Which substances have a constant melting point. Melting
ESSAY
"Melting bodies"
Performed:
Prisyazhnyuk Olga 9-A
Checked:
Nevzorova Tatyana Igorevna
Introduction
1) Calculation of the amount of heat
2) Melting
3) Specific heat of fusion
4) Melting of metals
5) Melting point and boiling point of water
6) Melts
7) Interesting about melting
Use of knowledge in other contexts, related conditions
The properties of matter and especially water play a role in many phenomena. Here, for example, the meteorological cycle of water and the evapotranspiration of living organisms should be mentioned. Icebergs are made up of pure water, which is why they are considered sources of drinking water. But also production fresh water from salt water in the seas is theoretically and technically not a difficulty and is a frequently applied procedure in countries with little drinking water but rich energy sources. The only problem is economic viability.
Conclusion (conclusions)
Introduction
Aggregate state - a state of matter characterized by certain qualitative properties: the ability or inability to maintain volume and shape, the presence or absence of long-range and short-range order, and others. A change in the state of aggregation may be accompanied by an abrupt change free energy, entropy, density and other basic physical properties.
On the Internet you will find many pages that say melting and evaporation. Of course, there are many more. But some questions remain as good as ever unanswered. Why do some substances have a very low melting point while others have a high melting point?
- What processes occur during melting and evaporation?
- So why do fabrics have such different points boiling?
What processes take place during melting?
Why do some substances not melt or boil, but decompose earlier? . The terms boiling and evaporation are used in the same way on this page. More information can be found on this page.
First step: how the solid is created
Every solid is made up of tiny particles. There are forces between the particles that hold them together. They are always electrostatic. The nature of the particles and the strength of the forces are very numerous. You can find the exact collection on my Bindings page. Here is a short summary.There are three main states of aggregation: solid, liquid and gas. Sometimes it is not quite correct to classify plasma as a state of aggregation. There are other states of aggregation, for example, liquid crystals or Bose-Einstein condensate.
Changes in the state of aggregation are thermodynamic processes called phase transitions. The following varieties are distinguished: from solid to liquid - melting; from liquid to gaseous - evaporation and boiling; from solid to gaseous - sublimation; from gaseous to liquid or solid - condensation. A distinctive feature is the absence of a sharp boundary of the transition to the plasma state.
Second step: what happens when a solid is heated below its melting point?
If a substance is heated by heating, the smallest particles absorb this energy. They say that their internal energy is growing. Internal energy can be of three types: vibrational energy, rotational energy, and electron energy. The electron energy is irrelevant for melting and boiling. Electrons that reach over high level energy, stay there only for a while, and then return to the original level. The energy is released and leaves the solid. Here, phenomena such as phosphorescence and fluorescence occur.
To describe various states in physics, a broader concept of a thermodynamic phase is used. Phenomena that describe transitions from one phase to another are called critical phenomena.
Solid: A state characterized by the ability to maintain volume and shape. Atoms of a solid body make only small vibrations around the state of equilibrium. There is both long-range and short-range order.
Let's look at vibration, shall we? and the rotational force is a little closer. Small particles move in a certain way. Some of the possibilities for these movements are now listed. Stretching. The 2 atoms connected by a bond vibrate along the bonding axis so that the bond periodically becomes shorter and longer. Only molecules can carry out stretching vibrations, since they only have a bond within the particle. Bending vibration. 3 bonded atoms vibrate so that the angle between the two bonds is periodically smaller and larger. Molecules of at least 3 atoms can cause bending vibrations. Larger and more complex molecules can perform complex complex vibrations. A simple example is the inversion of the ammonia molecule. The whole particle can rotate around its center of gravity. Part of the molecule can rotate around one bond.
- Vibrations of a particle around its central position.
- That is, an ion or molecule oscillates back and forth like a pendulum.
- Each of the smallest particles from the first step can carry out such vibrations.
Liquid: The state of a substance in which it has low compressibility, that is, it retains volume well, but is unable to retain its shape. The liquid easily takes the shape of the vessel in which it is placed. Atoms or molecules of a liquid vibrate near the equilibrium state, trapped by other atoms, and often jump to other free places. There is only short-range order.
