Properties of molecules in the gaseous state. Arrangement of molecules in liquids
Arrangement of molecules in liquids. Molecules in liquids are located at distances equal to the size of the molecules, maintaining the so-called short-range order, so liquids retain their volume. Molecules are constantly moving, making jumps, so liquids flow, taking the shape of a vessel.
Picture 9 from the presentation "3 states of matter" to physics lessons on the topic " thermal phenomena»Dimensions: 960 x 720 pixels, format: jpg. To download a picture for free physics lesson, right-click on the image and click "Save Image As...". To show pictures in the lesson, you can also download the presentation “3 States of Matter.ppt” for free with all the pictures in a zip archive. The size of the archive is 2714 KB.
Download presentationthermal phenomena
"Diffusion of substances" - Heraclitus. Observations. Diffusion in technology and nature. Tasks for biology lovers. The phenomenon of diffusion. We solve problems. Democritus. Scientists Ancient Greece. Proverbs. Diffusion. Thales of Miletus. Diffusion in gases. Fragrant leaves. Dark color.
"3 States of Matter" - Properties solids. Ice. Interesting Facts. Condensation. Properties of liquids. Arrangement of molecules in gases. Substance. Arrangement of molecules in liquids. Solve the crossword. Process examples. states. Vaporization. Crystallization. Arrangement of molecules in solids. Character of movement and interaction of particles.
"Diffusion in nature" - Widely used in the food industry when preserving vegetables and fruits. When smelting steel. The phenomenon of diffusion has important manifestations in nature, is used in science and in production. diffusion in nature. An example of diffusion is the mixing of gases or liquids. Diffusion in breath.
"Changing the aggregate states of matter" - Boiling. Melting and crystallization temperature. Boiling temperature. vaporization conditions. aggregate transformations. Specific heat vaporization. melting and solidification process. Explanation of the melting process. Vaporization. Aggregate transformations of matter. Three states. Calculation of the amount of heat.
"Thermal phenomena during dissolution" - Chemist. Phenomena. Dissolution. Mutual penetration of molecules. We wish you success in further knowledge of the laws of physics and chemistry. Check your roommate. Practical tasks. The value of solutions. Physico - chemical process. How did you feel during the lesson? exothermic process. Signs of chemical reactions.
"Interaction of molecules" - Repulsion prevents complete convergence. gaseous substances. Is it possible to connect two pieces of iron nail? Option I Natural mixtures do not include: a) clay; b) cement; c) soil. Goals and objectives of the lesson: To expand knowledge about the particles that form substances. Attraction holds the particles together.
Total in the topic 23 presentations
Full text search:
Physics->Lab work
Study of processes in electrical circuit With parallel connection receivers containing inductive and capacitive elements, with a different ratio...full>>
Physics->Lab work
Investigation of the influence of the coil inductance on the electrical parameters of a single-phase sinusoidal voltage circuit containing in series ... completely>>
Home > Abstract >Physics
1 Maintaining………………………………………………………..2
2 Main body
2.1 The structure of the liquid. Movement of liquid molecules………3
2.2 Pressure in the liquid……………………………………….4
2.3 Law of Archimedes …………………………………………….5
2.4 Evaporation………………………………………………………6
2.5 Boiling……………………………………………………….7
2.6 Surface tension of the liquid……………………….8
2.7 Liquid films……………………………………………9
2.8 Wetting and non-wetting…………………………….….10
2.9 Capillary phenomena………………………………………..12
2.10 Electricity in liquids…………………………..13
3 Conclusion………………………………………………………..14
References……………………………………………………16
1. Introduction
In everyday life, we usually encounter three phase states of matter - solid, liquid and gaseous. We have a fairly clear idea of the structure of gases and solid crystalline bodies. A gas is a collection of molecules moving randomly in all directions independently of each other. In a solid body, all molecules retain their mutual arrangement for a long time, making only small oscillations around certain equilibrium positions.
In this essay, I will take a closer look at liquid state substances. Main Feature This state of aggregation is that the liquid state, occupying an intermediate position between gases and crystals, combines some of the properties of both of these states. In particular, for liquids, as well as for crystalline bodies, the presence of a certain volume is characteristic, and at the same time, a liquid, like a gas, takes the form of the vessel in which it is located. Most people tend to think that liquids have no shape of their own. But this is not true. The natural shape of any liquid is a sphere. Usually, gravity prevents the liquid from taking this form, the liquid either spreads in a thin layer over the surface, or takes the form of a vessel if poured into it.
