".
Option 1.
A 1. What happens during the isothermal expansion of a gas at a constant mass?
give him some warmth his internal energy increases his pressure rises Work external forces positive.A 2. Which expression corresponds to the first law of thermodynamics applied to the isochoric process?
1. ΔU=Q.
2. ΔU=A.
3. ΔU = 0.
4. Q = -A.
At the same temperature, saturated water vapor in a closed vessel differs from unsaturated steam
An ideal gas received an amount of heat of 300 J, and the internal energy of the gas increased by 100 J. At the same time
To cool lemonade weighing 200 g, ice cubes at a temperature of 0 ° C are thrown into it. The mass of each cube is 8 g. The initial temperature of the lemonade is 30ºС. How many whole cubes must be thrown into lemonade to reach a temperature of 15º C? Ignore heat loss. The specific heat capacity of lemonade is the same as that of water.
Match the title physical quantity and the formula by which it can be determined.
TITLE | FORMULA |
||
The amount of heat required to heat up a body | |||
Specific heat melting crystalline substance | |||
The amount of heat released during the combustion of fuel | |||
0.5 kJ | 1.0 kJ | 1.5 kJ | 2.0 kJ |
The heat engine has an efficiency of 25%. The average power of heat transfer to the refrigerator during its operation is 3 kW. How much heat is received by the working body of the machine from the heater in 10 s?
0.4 J | 400 J | 40 kJ |
Test work on the topic "Thermodynamics"
Option 2.
The figure shows a graph of absolute temperatureT water massm from timet in the implementation of heat removal with constant power P. At the time t = 0 water was in gaseous state. Which of the following expressions defines specific heat ice according to the results of this experiment?
0 "style="border-collapse:collapse">
A monatomic ideal gas in an amount of 4 moles absorbs an amount of heat of 2 kJ. In this case, the gas temperature rises by 20 K. The work done by the gas in this process is equal to
0.5 kJ | 1.0 kJ | 1.5 kJ | 2.0 kJ |
What is the work done by the gas during the transition from state 1 to state 2 (see Fig.)?
four). There is not enough data to calculate the work.

In a heat engine, the heating temperature is K, the temperature of the refrigerator is 200 K lower than that of the heater. Maximum possible machine efficiency equals
1) 0,/3 3) 0,5 4) 1/3
The hot liquid was slowly cooling in the glass. The table shows the results of measurements of its temperature over time.
Time, min | ||||||||
Temperature, °С |
In the beaker, 7 min after the start of measurements, there was a substance
only in liquid state |
|
only in solid state |
|
in both liquid and solid states |
|
in both liquid and gaseous states |
|
Using the first law of thermodynamics, establish a correspondence between the features of the isoprocess described in the first column in an ideal gas and its name.
FEATURES OF THE ISOPROCESS | NAME OF THE ISOPROCESS |
||
All the amount of heat transferred to the gas is used to perform work, and the internal energy of the gas remains unchanged. | isothermal isobaric isochoric |
||
Change internal energy gas occurs only due to the performance of work, since there is no heat exchange with the surrounding bodies. | adiabatic |
Transfer the resulting sequence of numbers to the answer sheet (without spaces or any symbols).
A man with glasses entered the warm room from the street and found that his glasses were fogged up. What should be the outside temperature for this phenomenon to occur? The room temperature is 22°C and relative humidity air 50%. Explain how you got the answer.
(When answering this question, use the table for the pressure of saturated vapors of water.)
Pressure saturated vapors water at different temperatures
p, kPa |
p, kPa |
What is the work done by the gas during the transition from the state 1 into a state 3 (see picture)?
10 kJ |
|
20 kJ |
|
30 kJ |
|
40 kJ |

The vessel contains a constant amount ideal gas. How will the temperature of the gas change if it passes from the state 1 into a state 2 (see picture)?
T 2 = 4T 1 |
|
T 2 = 14T 1 |
|
T 2 = 43T 1 |
|
T 2 = 34T 1 |

In a heat-insulated vessel with a large amount of ice at a temperature of 00C, pour 1 kg of water at a temperature of 440C. What mass of ice will melt when thermal equilibrium is established? Express your answer in grams.
