What is called saturated steam. Big encyclopedia of oil and gas
Before answering the question posed in the title of the article, let's figure out what steam is. The images that most people have with this word: a boiling kettle or saucepan, a steam room, a hot drink, and many more similar pictures. One way or another, in our ideas there is a liquid and a gaseous substance rising above its surface. If you are asked to give an example of steam, you will immediately remember water vapor, vapors of alcohol, ether, gasoline, acetone.

There is another word for gaseous states - gas. Here we usually think of oxygen, hydrogen, nitrogen and other gases without associating them with the corresponding liquids. It is well known that they also exist in liquid state. At first glance, the differences lie in the fact that steam corresponds to natural liquids, and gases must be liquefied on purpose. However, this is not entirely true. Moreover, the images that arise with the word steam are not steam. To give a more precise answer, let's look at how steam is created.
How is steam different from gas?
The state of aggregation of a substance is set by temperature, more precisely by the ratio between the energy with which its molecules interact and the energy of their thermal chaotic motion. Approximately, we can assume that if the interaction energy is much greater - solid state, if the energy of thermal motion is much greater - gaseous, if the energies are comparable - liquid.

It turns out that in order for a molecule to break away from a liquid and participate in the formation of vapor, the value of thermal energy must be greater than the interaction energy. How can this happen? The average speed of thermal motion of molecules is equal to a certain value, depending on temperature. However, the individual velocities of the molecules are different: most of them have velocities close to the average value, but some of them have velocities greater than the average, some less.
Faster molecules can have thermal energy greater than the interaction energy, which means that, having hit the surface of the liquid, they are able to break away from it, forming steam. This type of vaporization is called evaporation. Because of the same distribution of velocities, there is an opposite process - condensation: molecules from a vapor pass into a liquid. By the way, the images that usually appear with the word steam are not steam, but the result of the opposite process - condensation. You can't see the couple.

Steam under certain conditions can become a liquid, but for this its temperature must not exceed a certain value. This value is called the critical temperature. Steam and gas are gaseous states that differ in the temperature at which they exist. If the temperature does not exceed the critical - steam, if it exceeds - gas. If you keep the temperature constant and reduce the volume, the vapor liquefies, the gas does not liquefy.
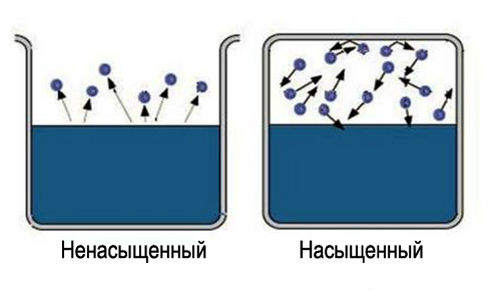
What is saturated and unsaturated steam
The very word "saturated" carries certain information, it is difficult to saturate a large area of space. So to get saturated steam, necessary limit the space in which the liquid is located. In this case, the temperature should be less than the critical one for the given substance. Now the evaporated molecules remain in the space where the liquid is located. At first, most transitions of molecules will occur from the liquid, while the vapor density will increase. This in turn will cause more reverse transitions of molecules into liquid, which will increase the rate of the condensation process.
Finally, a state is established for which the average number of molecules passing from one phase to another will be equal. Such a state is called dynamic balance. This state is characterized by the same change in the magnitude and direction of the rates of evaporation and condensation. This state corresponds to saturated steam. If the state of dynamic equilibrium is not reached, this corresponds to an unsaturated steam.
They begin the study of some object, always with its simplest model. In molecular kinetic theory, this is an ideal gas. The main simplifications here are the neglect of the intrinsic volume of molecules and the energy of their interaction. It turns out that this model satisfactorily describes unsaturated steam. Moreover, the less saturated it is, the more legitimate its use. An ideal gas is a gas; it cannot become either a vapor or a liquid. Therefore, for saturated steam, such a model is not adequate.
The main differences between saturated and unsaturated steam
- Saturated means that the given object has the largest possible value of some parameters. For a couple it is density and pressure. These parameters for unsaturated steam have smaller values. The farther the vapor is from saturation, the smaller these values. One clarification: the reference temperature must be constant.