The intermediate position of liquids is due to the fact that the liquid state is particularly complex in its properties. Although liquids have been the subject of scientific study since at least the time of Archimedes, that is, 2200 years ago, the analysis of the behavior of liquids is still one of the most difficult areas of applied science. Until now, there is no completely complete and generally accepted theory of liquids.
2 .Main part.
To understand the basic properties and regularities of the liquid state of a substance, it is necessary to consider the following aspects:
2.1. The structure of the liquid. Movement of liquid molecules.
A fluid is something that can flow.
The so-called short-range order is observed in the arrangement of liquid particles. This means that with respect to any particle, the location of its nearest neighbors is ordered. However, as one moves away from a given particle, the arrangement of other particles in relation to it becomes less and less ordered, and rather quickly the order in the arrangement of particles completely disappears. Liquid molecules move much more freely than solid molecules, though not as freely as gas molecules. Each molecule of the liquid for some time moves here and there, without moving away, however, from its neighbors. But from time to time a liquid molecule breaks out of its environment and goes to another place, getting into a new environment, where again for some time it makes movements similar to oscillations. Significant merit in the development of a number of problems in the theory of the liquid state belongs to the Soviet scientist Ya. I. Frenkel. According to Frenkel, thermal motion in liquids has the following character. Each molecule oscillates around a certain equilibrium position for some time. From time to time, the molecule changes its place of equilibrium, jumping to a new position, separated from the previous one by a distance of the order of the size of the molecules themselves. That is, the molecules only slowly move inside the liquid, staying part of the time near certain places. Thus, the movement of liquid molecules is something like a mixture of movements in a solid and in a gas: oscillatory movement in one place is replaced by a free transition from one place to another.
2.2 Pressure in a liquid
Everyday experience teaches us that liquids act with known forces on the surface of solids in contact with them. These forces are called fluid pressure forces.
Covering the opening of an open water tap with a finger, we feel the force of the pressure of the liquid on the finger. The pain in the ears experienced by a swimmer diving to great depths is caused by the forces of water pressure on the eardrum. Deep sea thermometers must be very strong so that the pressure of the water cannot crush them.
The pressure in a liquid is due to a change in its volume - compression. In relation to a change in volume, liquids have elasticity. The elastic forces in a fluid are pressure forces. Thus, if a fluid acts with pressure forces on bodies in contact with it, this means that it is compressed. Since the density of a substance increases during compression, it can be said that liquids have elasticity with respect to a change in density.
The pressure in a liquid is perpendicular to any surface placed in the liquid. The pressure in the liquid at depth h is equal to the sum of the pressure on the surface and a value proportional to the depth:
Due to the fact that liquids can transmit static pressure, practically no less than their density, they can be used in devices that give a gain in strength: the hydraulic press.
2.3.Law of Archimedes
Pressure forces act on the surface of a solid body immersed in a liquid. Since pressure increases with depth, the upward pressure forces on the bottom of the fluid are greater than the downward forces on the top, and we can expect the resultant of the pressure forces to be upward. The resultant force of pressure on a body immersed in a liquid is called the supporting force of the liquid.
If a body immersed in a liquid is left to itself, then it will sink, remain in equilibrium, or float to the surface of the liquid, depending on whether the supporting force is less than the force of gravity acting on the body, equal to it or greater than it.
Archimedes' principle states that a body in a fluid is subjected to an upward buoyant force equal to the weight of the displaced fluid. A body immersed in a liquid is subjected to a buoyant force (called the Archimedes force)
where ρ is the density of the liquid (gas), is the acceleration free fall, a V- the volume of the submerged body (or the part of the volume of the body below the surface).
If a body immersed in a liquid is suspended from a scale, then the scale shows the difference between the weight of the body in air and the weight of the displaced liquid. Therefore, the Archimedes law is sometimes given the following formulation: a body immersed in a liquid loses as much in its weight as the liquid displaced by it weighs.
It is interesting to note such an experimental fact that, being inside another liquid of a larger specific gravity, the liquid according to the law of Archimedes "loses" its weight and takes its natural, spherical shape.