After the stove was heated in the room, the temperature rose from 150C to 270C. By what percentage has the number of air molecules in the room changed?
Answers to the test work on "Thermodynamics"
Option 1.
A 1. - 1
A 2. - 2
A 3. - 1
A 4. - 2
A 5. - 4
A 6. - 2
A 7. - 4
B 1 - 415
AT 21
From 1 - decreased by 6 times.
C 2 - 62.7%
Option 2.
A 1 - 4
A 2 - 2
A 3 - 2
A 4 - 4
A 5 - 3
A 6 - 1
A 7 - 1
At 1 - 14
At 2 - 560
C 1 - not higher than 110C
From 2 - by 4% decreased
Project. Demonstration version of exam tasks
Work instructions
To complete the examination paper in physics, 3.5 hours (210 minutes) are allotted. The work consists of three parts, including 35 tasks.
Part 1 contains 25 tasks ( BUT 1–BUT 25). Each question has 4 possible answers, of which only one is correct.
Part 2 contains 5 tasks ( AT 1–AT 5), to which you should give a short answer in the form of a set of numbers.
Part 3 consists of 5 tasks ( FROM 1–FROM 5), which require a detailed answer.
When calculating, it is allowed to use a non-programmable calculator.
Read each question carefully and the suggested answers, if any. Answer only after you have understood the question and analyzed all possible answers.
Complete the tasks in the order in which they are given. If a task is difficult for you, skip it. You can return to missed tasks if you have time.
For completing tasks of various complexity, 1 or more points are given. The points you get for completed tasks are summed up. Try to complete as many tasks as possible and score the most points.
We wish you success!
The following are reference data that you may need to get the job done.
Decimal Prefixes
Name |
Designation |
Factor |
Name |
Designation |
Factor |
| giga- | G | 10 9 | centi | With | 10 –2 |
| mega- | M | 10 6 | milli | m | 10 –3 |
| kilo- | to | 10 3 | micro | mk | 10 –6 |
| hecto | G | 10 2 | nano | n | 10 –9 |
| deci- | d | 10 –1 | pico- | P | 10 –12 |
Constants
Ratio between different units
Particle mass
Density, kg / m 3
Normal conditions:
pressure 10 5 Pa, temperature 0 °С
Molar mass, kg/mol
Part 1
When completing the tasks of part 1 in the answer sheet No. 1 under the number of the task you are performing ( BUT 1–BUT 25) put the sign "" in the box, the number of which corresponds to the number of the answer you have chosen.
BUT 1. The figures show graphs of the dependence of the acceleration module on time for different types movement. Which graph corresponds to uniformly accelerated motion?
BUT 2. An airplane flies in a straight line at a constant speed at an altitude of 9000 m. The frame of reference associated with the Earth is considered to be inertial. In this case:
1) the aircraft is not affected by gravity;
2) no forces act on the aircraft;
3) the sum of all forces acting on the aircraft is zero;
4) gravity is equal to the Archimedes force acting on the aircraft.
BUT 3. Two small balls of mass m each when the distance between their centers is r, are attracted to each other with force F. What is the force of gravitational attraction of the other two balls, if the mass of one is 3 m, the mass of another m/3, and the distance between their centers is 3 r?
BUT 4. When a spring is stretched by 0.1 m, an elastic force equal to 2.5 N arises in it. Determine the potential energy of this spring when stretched by 0.08 m.
1) 25 J; 2) 0.16 J; 3) 0.08 J; 4) 0.04 J.
BUT 5. If the weight of the mathematical pendulum is increased by 4 times, then the period of its small free oscillations:
1) will increase by 4 times; 2) will increase by 2 times;
3) decrease by 4 times; 4) will not change.
BUT 6. Bar mass M= 300 g connected to a block of mass m= 200 g of a weightless and inextensible thread thrown over a weightless block. What is the acceleration modulus of the bars? Ignore friction.