- For unsaturated steam, boyle-mariotte law: if the temperature and mass of the gas are constant, an increase or decrease in volume causes a decrease or increase in pressure by the same amount, pressure and volume are inversely related. From the maximum density and pressure at constant temperature their independence from the volume of saturated steam follows, it turns out that for saturated steam pressure and volume are independent of each other.
- For unsaturated steam density does not depend on temperature, and if the volume is conserved, the density value does not change either. For saturated steam, while maintaining volume, the density changes if the temperature changes. In this case, the relationship is direct. If the temperature increases, the density also increases; if the temperature decreases, the density also changes.
- If the volume is constant, unsaturated vapor behaves in accordance with Charles' law: as the temperature increases, the pressure increases by the same factor. This relationship is called linear. For saturated steam, as the temperature increases, the pressure increases faster than for unsaturated steam. The dependence is exponential.
Summing up, we can note significant differences in the properties of the compared objects. The main difference is that vapor, in a state of saturation, cannot be considered in isolation from its liquid. It is a two-component system to which most gas laws cannot be applied.
Evaporation - this is vaporization that occurs only from the free surface of a liquid adjoining a gaseous medium or vacuum.
The uneven distribution of the kinetic energy of the thermal motion of molecules leads to the fact that at any temperature the kinetic energy of some molecules of a liquid or solid can exceed the potential energy of their connection with the rest of the molecules.
Evaporation is the process by which molecules are ejected from the surface of a liquid or solid kinetic energy which exceeds the potential energy of interaction of molecules. Evaporation is accompanied by cooling of the liquid.
Let us consider the evaporation process from the point of view of molecular-kinetic theory. To leave the liquid, the molecules must do work by reducing their kinetic energy. Among the randomly moving molecules of a liquid in its surface layer, there will always be molecules that tend to fly out of the liquid. When such a molecule goes beyond surface layer, then a force arises that pulls the molecule back into the liquid. Therefore, only those molecules fly out of the liquid, in which the kinetic energy is greater than the work necessary to overcome the counteraction of molecular forces.
The rate of evaporation depends on:
a) the type of liquid;
b) on the area of its free surface. The larger this area, the faster the liquid evaporates.
c) the lower the vapor density of a liquid above its surface, the greater the rate of evaporation. Therefore, pumping vapors (wind) from the surface will accelerate its evaporation.
d) with increasing temperature, the rate of evaporation of the liquid increases.
vaporization is the transition of a substance from a liquid state to a gaseous state.
Condensation - is the transfer of matter from gaseous state into a liquid state.
During vaporization internal energy matter increases, and when condensed - decreases.
Heat of vaporization – is the amount of heat Q required to turn a liquid into vapor at a constant temperature.
Specific heat vaporization L is measured by the amount of heat required to turn a unit mass of liquid into steam at a constant temperature
Saturated and unsaturated steam. The evaporation of a liquid in a closed vessel at a constant temperature leads to a gradual increase in the concentration of molecules of the evaporating substance in the gaseous state. Some time after the start of the evaporation process, the concentration of a substance in the gaseous state reaches a value at which the number of molecules returning to the liquid per unit time becomes equal to the number of molecules leaving the surface of the liquid in the same time. A dynamic equilibrium is established between the processes of evaporation and condensation of matter.
The article tells about what steam is, what types of it are and how they are used in industry and everyday life.
Physics
One of the main sciences that help to find out the structure of the surrounding world and some of its processes is physics. There are many interesting reactions going on around us every second. We have long been accustomed to many of them and do not pay attention to them. Moreover, in Everyday life few people think about the nature of the burning of the Sun, or the occurrence of water vapor, which has a huge impact on the climate. And although the processes described above have been studied quite well, we will still consider the question of what steam is. By the way, it can be formed as a result of not only boiling or other reactions of various substances, but first things first.
Definition
Vapor is the gaseous state of a substance, provided that this phase of it is in equilibrium with other aggregate states of this matter. The process itself, as a result of which steam appears, is usually called vaporization, and the reverse process is called condensation. So now we know what steam is. By the way, usually when the word “steam” is mentioned, people almost always mean the steam that is obtained from ordinary water, although, as mentioned above, many substances can take this state of aggregation.