2.4 Evaporation
In the surface layer and near the surface of the liquid, forces act that ensure the existence of the surface and do not allow molecules to leave the volume of the liquid. Due to thermal motion, some of the molecules have high enough velocities to overcome the forces holding the molecules in the liquid and leave the liquid. This phenomenon is called evaporation. It is observed at any temperature, but its intensity increases with increasing temperature.
If the molecules that have left the liquid are removed from the space near the surface of the liquid, then, in the end, all the liquid will evaporate. If the molecules that have left the liquid are not removed, they form vapor. Vapor molecules that have fallen into the region near the surface of the liquid are drawn into the liquid by the forces of attraction. This process is called condensation.
Thus, if the molecules are not removed, the evaporation rate decreases with time. With a further increase in the vapor density, a situation is reached where the number of molecules leaving the liquid in a certain time will be equal to the number of molecules returning to the liquid in the same time. There comes a state of dynamic equilibrium. Vapor in a state of dynamic equilibrium with a liquid is called saturated.
With increasing temperature, density and pressure saturated steam increase. The higher the temperature, the greater the number of liquid molecules has sufficient energy to evaporate, and the greater the vapor density must be in order for condensation to equal evaporation.
2.5 Boiling
When a liquid is heated to a temperature at which the pressure saturated vapors equal to the external pressure, an equilibrium is established between the liquid and its saturated steam. When an additional amount of heat is communicated to the liquid, the corresponding mass of liquid is immediately converted into steam. This process is called boiling.
Boiling is the intense evaporation of a liquid, which occurs not only from the surface, but throughout its entire volume, inside the resulting vapor bubbles. To go from liquid to vapor, molecules must acquire the energy needed to overcome the attractive forces that hold them in the liquid. For example, to evaporate 1 g of water at a temperature of 100 ° C and a pressure corresponding to atmospheric pressure at sea level, it is required to spend 2258 J, of which 1880 go to separate molecules from the liquid, and the rest go to work to increase the volume occupied by the system, against atmospheric pressure forces (1 g of water vapor at 100 ° C and normal pressure occupies a volume of 1.673 cm 3, while 1 g of water under the same conditions is only 1.04 cm 3).
The boiling point is the temperature at which the vapor pressure becomes equal to the external pressure. As pressure increases, the boiling point increases, and as pressure decreases, it decreases.
Due to the change in pressure in a liquid with the height of its column, boiling at different levels in the liquid occurs, strictly speaking, at different temperatures. Only saturated steam above the surface of a boiling liquid has a certain temperature. Its temperature is determined only by external pressure. It is this temperature that is meant when talking about the boiling point.
The boiling points of various liquids are very different from each other, and this is widely used in technology, for example, in the distillation of petroleum products.
The amount of heat that must be supplied in order to isothermally turn a certain amount of liquid into vapor, at an external pressure equal to the pressure of its saturated vapors, is called the latent heat of vaporization. Usually this value is related to one gram, or one mole. The amount of heat required for the isothermal evaporation of a mole of liquid is called molar latent heat vaporization. If this value is divided by the molecular weight, then the specific latent heat of vaporization will be obtained.
2.6 Surface tension of liquid
The property of a liquid to reduce its surface to a minimum is called surface tension. Surface tension is a phenomenon of molecular pressure on a liquid, caused by the attraction of surface layer molecules to molecules inside the liquid. At the surface of a liquid, molecules experience forces that are not symmetrical. On the molecule inside the liquid, on average, the force of attraction, cohesion, acts on average uniformly from all sides. If the surface of the liquid is increased, then the molecules will move against the action of the holding forces. Thus, the force tending to shorten the surface of the liquid acts in the opposite direction to the external tensile force on the surface. This force is called the surface tension force and is calculated by the formula:
 - surface tension coefficient
- surface tension coefficient
 (
( )
)
 - length of the boundary of the liquid surface
- length of the boundary of the liquid surface
Note that easily evaporating liquids (ether, alcohol) have a lower surface tension than non-volatile liquids (mercury). The surface tension of liquid hydrogen and, especially, liquid helium is very low. For liquid metals surface tension on the contrary, it is very large. The difference in the surface tension of liquids is explained by the difference in the cohesive forces of different molecules.
Measurements of the surface tension of a liquid show that the surface tension depends not only on the nature of the liquid, but also on its temperature: with an increase in temperature, the difference in the density of the liquid decreases, and therefore the coefficient of surface tension - decreases.