1) 6 m / s 2; 2) 2 m / s 2; 3) 3 m / s 2; 4) 4 m/s 2 .
BUT 7.
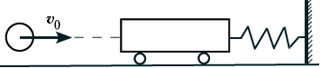 A plasticine ball with a mass of 0.1 kg has a speed of 1 m/s. It hits a stationary trolley with a mass of 0.1 kg, attached to a spring, and sticks to the trolley. What is the total mechanical energy of the system during its further vibrations? Ignore friction.
A plasticine ball with a mass of 0.1 kg has a speed of 1 m/s. It hits a stationary trolley with a mass of 0.1 kg, attached to a spring, and sticks to the trolley. What is the total mechanical energy of the system during its further vibrations? Ignore friction.
1) 0.025 J; 2) 0.05 J; 3) 0.5 J; 4) 0.1 J.
BUT 8. At a temperature T 0 and pressure p 0 1 mole of an ideal gas occupies a volume V 0 . What is the volume of 2 moles of gas at the same pressure p 0 and temperature 2 T 0 ?
1) 4V 0 ; 2) 2V 0 ; 3) V 0 ; 4) 8V 0 .
BUT 9. At the same temperature, saturated water vapor in a closed vessel differs from unsaturated vapor:
1) concentration of molecules;
2) the speed of movement of molecules;
3) average energy of chaotic motion of molecules;
4) the absence of admixture of foreign gases.
BUT 10.
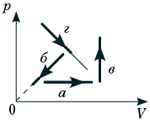 The figure shows graphs of four processes of changing the state of an ideal gas. Isochoric heating is a process:
The figure shows graphs of four processes of changing the state of an ideal gas. Isochoric heating is a process:
1) a; 2) b; 3) in; 1) G.
BUT 11. An ideal gas received an amount of heat of 300 J, and the internal energy of the gas increased by 100 J. At the same time:
1) the gas did the work 400 J;
2) the gas did the work 200 J;
3) work done on the gas was 400 J;
4) 100 J have done work on the gas.
BUT 12. To cool a lemonade weighing 200 g, ice cubes at a temperature of 0 ° C are thrown into it. The mass of each cube is 8 g. The initial temperature of the lemonade is 30 °C. How many whole cubes must be thrown into the lemonade to bring the temperature to 15°C? Ignore heat loss. The specific heat capacity of lemonade is the same as that of water.
1) 1; 2) 2; 3) 3; 4) 4.
BUT 13 . How will the force of the Coulomb interaction of two point charges change if the distance between them is reduced by 2 times?
1) will increase by 2 times; 2) will decrease by 2 times;
3) will increase by 4 times; 4) will decrease by 4 times.
BUT 14.

A direct current flows through the circuit I\u003d 4 A. What does the ammeter show? Ignore the resistance of the ammeter.
1) 1 A; 2) 2 A; 3) 3 A; 4) 1.5 A.
BUT 15.
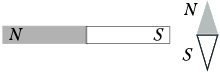 Towards a magnetic needle (the north pole is shaded) that can turn around vertical axis, perpendicular to the plane of the drawing, brought a permanent bar magnet. In this case, the arrow:
Towards a magnetic needle (the north pole is shaded) that can turn around vertical axis, perpendicular to the plane of the drawing, brought a permanent bar magnet. In this case, the arrow:
1) rotate 90° counterclockwise;
2) rotate 90° clockwise;
3) turn 180°;
4) will remain in the same position.
BUT 16.
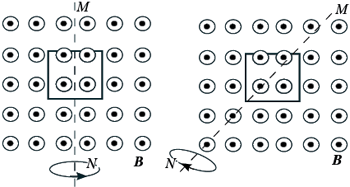
The figure shows two ways of rotating a wire frame in a uniform magnetic field, the lines of induction of which go from the plane of the drawing. Rotation occurs around an axis MN. Loop current:
1) exists in both cases;
2) does not exist in any of the cases;
3) exists only in the first case;
4) exists only in the second case.
BUT 17.