In addition, it is customary to distinguish two main types: saturated and unsaturated. But these definitions apply only to substances that are chemically pure. Let's analyze them in more detail.
What is unsaturated steam?

So they call it when it could not reach an equilibrium, called dynamic, in relation to the fluid from which it was formed. Often such a definition is confused with thermodynamic, which is wrong. If you compare the pressure of unsaturated vapor with saturated, then it will always have a lower value.
When unsaturated vapor appears on the surface of a liquid, the process of its formation proceeds faster and prevails over the reverse process (as we have already said, it is called condensation). And as a result, gradually the liquid becomes less and less.
Now let's look at what is
Saturated steam

Saturated steam is called when it was able to achieve dynamic equilibrium with the liquid from which it was obtained. Simply put, in this case, evaporation equals condensation, and unlike the situation with unsaturated steam, the amount of liquid can remain unchanged. Now we know what steam is and its types.
If, for example, you compress a vapor that is in equilibrium with its liquid, then this balance will gradually disappear, and the condensation will become stronger and stronger until, due to a change in the density of the gaseous substance, dynamic equilibrium is restored again.
Depending on the type of liquid, dynamic equilibrium with vapor manifests itself at different values of its density. This is due to the fact that all substances have a different force of intermolecular attraction.
water vapor

And yet, most often people understand this word as the gaseous state of water that is familiar to everyone. If you look into the encyclopedia, you can find the main defining characteristics of steam: the absence of color, smell and getting it, in fact, from water.
We've all noticed it repeatedly, whether it's boiling water for cooking or evaporating moisture off hot pavement after rain. But if you think about it and remember the periods of the industrial and technological revolution, it becomes clear that great importance in a person's life has steam. The physics of this phenomenon is such that it occupies a volume ten times larger than its original liquid form. It was this observation that at one time gave impetus to the development of technology.
It all started with the first and a little later "self-propelled" carts, as vehicles with such motors were called. But such cars were brought to working condition for a very long time, had low speed and poor handling. Everything changed only with the invention of steam locomotives.
In addition, water is also a good heat carrier and is used in many cooling systems, and if their circuit is not closed, then steam also appears as a result. In our time, it is sometimes specially brought to a gaseous state, but not in primitive engines, but at nuclear power plants, where turbines of electric generators rotate with steam.
Evaporated water is also of great importance in the climate. Having risen to a height where the temperature is much lower, the steam condenses and falls to the surface of the earth in the form of rain. Snow is almost the same.
Well, in the end, they cook dietary and simply healthy food for a couple.
Under the right conditions, the steam forms a fog at the surface of the earth.
Now we know what steam is and how it happens.
Topic 2. PHASE TRANSITIONS.
Phase transition ( phase transformation) is the transition of a substance from one phase to another with a change in external conditions (for example, temperature, pressure, magnetic and electric fields, etc.), accompanied by a change physical properties and substance parameters.
The value of temperature, pressure or some other physical quantity at which the phase transition occurs is called the transition point. There are two types of phase transition.
PHASE TRANSITIONS OF THE FIRST KIND
During a phase transition of the first kind, such thermodynamic
characteristics of a substance, such as density, concentration of components, specific volume, the amount of stored internal energy, i.e. a certain amount of heat is released or absorbed, which is called the heat of transition. Moreover, this refers to the abrupt change in these quantities not in time, but with a change in temperature, pressure, etc. The most common examples of first-order phase transitions are:
- melting and crystallization
- evaporation and condensation
- sublimation and desublimation
PHASE TRANSITIONS OF THE SECOND KIND
During a second-order phase transition, the density and internal energy do not change, so that naked eye such phase transition may be invisible. The jump is experienced by their derivatives with respect to temperature and pressure: heat capacity, coefficient of thermal expansion, various susceptibilities, etc. I.e. phase transitions of the second kind are accompanied by a change in the symmetry of the structure of the substance, and not by the release or absorption of energy (heat). The most common examples of second-order phase transitions are:
- passage of the system through a critical point
- paramagnetic-ferromagnetic transition
- transition of metals and alloys to the state of superconductivity
- transition of liquid helium to a superfluid state
- transition of amorphous materials to a glassy state
Modern physics also investigates systems that have phase transitions of the third or higher order. Recently wide use received the concept of a quantum phase transition, i.e. phase transition controlled not by classical thermal fluctuations, but by quantum ones, which exist even at absolute zero temperatures, where the classical phase transition cannot be realized due to the Nernst theorem.