At low temperatures, particles carry only slow, low-amplitude oscillations and rotate slowly. If the solid is heated, the vibrations and rotations become faster and the vibrations increase in amplitude. Therefore, the smallest particles absorb the heat of heat and convert it into energy of motion.
Third step: What processes take place during melting?
Can the smallest particles absorb an unlimited amount of heat? In the previous two paragraphs, we saw two properties of the smallest particles in a solid. Electrostatic attractors work between them. They lead to a certain binding energy. It does not depend on temperature. On the other hand, kinetic energy contained in the particles. This is more the higher the temperature.
Gas: A state characterized by good compressibility, lacking the ability to retain both volume and shape. Gas tends to occupy the entire volume provided to it. Atoms or molecules of a gas behave relatively freely, the distances between them are much greater than their size.
Other states: Upon deep cooling, some (by far not all) substances pass into a superconducting or superfluid state. These states, of course, are separate thermodynamic phases, but they hardly deserve to be called new aggregate states of matter due to their non-universality. Inhomogeneous substances such as pastes, gels, suspensions, aerosols, etc., which under certain conditions exhibit the properties of both solids and liquids and even gases, are usually classified as dispersed materials, and not to any specific aggregate states substances.
If a solid body is heated, the kinetic energy of its smallest particles increases. At some point, it is greater than the bond energy. It can be imagined that the attractive forces between particles are no longer strong enough to withstand the forces caused by thermal fluctuations. The particles now move independently of each other, and this is precisely the characteristic of the liquid. The solid melts.
Why are liquids liquid?
In a liquid, the particles are thus no longer held together by the forces of electrostatic attraction. The red arrow shows what happens when you. the solid is heated at low pressure. This process is called sublimation. This is easy to understand because the line of argument is simple. The binding energy is too low, the attractive forces are too weak to hold the particles together, so they move apart. However, if you do not just know the information about school books, you will find a contradiction here and ask yourself a question.
Melting
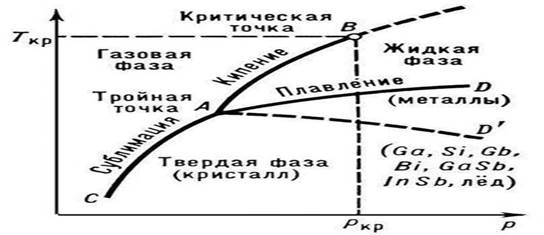
Rice. 1. State of pure matter (diagram)
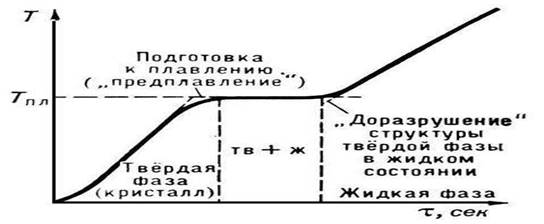
Rice. 2. Melting temperature of a crystalline body
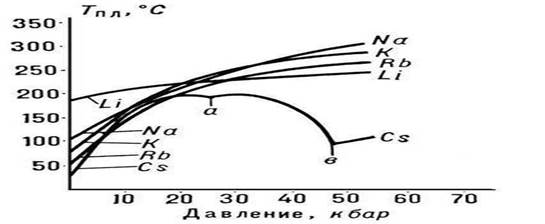
Rice. 3. Melting point alkali metals
Melting - the transition of a substance from a crystalline (solid) state to a liquid; occurs with the absorption of heat (phase transition of the first order). The main characteristics of P. of pure substances are the melting point (Tmelt) and the heat that is necessary for the implementation of the P. process (heat of melting Qmelt).
In a liquid, the particles are almost always not separated from each other, they are still almost as close as in a solid. Why don't they film themselves like they're in gas? In other words, why does the solid not immediately go into a gaseous state during melting?
Pressure plays a key role
They do it at low pressure. Only at high pressure do they become liquid. Is it the pressure that keeps solid particles from directly entering the gaseous state? Is pressure the reason liquids exist at all?