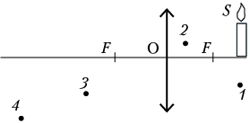
At which of the points shown in the figure ( 1 , 2 , 3 or 4 ), there will be an image of a candle flame ( S) produced by a converging lens?
1) At the point 1 ; 2) at the point 2 ; 3) at the point 3 ; 4) at the point 4 .
BUT 18.
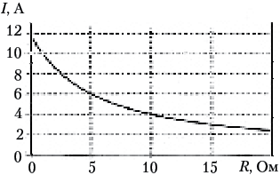
An electrical circuit consists of a current source and a resistor. The figure shows a graph of the dependence of the current in the circuit on the resistance of the resistor. What is the internal resistance of the current source?
1) 12 Ohm; 2) 5 ohm; 3) 0.5 ohm; 4) 0.4 ohm.
BUT 19. A normally parallel beam of white light is incident on a diffraction grating with a period of 2 × 10–5 m. The spectrum is observed on a screen behind a grating at a distance of 2 m from it. What is the distance between the red and violet portions of the first-order spectrum (the first color strip on the screen), if the wavelengths of red and violet light are approximately equal to 8 × 10–7 m and 4 × 10–7 m, respectively? Read sin = tg.
1) 4 cm; 2) 8 cm; 3) 1 cm; 4) 0.4 cm.
BUT 20. In which of the electromagnetic radiation ranges listed below is the photon energy the lowest?
1) x-rays;
2) ultraviolet radiation;
3) visible light;
4) infrared radiation.
BUT 21. E. Rutherford's model of the atom describes the atom as:
1) a homogeneous electrically neutral body of a very small size;
2) a ball of protons surrounded by a layer of electrons;
3) a solid homogeneous positively charged sphere with inclusions of electrons;
4) a positively charged small nucleus around which electrons move.
BUT 22. The half-life of some radioactive isotope equals 1 month. How long will it take for the initially large number of nuclei of this isotope to decrease by a factor of 32?
1) 3 months; 2) 4 months; 3) 5 months; 4) 6 months.
BUT 23. The work function of electrons for the metal under study is 3 eV. What is the maximum kinetic energy of photoelectrons emitted from the surface of a metal plate under the action of light whose wavelength is 2/3 of the wavelength corresponding to the red border of the photoelectric effect for this metal?
BUT 24.
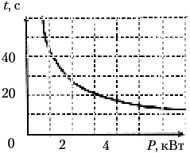
The dependence of the boiling time of a certain amount of water on the power of the boiler was experimentally studied. Based on the measurement results, a graph is constructed, shown in the figure. What conclusion can be drawn from the results of the experiment?
1) Heating time is directly proportional to the heater power;
2) with an increase in the power of the heater, the water heats up faster;
3) heater power decreases over time;
4) the heat capacity of water is 4200 J/(kg °C).
BUT 25. In the laboratory, the dependence of the voltage on the capacitor plates on the charge of this capacitor was studied. The measurement results are presented in the table.
Measurement errors q and U were 0.05 μC and 0.25 kV, respectively. The capacitance of the capacitor is approximately equal to:
1) 250 pF; 2) 10 nF; 3) 100 pF; 4) 750 uF.
Answers(for inspectors)
| job number | Answer | job number | Answer |
| BUT 1 | 4 | BUT 14 | 3 |
| BUT 2 | 3 | BUT 15 | 1 |
| BUT 3 | 2 | BUT 16 | 1 |
| BUT 4 | 3 | BUT 17 | 4 |
| BUT 5 | 4 | BUT 18 | 2 |
| BUT 6 | 2 | BUT 19 | 1 |
| BUT 7 | 1 | BUT 20 | 4 |
| BUT 8 | 1 | BUT 21 | 4 |
| BUT 9 | 1 | BUT 22 | 3 |
| BUT 10 | 3 | BUT 23 | 2 |
| BUT 11 | 2 | BUT 24 | 2 |
| BUT 12 | 4 | BUT 25 | 3 |
| BUT 13 | 3 |