Due to surface tension, any volume of liquid tends to reduce the surface area, thus reducing the potential energy. Surface tension is one of the elastic forces responsible for the movement of ripples on water. In bulges, surface gravity and surface tension pull the water particles downward, tending to make the surface smooth again.
2.7 Liquid films
Everyone knows how easy it is to get lather out of soapy water. Foam is a set of air bubbles bounded by the thinnest film of liquid. A separate film can easily be obtained from the foam-forming liquid.
These films are very interesting. They can be extremely thin: in the thinnest parts, their thickness does not exceed a hundred-thousandth of a millimeter. Despite their thinness, they are sometimes very stable. The soap film can be stretched and deformed, and a stream of water can flow through the soap film without destroying it.
How can one explain the stability of films? An indispensable condition for the formation of a film is the addition of substances soluble in it to a pure liquid, moreover, those that greatly reduce the surface tension
In nature and technology, we usually meet not with individual films, but with a collection of films - foam. You can often see in streams, where small streams fall into calm water, an abundant formation of foam. In this case, the ability of water to foam is associated with the presence in the water of a special organic substance released from the roots of plants. In construction equipment, materials that have a cellular structure, such as foam, are used. Such materials are cheap, light, do not conduct heat and sound well, and are strong enough. For their manufacture, substances that promote foaming are added to the solutions from which building materials are formed.
2.8 Wetting
Small droplets of mercury placed on a glass plate take on a spherical shape. This is the result of molecular forces tending to reduce the surface of the liquid. Mercury placed on the surface of a solid does not always form round droplets. It spreads over the zinc plate, and the total surface of the drop will undoubtedly increase.
An aniline drop is also spherical only when it does not touch the wall of the glass vessel. As soon as she touches the wall, she immediately sticks to the glass, stretching along it and acquiring a large common surface.
This is explained by the fact that in the case of contact with a solid body, the adhesion forces of liquid molecules with solid body molecules begin to play a significant role. The behavior of a liquid will depend on which is greater: the adhesion between liquid molecules or the adhesion of a liquid molecule to a solid molecule. In the case of mercury and glass, the cohesive forces between mercury and glass molecules are small compared to the cohesive forces between mercury molecules, and the mercury collects in a drop. Such a liquid is called non-wetting solid. In the case of mercury and zinc, the cohesive forces between the molecules of the liquid and the solid exceed the cohesive forces acting between the molecules of the liquid, and the liquid spreads over the solid. In this case, the liquid is called wetting solid.
From this it follows that, speaking of the surface of a liquid, one must have in mind not only the surface where the liquid borders on air, but also the surface bordering on other liquids or on a solid body.
Depending on whether the liquid wets the walls of the vessel or not, the shape of the surface of the liquid at the point of contact with the solid wall and the gas has one form or another. In the case of non-wetting, the shape of the liquid surface at the edge is round, convex. In the case of wetting, the liquid at the edge takes a concave shape.
2.9. Capillary phenomena.
In life, we often deal with bodies pierced by many small channels (paper, yarn, leather, various building materials, soil, wood). Coming into contact with water or other liquids, such bodies often absorb them. This is the basis for the action of a towel when drying hands, the action of a wick in a kerosene lamp, etc. Similar phenomena can also be observed in narrow glass tubes. Narrow tubes are called capillary or hair.
When such a tube is immersed with one end into a wide vessel into a wide vessel, the following occurs: if the liquid wets the walls of the tube, then it will rise above the level of the liquid in the vessel and, moreover, the higher, the narrower the tube; if the liquid does not wet the walls, then, on the contrary, the liquid level in the tube is set lower than in a wide vessel. The change in the height of the liquid level in narrow tubes or gaps is called capillarity. In a broad sense, capillary phenomena are understood as all phenomena due to the existence of surface tension.
The height of liquid rise in capillary tubes depends on the radius of the channel in the tube, the surface tension and the density of the liquid. Between the liquid in the capillary and in the wide vessel, such a level difference h is established so that the hydrostatic pressure gh balances the capillary pressure:
where is the surface tension of the liquid
R is the capillary radius.