P.'s temperature depends on the external pressure p; on the state diagram of a pure substance, this dependence is depicted by the melting curve (the curve of the coexistence of solid and liquid phases, AD or AD "in Fig. 1). The melting of alloys and solid solutions occurs, as a rule, in the temperature range (with the exception of eutectics with a constant Tmelt) The dependence of the temperature of the beginning and end of the P. of an alloy on its composition at a given pressure is depicted on state diagrams by special lines (liquidus and solidus curves, see Binary systems). from a solid crystalline state to an isotropic liquid occurs in stages (in a certain temperature range), each stage characterizes a certain stage of destruction crystal structure.
Let's take a closer look at the role of pressure. Particles inside are surrounded by neighbors. surrounded and attracted by them. Particles on the surface have several neighbors. Look at the picture on the right. It shows part of the liquid, but the ratios in solids are basically the same.
The particles in the image are spherical, but the ratios are the same for molecules or ions of different shapes. First, look at the particle inside. It is surrounded by other particles in all directions. There are attractors between the particle and all its neighbors. These are the Coulomb forces, which were described in detail in the first step. Naturally, they are present in both liquid and solid, and they are still as strong as they were before melting. They are represented by black lines in the figure. One particle is highlighted, it has 7 neighbors, which are affected by Coulomb forces.
The presence of a certain temperature P. is an important sign of the correct crystal structure solid bodies. On this basis, they are easy to distinguish from amorphous solids, which do not have a fixed Tm. Amorphous solids pass into the liquid state gradually, softening with increasing temperature (see Amorphous state). Tungsten has the highest temperature among pure metals (3410°C), and mercury has the lowest temperature (-38.9°C). Particularly refractory compounds include: TiN (3200 °C), HfN (3580 °C), ZrC (3805 °C), TaC (4070 °C), HfC (4160 °C), etc. As a rule, for substances with high Tm is characterized by higher values of Qm. The impurities present in crystalline substances ah, lower their Tm. This is used in practice to obtain alloys with low Tmelt (see, for example, Wood's alloy with Tmelt = 68 °C) and cooling mixtures.
One of them had already moved away from him. If it moves further, it will completely leave the medium of the first particle. But it is still in the liquid because it will get close to another particle and a bond between them will form for a short time. Particles inside the liquid cannot leave them, as they barely move from one neighboring particle, they are attracted by another.
In the case of particles on the liquid surface, the situation is different. The image shows two such particles. They have neighbors only inside the liquid, and only for them the Coulomb forces. Outwardly, there are hardly any neighbors. There is air or some other gas. It has a very low density compared to a liquid, so it contains far fewer particles per unit volume. You will find that you need to take a closer look at the particles on the surface. There is a key to the answer.
P. begins when the crystalline substance reaches Tpl. From the beginning of P. until its completion, the temperature of the substance remains constant and equal to Tmelt, despite the transfer of heat to the substance (Fig. 2). Under normal conditions, it is not possible to heat a crystal to T > Tmelt (see Overheating), while significant supercooling of the melt is relatively easy to achieve during crystallization.
Particles with more or less energy
Energy distribution of particles in a liquid. We have always said that particles have a certain energy due to thermal fluctuations, and one might think that all particles in a drop of liquid have the same energy. Some of them have a higher energy, others have a lower one. This is easy to understand given that the particles collide. Depending on the speed and direction in which they collide, energy is transferred from one particle to another. Since the encounters are fairly random, there are winners whose energy rises from punch to punch, as well as losers.
The nature of the dependence of Tm on pressure p is determined by the direction of volumetric changes (DVm) at P. (see Clapeyron-Clausius equation). In most cases, P. of a substance is accompanied by an increase in their volume (usually by several percent). If this is the case, then an increase in pressure leads to an increase in Tm (Fig. 3). However, in some substances (water, a number of metals and metallides, see Fig. 1), during P., a decrease in volume occurs. The temperature of P. of these substances decreases with increasing pressure.