Let us consider in more detail the phenomena of interest to us, which are associated with first-order phase transitions.
EVAPORATION, CONDENSATION, BOILING.
SATURATED AND UNSATURATED PAIRS.
Any substance under certain conditions can be in various states of aggregation - solid, liquid and gaseous. Transitions from one state of aggregation to the second are phase transitions of the first kind.
Evaporation and condensation are phase transitions between the liquid and gaseous phases of a substance.
All real gases(oxygen, nitrogen, hydrogen, etc.) under certain conditions are able to turn into a liquid. However, such a transformation can only occur at temperatures below a certain, so-called critical temperature T cr. For example, for water, the critical temperature is 647.3 K, for nitrogen 126 K, for oxygen 154.3 K. At room temperature (≈ 300 K), water can be in both liquid and gaseous states, while nitrogen and oxygen exist only in the form of gases.
by evaporation is called the phase transition from liquid to gaseous state. From the point of view of molecular kinetic theory, evaporation is a process in which the fastest molecules fly out from the surface of a liquid, the kinetic energy of which exceeds the energy of their connection with the rest of the liquid molecules. This leads to a decrease in the average kinetic energy of the remaining molecules, i.e., to cooling of the liquid (if there is no energy supply from the surrounding bodies).
Condensation is the reverse process of evaporation. During condensation, the vapor molecules return to the liquid.
In a closed vessel, a liquid and its vapor can be in a state dynamic balance, when the number of molecules leaving the liquid is equal to the number of molecules returning to the liquid from vapor, i.e., when the rates of evaporation and condensation are the same. Such a system is called two-phase . A vapor in equilibrium with its liquid is called rich.
The number of molecules emitted from a unit surface area of a liquid in one second depends on the temperature of the liquid. The number of molecules returning from vapor to liquid depends on the concentration of vapor molecules and on average speed their thermal motion, which is determined by the temperature of the steam. It follows that for a given substance, the concentration of vapor molecules at equilibrium of a liquid and its vapor is determined by their equilibrium temperature. The establishment of dynamic equilibrium between the processes of evaporation and condensation with increasing temperature occurs at higher concentrations of vapor molecules. Since the pressure of a gas (steam) is determined by its concentration and temperature, we can conclude: pressure saturated steam p 0 of a given substance depends only on its temperature and does not depend on volume. Therefore, the isotherms of real gases on the plane ( p, V) contain horizontal sections corresponding to a two-phase system (Fig. 3.4.1).
As the temperature rises, the saturation vapor pressure and its density increase, while the density of the liquid decreases due to thermal expansion. At a temperature equal to the critical temperature T kr for a given substance, the vapor and liquid densities become the same. At T > T kr, the physical differences between the liquid and its saturated vapor disappear.
If unsaturated vapor is isothermally compressed at T < T cr, then its pressure will increase until it becomes equal to the pressure of saturated vapor. With a further decrease in volume, a liquid is formed at the bottom of the vessel and a dynamic equilibrium is established between the liquid and its saturated vapor. With a decrease in volume, an increasing part of the vapor condenses, and its pressure remains unchanged (horizontal section on the isotherm). When all the vapor turns into a liquid, the pressure increases sharply with a further decrease in volume due to the low compressibility of the liquid.
It is possible to pass from the gaseous state to the liquid one bypassing the two-phase region. To do this, you need to complete the process bypassing the critical point K. One of the possible processes of this kind is shown in Fig. 1 by a broken line ABC.
AT atmospheric air water vapor is always present at some partial pressure p, which is usually less than the saturation vapor pressure p 0 . Attitude p / p 0 , expressed as a percentage, is called relative humidity air.
| |
Unsaturated steam can be theoretically described using the equation of state ideal gas under the usual restrictions for real gases: the vapor pressure should not be too high (practically p≤ (10 6 –10 7) Pa), and its temperature is higher than some value defined for each substance. The laws of an ideal gas can also be approximately applied to saturated steam, provided that for each temperature T pressure p 0 saturated steam is determined by balance curvep 0 (T) for a given substance.