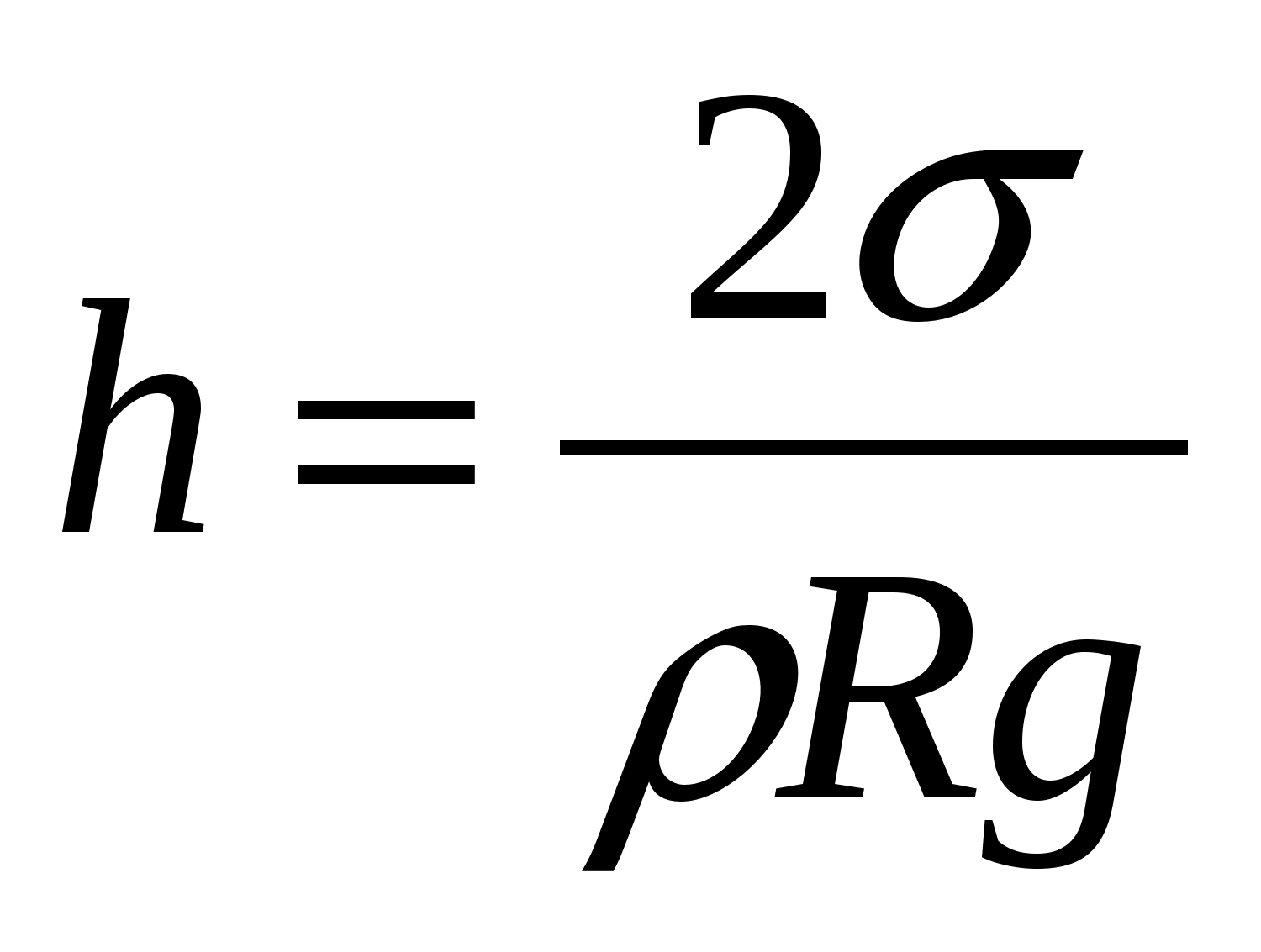
The height of the rise of a liquid in a capillary is proportional to its surface tension and inversely proportional to the radius of the capillary channel and the density of the liquid (Jurin's law)
2.10. Electric current in liquids.
Pure liquids do not conduct electric current, that is, they are dielectrics, since each of the liquid molecules is neutral and does not move in an electric field.