Particle energy distribution in one. Both distributions differ slightly. The particles evaporate. The area to the right of the red line at the bottom. Therefore, at 20°C, fewer particles have the energy needed to evaporate. can be both at 50 ° C, and at this temperature, evaporation is more stable. What happens to the winners? Their energy is so great that they can overcome the Coulomb forces of their neighbors. Just because there are so few neighbors, they stand a chance of overpowering the attractiveness of all the neighbors.
And just because their energy is so great, they can not only overcome these sights for a short time, but also catch them again, but forever. These few high energy particles leave liquid droplets. They move as far as possible and never return to their drops. Everyone knows the phenomenon described above.
P. is accompanied by a change in the physical properties of the substance: an increase in entropy, which reflects the disorder of the crystalline structure of the substance; an increase in heat capacity electrical resistance[the exception is some semimetals (Bi, Sb) and semiconductors (Ge), in liquid state with higher electrical conductivity. During P., the shear resistance drops to almost zero (transverse elastic waves cannot propagate in the melt, see Liquid), the speed of propagation of sound (longitudinal waves), etc. decreases.
You have just learned why a drop of water falls. Thermal energy some particles on the surface are large enough to leave droplets. Since we're just talking about evaporation, you can also clean up quickly, as it depends on the temperature. Already directly above the melting point there will be several particles that have enough energy to leave a drop. The higher the temperature, the higher the average particle energy, and more and more particles will have the energy needed to exit the surface.
Closed systems they bring more clarity
As a result, the drop evaporates faster. The illustration below illustrates this. Once again, we were able to explain everyday experiences. An open system is a system for exchanging matter and energy with environment. For example, a drop of water is open system. Against, closed systems and closed systems. The pattern mineral water bottle is good example closed system. It is about one third filled with liquid. To avoid making an unnecessarily complicated argument, it should be filled with a clean liquid like water, not a mixture like mineral water or gasoline.
According to molecular and kinetic representations, P. is carried out as follows. When heat is applied to a crystalline body, the energy of vibrations (oscillation amplitude) of its atoms increases, which leads to an increase in body temperature and contributes to the formation of various kinds of defects in the crystal (unfilled nodes of the crystal lattice - vacancies; violations of the periodicity of the lattice by atoms embedded between its nodes, etc. ., see Defects in crystals). In molecular crystals, partial disordering of the mutual orientation of the axes of molecules can occur if the molecules do not have a spherical shape. A gradual increase in the number of defects and their association characterize the premelting stage. When Tmelt is reached, a critical concentration of defects is created in the crystal, crystallization begins—the crystal lattice breaks up into easily mobile submicroscopic regions. The heat supplied during P. is used not to heat the body, but to break interatomic bonds and destroy long-range order in crystals (see Long-range order and short-range order). In the submicroscopic regions themselves, on the other hand, the short-range order in the arrangement of atoms does not change significantly at melting point (the coordination number of the melt at Tmelt in most cases remains the same as that of the crystal). This explains the lower values of the heats of fusion Qmelt compared to the heats of vaporization and the relatively small change in a number of physical properties of substances during their melting.
The pyrolysis process plays an important role in nature (the pyrolysis of snow and ice on the earth's surface, the mineralization of minerals in its depths, and so on) and in technology (the production of metals and alloys, mold casting, etc.).
Specific heat of fusion
Specific heat of fusion (also: enthalpy of fusion; there is also an equivalent concept of specific heat of crystallization) - the amount of heat that must be imparted to one unit of mass of a crystalline substance in an equilibrium isobaric-isothermal process in order to transfer it from a solid (crystalline) state to a liquid (same the amount of heat released during the crystallization of a substance). Melting heat - special case warmth phase transition I kind. Distinguish specific heat of fusion (J/kg) and molar (J/mol).
The specific heat of fusion is denoted by the letter
(Greek letter lambda) The formula for calculating the specific heat of fusion: , - specific heat of fusion, - the amount of heat received by the substance during melting (or released during crystallization), - the mass of the melting (crystallizing) substance.