Pressure p 0 of saturated steam increases very rapidly with increasing temperature T. Addiction p 0 (T) cannot be obtained from the ideal gas laws. The pressure of a gas at a constant concentration of molecules increases in direct proportion to the temperature. In saturated steam, as the temperature rises, not only the average kinetic energy of molecular motion increases, but also their concentration. Therefore, the pressure of saturated vapor increases faster with increasing temperature than the pressure of an ideal gas at a constant concentration of molecules.
Evaporation can occur not only from the surface, but also in the bulk of the liquid. Liquids always contain tiny gas bubbles. If the saturation vapor pressure of a liquid is equal to or greater than the external pressure (i.e., the pressure of the gas in the bubbles), the liquid will evaporate into the bubbles. The bubbles filled with steam expand and float to the surface. This process is called boiling . Thus, the boiling of a liquid begins at a temperature at which its pressure saturated vapors becomes equal to the external pressure.
In particular, under normal atmospheric pressure water boils at 100°C. This means that at this temperature the saturated vapor pressure of water is 1 atm. When climbing mountains, atmospheric pressure decreases, and therefore the boiling point of water decreases (approximately 1 ° C for every 300 meters of height). At an altitude of 7 km, the pressure is approximately 0.4 atm, and the boiling point drops to 70 °C.
In a hermetically sealed vessel, the liquid cannot boil, because at each temperature value, an equilibrium is established between the liquid and its saturated vapor. Along the equilibrium curve p 0 (T) it is possible to determine the boiling point of a liquid at various pressures.
The picture of real gas isotherms shown in Fig. 1 describes the processes of evaporation and condensation, i.e., the phase transition between the gaseous and liquid phases of a substance. In fact, this picture is incomplete, since any substance can go from gaseous and liquid to a solid state. At a given temperature T thermodynamic equilibrium between two phases of the same substance is possible only at a certain pressure in the system. The dependence of equilibrium pressure on temperature is called phase equilibrium curve . An example is the equilibrium curve p 0 (T) saturated vapor and liquid. If the equilibrium curves between different phases of a given substance are plotted on the plane ( p, T), then they divide this plane into separate regions in which matter exists in a homogeneous state of aggregation- solid, liquid or gaseous (Fig. 2). Depicted in the coordinate system ( p, T) equilibrium curves are called phase diagram .
Curve 0 T, corresponding to the equilibrium between the solid and gaseous phases, is called sublimation curve. Curve TK equilibrium between liquid and vapor is called evaporation curve, it breaks off at critical point K. Curve TM balance between solid and it's called a liquid melting curve.
Equilibrium curves converge at a point T, in which all three phases can coexist in equilibrium. This point is called triple point.
For many substances the pressure p tr at the triple point is less than 1 atm ≈ 10 5 Pa. Such substances melt when heated at atmospheric pressure. For example, triple point water (Fig. 3) has coordinates T tr = 273.16 K, p tr \u003d 6.02 10 2 Pa and is used as a reference for calibrating the absolute temperature scale of Kelvin.
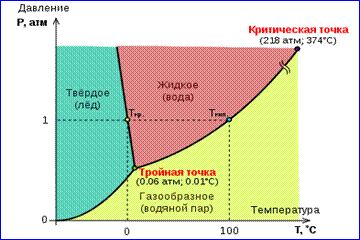 There are, however, also such 3 Phase diagram of water
There are, however, also such 3 Phase diagram of water
substances that have p tr
exceeds 1 atm. So for
carbon dioxide (CO 2) pressure
p tr = 5.11 atm and temperature
T tr = 216.5 K. Therefore, at atmospheric
pressure, solid carbon dioxide can
exist only at low temperatures, and in the liquid state at p= 1 atm it does not exist at all. In the solid state, carbon dioxide is in equilibrium with its vapor at atmospheric pressure at a temperature of 173 K or -80 °C. This is a widely used "dry ice" that never melts, but only evaporates (sublimes).