Fluids that conduct electricity are called electrolytes. Electric current in liquids is formed as a result of the directed movement of salt ions. The phenomenon of the release of a substance on the electrodes when a current passes through the electrolyte is called electrolysis. On a negatively charged electrode cathode there is an electrochemical reduction of particles (atoms, molecules, cations), and on a positively charged electrode - anode there is an electrochemical oxidation of particles (atoms, molecules, anions). In 1832, Faraday found that the mass M of the substance released on the electrode is directly proportional to the electric charge Q that has passed through the electrolyte:
if it is passed through the electrolyte for a time t D.C. with current I.
The coefficient of proportionality k is called the electrochemical equivalent of a substance. It is numerically equal to the mass of the substance released during the passage of a single electric charge through the electrolyte, and depends on the chemical nature of the substance
Faraday's second law says: The electrochemical equivalents of various substances are treated as their chemical equivalents. The chemical equivalent of an ion is the ratio of the molar mass A of the ion to its valence z. Therefore, the electrochemical equivalent is:
![]() ,
,
where F is Faraday's constant
The phenomenon of electrolysis is widely used in modern industry. In particular, electrolysis is one of the methods for the industrial production of hydrogen, as well as sodium hydroxide, chlorine, organochlorine compounds, manganese dioxide, and hydrogen peroxide. A large number of metals are extracted from ores and processed using electrolysis (electroextraction, electrorefining). Electrolysis is used for wastewater treatment (electrocoagulation, electroextraction, electroflotation processes).
3.Conclusion
Thus, a liquid is an intermediate state of matter between the solid and gaseous states. This determines that liquids have properties characteristic of both solid and gaseous states. A vivid example of the state of matter, which combines the properties of liquid and solid states, are liquid crystals, which are widely used in industry and technology (liquid crystal displays). In this regard, the description of the state of a liquid requires the synthesis of mathematical methods used to describe the solid and gaseous states, which complicates and makes it difficult to thoroughly describe many physical and chemical phenomena.
At present, many properties of liquids are widely used in industry and technology. For example, the property of a liquid to increase pressure in its entire volume is used in hydraulically driven hoisting machines. But further deep study of the theory of the liquid state of matter is also necessary. Thus, the relevance of studying the flow of a boiling liquid is associated with the needs of nuclear energy, with the problem of the safety of power plants.
Of particular interest in the study of physical and chemical processes in the liquid state is due to the fact that we ourselves are 90% water, the most common liquid on Earth. And all the vital processes in the animal and flora occur in a liquid, namely water. Therefore, the study of this state of matter is important and relevant for all people.
Bibliography:
I.V. Savelyev "Course of General Physics"
Cl.E. Swartz "Extraordinary Physics of Ordinary Phenomena"
Elementary textbook of physics, edited by Academician G.S. Landsberg
T.I. Trofimov "Course of Physics"
ME AND. Perelman "Entertaining Physics"
Arrangement of molecules in solids. In solids, the distances between molecules are equal to the size of the molecules, so solids retain their shape. Molecules are arranged in a certain order, called a crystal lattice, so under normal conditions, solids retain their volume.
Picture 5 from the presentation "3 states of matter" to physics lessons on the topic "Thermal phenomena"Dimensions: 960 x 720 pixels, format: jpg. To download a picture for a physics lesson for free, right-click on the image and click "Save Image As...". To show pictures in the lesson, you can also download the presentation “3 States of Matter.ppt” for free with all the pictures in a zip archive. The size of the archive is 2714 KB.
Download presentationthermal phenomena
"Diffusion in nature" - Widely used in the food industry when preserving vegetables and fruits. When smelting steel. An example of diffusion is the mixing of gases or liquids. What is diffusion? Diffusion in breath. The phenomenon of diffusion has important manifestations in nature, is used in science and in production.
"Changing the aggregate states of matter" - Aggregate transformations of matter. Specific heat of vaporization. Boiling temperature. Boiling. Temperature graph of changes in the aggregate states of water. Melting and crystallization temperature. vaporization conditions. aggregate transformations. Vaporization. Calculation of the amount of heat. melting and solidification process.
"3 States of Matter" - Solve the crossword puzzle. Crystallization. Arrangement of molecules in solids. Process examples. states. Substance. Properties of gases. Vaporization. Questions for the crossword. Properties of liquids. Arrangement of molecules in liquids. Ice. Properties of solids. Condensation. Character of movement and interaction of particles.