Melting of metals
When melting metals, certain rules must be observed. Suppose that they are going to melt lead and zinc. Lead will melt quickly, having a melting point of 327°; zinc, on the other hand, will remain solid for a long time, since its melting point is above 419 °. What will happen to the lead with such overheating? It will begin to be covered with a film of iridescent color, and then its surface will be hidden under a layer of non-melting powder. Lead burned out from overheating, oxidized by combining with oxygen in the air. This process, as you know, occurs at ordinary temperature, but when heated, it goes much faster. Thus, by the time the zinc begins to melt, there will be very little metallic lead left. The alloy will turn out to be completely different composition, as expected, and a large amount of lead will be lost in the form of waste. It is clear that we must first melt the more refractory zinc and then put lead into it. The same thing will happen if zinc is alloyed with copper or brass, first heating the zinc. Zinc will burn by the time the copper melts. This means that you must always first melt the metal with a higher melting point.
But this one can not avoid the frenzy. If a properly heated alloy is kept on fire for a long time, a film is again formed on the surface of the liquid metal as a result of fumes. It is clear that the more fusible metal will again turn into oxide and the composition of the plug will change; This means that the metal cannot be overheated for a long time unnecessarily. Therefore, they try in every possible way to reduce the waste of the metal, laying it in a compact mass; small pieces, sawdust, shavings are first “packaged”, pieces of more or less the same size are melted, they are heated at a sufficient temperature, and the metal surface is protected from contact with air. For this purpose, the master can take a borax or simply cover the surface of the metal with a layer of ash, which will always float on top (due to its smaller specific gravity) and will not hurt when pouring metal. When the metal solidifies, another phenomenon occurs, probably also familiar to young craftsmen. The metal, solidifying, decreases in volume, and this decrease occurs due to internal, not yet solidified metal particles. A more or less significant funnel-shaped depression, the so-called shrinkage cavity, is formed on the surface of the casting or inside it. Usually, the mold is made in such a way that shrinkage holes are formed in those places of the casting, which are subsequently removed, trying to protect the product itself as much as possible. It is clear that shrinkage holes spoil the casting and can sometimes make it unusable. After melting, the metal is slightly superheated so that it is thinner and hotter and therefore better fills the details of the mold and does not freeze prematurely from contact with a colder mold.
Since the melting point of alloys is usually lower than the melting point of the most refractory of the constituent metals of the alloy, it is sometimes beneficial to do the opposite: first melt the more fusible metal, and then the more refractory. However, this is permissible only for metals that are not strongly oxidized, or provided that these metals are protected from excessive oxidation. It is necessary to take more metal than is required for the thing itself, so that it fills not only the mold, but also the sprue channel. It is clear that you must first calculate the required amount of metal.
Melting and boiling point of water
The most surprising and blissful property of water for living nature is its ability to be a liquid under "normal" conditions. Molecules of compounds very similar to water (for example, H2S or H2Se molecules) are much heavier, but form a gas under the same conditions. Thus, water seems to contradict the laws of the periodic table, which, as you know, predicts when, where and what properties of substances will be close. In our case, it follows from the table that the properties hydrogen compounds elements (called hydrides) located in the same vertical columns should change monotonically with increasing mass of atoms. Oxygen is an element of the sixth group of this table. In the same group are sulfur S (with an atomic weight of 32), selenium Se (with an atomic weight of 79), tellurium Te (with an atomic weight of 128) and pollonium Po (with an atomic weight of 209). Consequently, the properties of the hydrides of these elements should change monotonously when passing from heavy elements to lighter ones, i.e. in the sequence H2Po → H2Te → H2Se → H2S → H2O. Which is what happens, but only with the first four hydrides. For example, the boiling and melting points rise as the atomic weight of the elements increases. In the figure, the crosses mark the boiling points of these hydrides, and the circles mark the melting points.

As can be seen, as the atomic weight decreases, the temperatures decrease quite linearly. The area of existence of the liquid phase of hydrides becomes more and more "cold", and if the oxygen hydride H2O were a normal compound, similar to its neighbors in the sixth group, then liquid water would exist in the range from -80 ° C to -95 ° C. At more At high temperatures, H2O would always be a gas. Fortunately for us and all life on Earth, water is anomalous, it does not recognize a periodic pattern, but follows its own laws.
This is explained quite simply - most of the water molecules are connected by hydrogen bonds. It is these bonds that distinguish water from liquid hydrides H2S, H2Se, and H2Te. If they were not, then the water would boil already at minus 95 ° C. The energy of hydrogen bonds is quite high, and they can be broken only at much higher high temperature. Even in gaseous state big number H2O molecules retain their hydrogen bonds, uniting into (H2O)2 dimers. Fully hydrogen bonds disappear only at a water vapor temperature of 600 °C.
Recall that boiling consists in the fact that vapor bubbles form inside a boiling liquid. At normal pressure, pure water boils at 100 "C. If heat is supplied through the free surface, the process of surface evaporation will be accelerated, but volumetric evaporation characteristic of boiling does not occur. Boiling can also be carried out by lowering the external pressure, since in this case the pressure vapor equal to the external pressure is achieved at a lower temperature.At the top is very high mountain the pressure and, accordingly, the boiling point are so low that the water becomes unsuitable for cooking food - the required water temperature is not reached. With a high enough pressure, water can be heated enough to melt lead (327°C) and still not boil.
In addition to the super-high boiling points of melting (and the latter process requires too much heat of fusion for such a simple liquid), the very range of existence of water is anomalous - one hundred degrees, by which these temperatures differ - a rather large range for such a low molecular weight liquid as water. The limits of allowable values of hypothermia and overheating of water are unusually large - with careful heating or cooling, water remains liquid from -40 ° C to +200 ° C. This extends the temperature range in which water can remain liquid to 240 °C.
When ice is heated, its temperature first rises, but from the moment a mixture of water and ice is formed, the temperature will remain unchanged until all the ice has melted. This is explained by the fact that the heat supplied to the melting ice is primarily spent only on the destruction of crystals. The temperature of melting ice remains unchanged until all crystals are destroyed (see latent heat of fusion).
melts
Melts are a liquid molten state of substances at temperatures within certain limits remote from critical point melting point and located closer to the melting point. The nature of melts is inherently determined by the type chemical bonds elements in the molten substance.
Melts are widely used in metallurgy, glass-making and other fields of technology. Melts usually have a complex composition and contain various interacting components (see phase diagram).
Melts are
1. Metallic (Metals (the name comes from the Latin metallum - mine, mine) - a group of elements with characteristic metallic properties, such as high thermal and electrical conductivity, positive temperature coefficient of resistance, high ductility and metallic luster);
2. Ionic (Ion (ancient Greek ἰόν - going) - a monatomic or polyatomic electrically charged particle formed as a result of the loss or addition of one or more electrons to an atom or molecule. Ionization (the process of formation of ions) can occur at high temperatures, under impact electric field);
3.Semiconductor with covalent bonds between atoms (Semiconductors are materials that, in terms of their specific conductivity, occupy an intermediate position between conductors and dielectrics and differ from conductors by a strong dependence of specific conductivity on impurity concentration, temperature, and various kinds radiation. The main property of these materials is an increase in electrical conductivity with increasing temperature);
4. Organic melts with van der Waals bonds;
5. High polymer (Polymers (Greek πολύ- - many; μέρος - part) - inorganic and organic, amorphous and crystalline substances obtained by repeated repetition of various groups of atoms, called "monomeric units", connected into long macromolecules by chemical or coordination bonds)
Melts by type chemical compounds there are:
1. Salt;
2.Oxide;
3. Oxide-silicate (slag), etc.
Melts with special properties:
1.Eutectic
Interesting about melting
Ice grains and stars.
Enter a piece pure ice into a warm room and watch it melt. It will quickly become clear that the ice, which seemed monolithic and homogeneous, breaks up into many small grains - individual crystals. In the volume of ice, they are located randomly. Not less than interesting picture can be seen when the ice melts from the surface.