"Diffusion of substances" - Fragrant leaves. Dark color. Proverbs. Thales of Miletus. Heraclitus. We solve problems. Scientists of Ancient Greece. Diffusion in technology and nature. Tasks for biology lovers. Diffusion. The phenomenon of diffusion. Democritus. Observations. Diffusion in gases.
"Thermal phenomena during dissolution" - D.I. Mendeleev. Briefing. Dissolution of potassium permanganate in water. exothermic process. Check your roommate. We wish you success in further knowledge of the laws of physics and chemistry. diffusion rate. What is called thermal motion. Mutual penetration of molecules. The value of solutions. Practical tasks.
"Interaction of molecules" - Is it possible to connect two pieces of an iron nail? Attraction holds the particles together. Option I Natural mixtures do not include: a) clay; b) cement; c) soil. gaseous substances. Option II An artificial mixture is: a) clay; b) cement; c) soil. Distance between gas molecules more sizes the molecules themselves.
Total in the topic 23 presentations
Molecules in a liquid are closer together than in a gas. This follows from the fact that 1 volume of water is formed as a result of the condensation of 1300 volumes of steam. The formation of a liquid from a gas is a consequence of the appearance of attractive forces between the molecules. They must be significant in order to overcome the kinetic energy of the molecules. The cooling of the gas is accompanied by a loss of kinetic energy by the molecules until they reach a temperature at which their kinetic energy no longer overlaps the forces of intermolecular attraction, and as a result, the gas begins to liquefy.
The liquid state is much closer to the solid than to the gaseous state. Particles in the liquid state are more distant from each other than in the solid state and are less ordered. The melting of a solid is usually accompanied by a 10% increase in volume (This statement does not apply to substances such as water, bismuth, cast iron, the melting of which is accompanied by a decrease in volume).
The amount of heat required to convert 1 mole of liquid to vapor at 1 atm is called standard enthalpy of vaporization:∆H° app. The same amount of heat is released during the condensation of 1 mol of vapor at 1 atm. The amount of heat spent on the transformation of 1 mole of a solid into a liquid at 1 atm is called standard enthalpy of fusion:∆H° square The same amount of heat is released when 1 mol of liquid solidifies at 1 atm. The standard enthalpies of fusion are much smaller than the standard enthalpies of vaporization, since the transition from solid state to a liquid is accompanied by a lesser violation of intermolecular attraction than the transition from a liquid to a gaseous state.
Rice. 8.1 Distance between liquid molecules:
a- distance between liquid molecules; b- imaginary compression of the liquid, leading to a greater convergence of its molecules;
1 - forces of attraction between molecules; 2 - mutual overlapping of electron clouds, leading to the repulsion of molecules.
Liquids have a certain volume, and their compressibility is negligible.
In a liquid, the distance between molecules is due to the balance between their attractive forces and the repulsive forces of neighboring electron clouds (Fig. 8.1). The forces of intermolecular attraction in a liquid are smaller compared to those in solids, but not strong enough to give the liquid a particular shape. That is why the liquid flows and takes the form of the vessel containing it.
Gases can diffuse through high speed the movement of their molecules. The rate of diffusion of liquids is much less than that of gases; for solids it is even smaller. And although the molecules of a liquid move quickly, they cannot move freely from one part of the liquid to another, as they are held by attractive forces that bind the molecules to neighboring molecules in a loosely packed network. Occasionally, however, a molecule "breaks out" of this network and moves into another region of the liquid, i.e., diffuses. Since the molecules of a liquid are constantly moving, some of them have enough energy to escape from the liquid into vapor. (Concept steam refers to a gas at a temperature below its critical temperature.).
The energy of the escaping molecules is much greater than the average energy and is sufficient to overcome the attractive forces of other molecules. The energy of the molecules remaining in the liquid is less than the energy of the molecules escaping, so the temperature of the liquid decreases.

Rice. 8.2 The liquid and its vapor reach equilibrium in a closed vessel: the rates of evaporation and condensation become equal.
Since the proportion of high-energy molecules increases as the temperature rises, the rate of evaporation also increases. Evaporation continues as long as there is liquid. However, if the liquid is in a closed vessel, the vapor molecules collide with the walls of the vessel; some of them move towards the liquid, while some return to it, i.e., comes condensation(Fig. 8.2). Equilibrium is reached when the rates of liquid evaporation and vapor condensation become equal.