Hold a smooth piece of ice near the lamp and wait until it begins to melt. When melting touches the inner grains, very fine patterns will begin to appear there. With a strong magnifying glass, you can see that they have the shape of hexagonal snowflakes. In fact, these are melted depressions filled with water. The shape and direction of their rays correspond to the orientation of ice single crystals. These patterns are called "Tyndall stars" after the English physicist who discovered and described them in 1855. "Tyndall stars", similar to snowflakes, are actually depressions on the surface of melted ice, about 1.5 mm in size, filled with water. In their center, air bubbles are visible, which have arisen due to the difference in the volumes of melted ice and melt water.
DID YOU KNOW?
There is a metal, the so-called Wood's alloy, which can be easily melted even in warm water (+68 degrees Celsius). So when stirring sugar in a glass, a metal spoon made of this alloy will melt faster than sugar!
The most refractory substance, tantalum carbide TaCO-88, melts at a temperature of 3990°C.
In 1987, German researchers were able to supercool water to -700C while keeping it in a liquid state.
Sometimes, to make the snow on the sidewalks melt faster, they are sprinkled with salt. The melting of ice occurs because a solution of salt in water is formed, the freezing point of which is lower than the air temperature. The solution just flows off the sidewalk.
Interestingly, feet get colder more on wet pavement, as the temperature of the salt-water solution is lower than that of pure snow.
If you pour tea from a teapot into two mugs: with sugar and without sugar, then the tea in a mug with sugar will be colder, because. the dissolution of sugar (the destruction of its crystal lattice) also consumes energy.
In severe frosts, to restore the smoothness of the ice, the rink is watered hot water.. Hot water melts a thin top layer of ice, does not freeze so quickly, has time to spread, and the ice surface is very smooth.
Conclusion (conclusions)
Melting is the transition of a substance from solid state into liquid.
When heated, the temperature of the substance increases, and the speed of thermal motion of particles increases, while the internal energy of the body increases.
When the temperature of a solid reaches its melting point, the crystal lattice solid starts to break down. Thus, the main part of the energy of the heater, conducted to the solid body, is spent on reducing the bonds between the particles of the substance, i.e., on the destruction of the crystal lattice. In this case, the energy of interaction between particles increases.
The molten substance has a large supply internal energy than in the solid state. The remaining part of the heat of fusion is spent on doing work to change the volume of the body during its melting.
When melted, the volume of the majority crystalline bodies increases (by 3-6%), and decreases during hardening. But, there are substances in which, when melted, the volume decreases, and when solidified, it increases. These include, for example, water and cast iron, silicon and some others. . That is why ice floats on the surface of the water, and solid cast iron - in its own melt.
Solids called amorphous (amber, resin, glass) do not have a specific melting point.
The amount of heat required to melt a substance is equal to the product of the specific heat of fusion times the mass of the substance.
The specific heat of fusion shows how much heat is needed to completely convert 1 kg of a substance from a solid state to a liquid, taken at the melting rate.
The unit of specific heat of fusion in SI is 1J/kg.
During the melting process, the temperature of the crystal remains constant. This temperature is called the melting point. Each substance has its own melting point.
The melting point for a given substance depends on atmospheric pressure.
List of used literature
1) Data from the electronic free encyclopedia "Wikipedia"
http://ru.wikipedia.org/wiki/Main_page
2) Site "Class! Physics for the curious" http://class-fizika.narod.ru/8_11.htm
3) Site " Physical Properties water"
http://all-about-water.ru/boiling-temperature.php
4) Website "Metals and structures"
http://metaloconstruction.ru/osnovy-plavleniya-metallov/
There's no such thing solid body, which would resist the increase in temperature as much as necessary. Sooner or later a solid piece turns into a liquid; right, in some cases we will not be able to get to the melting point - chemical decomposition can occur.
As the temperature rises, the molecules move faster and faster. Finally, there comes a moment when maintaining order "among strongly" swung "molecules becomes impossible. The solid melts. Tungsten has the highest melting point: 3380 ° C. Gold melts at 1063 ° C, iron at 1539 ° C. However, there are also fusible metals. Mercury, as is well known, melts already at a temperature of -39 ° C. Organic substances do not have high melting points. Naphthalene melts at 80 ° C, toluene - at -94.5 ° C.
It is not at all difficult to measure the melting point of a body, especially if it melts in the temperature range that is measured with an ordinary thermometer. It is not at all necessary to follow the melting body with your eyes. It is enough to look at the mercury column of the thermometer. Until melting has begun, the body temperature rises (Fig. 4.5). As soon as melting starts, the temperature rise stops and the temperature will remain unchanged until the melting process is complete.
Like the transformation of a liquid into vapor, the transformation of a solid into a liquid requires heat. The heat required for this is called latent heat melting. For example, melting one kilogram of ice requires 80 kcal.
Ice is one of the bodies with a high heat of fusion. Melting ice requires, for example, 10 times more energy than melting the same mass of lead. Of course we are talking about the melting itself, we do not say here that before the melting of lead begins, it must be heated to + 327 ° C. Due to the high heat of melting ice, the melting of snow slows down. Imagine that the heat of melting would be 10 times less. Then spring floods would bring unimaginable disasters every year.
So, the heat of fusion of ice is great, but it is also small when compared with specific heat vaporization at 540 kcal / kg (seven times less). However, this difference is quite natural. When converting a liquid into vapor, we have to tear the molecules one from the other, and when melting, we only have to destroy the order in the arrangement of the molecules, leaving them at almost the same distances. It is clear that less work is required in the second case.
The presence of a certain melting point is an important feature of crystalline substances. It is on this basis that they are easy to distinguish from other solids, called amorphous or glasses. Glasses are found among both inorganic and organic substances. Window panes are usually made from sodium and calcium silicates; often organic glass is placed on the desk (it is also called plexiglass).
Amorphous substances, in contrast to crystals, do not have a definite melting point. Glass does not melt, but softens. When heated, a piece of glass first becomes soft from hard, it can be easily bent or stretched; at a higher temperature, the piece begins to change its shape under the influence of its own gravity. As it heats up, the thick viscous mass of glass takes the shape of the vessel in which it lies. This mass is at first thick, like honey, then like sour cream, and, finally, it becomes almost as low-viscosity liquid as water. With all our desire, we cannot indicate here a specific temperature for the transition of a solid to a liquid. The reasons for this lie in the fundamental difference between the structure of glass and the structure of crystalline bodies. As mentioned above, the atoms in amorphous bodies arranged randomly. Glasses in structure resemble liquids. Even in solid glass, the molecules are arranged randomly. This means that an increase in the temperature of glass only increases the range of vibrations of its molecules, giving them gradually more and more freedom of movement. Therefore, the glass softens gradually and does not show a sharp "solid" - "liquid" transition, which is characteristic of the transition from the arrangement of molecules in a strict order to a random arrangement.
When it came to the boiling curve, we said that liquid and vapor can, albeit in an unstable state, live in foreign regions - vapor can be supercooled and transferred to the left of the boiling curve, liquid can be superheated and pulled to the right of this curve.
Are similar phenomena possible in the case of a crystal with a liquid? It turns out that the analogy here is incomplete.
If you heat the crystal, it will begin to melt at its melting point. The crystal cannot be overheated. On the contrary, by cooling the liquid, it is possible, if certain measures are taken, to “slip through” the melting point relatively easily. In some liquids, large subcoolings can be achieved. There are even liquids that are easy to supercool, but difficult to make crystallize. As such a liquid cools, it becomes more and more viscous and finally solidifies without crystallizing. Such is glass.
You can also recool water. Fog droplets may not freeze even in severe frosts. If a crystal of a substance, a seed, is thrown into a supercooled liquid, then crystallization will immediately begin.
Finally, in many cases delayed crystallization may be initiated by shaking or other random events. It is known, for example, that crystalline glycerol was first obtained during transportation through railway. Glasses after a long standing may begin to crystallize (devitrify, or "collapse", as they say in technology).






