Unsaturated steam formula. Saturated and unsaturated steam
Unsaturated and saturated vapors
Above the free surface of a liquid, there are always vapors of this liquid, which are formed due to evaporation. If the space above the free surface of the liquid is not limited by any walls, the molecules or atoms of the evaporated substance, making chaotic thermal motion, move away from the surface of the liquid. The removal of vapor particles from the surface of a liquid is facilitated by the phenomenon of diffusion, as well as natural or artificial convection of air layers. Vapor particle concentration at constant temperature under these conditions, it can vary over a wide range, both in the direction of decrease and increase. Such pairs are called unsaturated.
A different picture is observed if there is a limited space above the free surface of the liquid. Whether it is filled with any other gaseous substances, except for the vapors of the evaporating liquid, or not, it does not matter. It is important to note that the process of evaporation into a closed space can only occur up to a certain limit. The greater the concentration of molecules or atoms of the evaporating substance in the vapor state becomes, the greater will be the pressure of these vapors at a constant temperature. In this case, an increasing number of molecules or atoms can return through the free surface back into the liquid. If the amount of space given to the vapor is small enough and the liquid is large enough, dynamic equilibrium can set in: the number of particles leaving the liquid per unit time equals the number of particles returning to the liquid in the same time interval. In this case, a constant mass of vapor will be above the liquid, and a higher concentration of its particles under these conditions cannot be achieved. Such steam is called saturated.
Pressure saturated steam of some substance at a given temperature can have one - the only definite value.
A decrease in the volume of space provided to a saturated vapor of a given substance at a constant temperature leads to the condensation of part of the vapor into a liquid, since the concentration of its particles cannot exceed a certain value. This isothermal compression saturated vapors differs from the compression of unsaturated vapors, which behave like ordinary ideal gases. The Boyle-Mariotte law, therefore, does not hold for saturated vapors.
Gay–Lussac's law also does not apply to saturated vapors, since the volume provided to them is independent of temperature.
Not applicable to saturated vapor and Charles's law. With increasing temperature ideal gases or unsaturated vapors in the isochoric process, the average kinetic energy their particles, which leads to their more frequent collisions with each other and with the walls of the vessel, i.e. to an increase in pressure.
It is possible to speak with confidence that a given closed vessel contains exactly the saturated vapor of some substance only if the vessel contains this substance in liquid state and the mass of its liquid phase does not change.
For example, they put a closed glass vessel in front of us and ask us to determine without any measurements whether it contains saturated or unsaturated water vapor.
To answer this question, you need to wait a few minutes so that the contents of the vessel have time to come into thermodynamic equilibrium with the surrounding air. This is caused by the fact that we do not know at what temperature the vessel was brought from the room, and a change in the temperature of the steam at a constant volume can bring the steam from an unsaturated state to a saturated state and vice versa. If, after equilibrium has been established, there is no condensation of water on the inner walls of the vessel, we must say that at the temperature at which the observation is made, the vessel contains unsaturated vapor. If water droplets appear on the walls of the vessel, the steam is saturated.
Water vapor in the air
The Earth's atmosphere always contains water vapor. Their presence has to be reckoned with very often. In particular, air humidity must be accurately assessed in enclosed or poorly ventilated rooms, drying chambers, etc.
For quantification the content of water vapor in the air, two values \u200b\u200bare used - absolute humidity (f) and relative humidity(AT).
The absolute humidity is called physical quantity, measured by the mass of water vapor contained in one cubic meter of air. In this way, absolute humidity coincides with the density dimension, but in practice they usually use a unit - 1 g / m 3.
The latter circumstance is due to the fact that the absolute humidity f, expressed in g/m 3 , differs little in numerical value from the partial pressure of water vapor p under the same conditions, measured in millimeters of mercury.
The percentage ratio of the partial pressure p of water vapor in the air to the pressure of saturated water vapor p n.p. at a given temperature is called relative humidity:
When calculating relative humidity using this formula, the pressure p and p n.s. must be measured in the same units. Usually they are measured not in pascals, but in millimeters of mercury. The value of p n.s is determined from the tables.
The temperature at which air becomes saturated with water vapor during cooling is called the dew point.
Lecture #2
BASICS OF HEAT TRANSFER
Heat transfer is a science that studies the patterns of heat transfer processes between bodies and the distribution of heat within one body. The study of the laws of heat transfer is necessary to control heat flows that occur almost everywhere in the working processes of machines, engines, apparatuses, etc.
In the theory of heat transfer, two main questions are considered:
I. Determination of the amount of heat that is transferred from one body to another or passes from one part of the body to another under given conditions.
II. Determination of temperature in different parts of the body involved in the process of heat transfer.
A necessary and sufficient condition for heat transfer is the temperature difference.
Heat is transferred in three ways: conduction, convection and radiation.
Thermal conductivity is the process of distribution of thermal energy by direct contact of individual parts of the body with different temperatures.
Convection is the process of energy transfer when moving volumes of liquid or gas in space from an area with one temperature to an area with a different temperature.
Radiation (radiant heat transfer) is the process of energy transfer by electromagnetic waves. Heat transfer by radiation is a double transformation of energy: a hotter body radiates energy in the form of electromagnetic oscillations, another less heated body absorbs energy and heats up.
Usually, heat exchange between bodies takes place in all three ways simultaneously. The combination of them can be the most diverse. In this case, one method may prevail over the other, depending on the conditions under which heat transfer occurs.
However, when studying heat transfer processes, one should clearly distinguish and separately consider various methods of heat transfer (thermal conduction, convection and radiation), since they obey different laws.
Heat transfer processes in heat engineering devices can proceed both under steady (stationary) and unsteady (non-stationary) modes. A stationary (steady) thermal regime is a regime in which the temperature at any point of the body does not depend on time. A stationary regime is always preceded by a non-stationary regime.
The processes occurring in the conditions of non-stationary thermal conditions (processes of heating and cooling) are very complex, and their consideration is not included in the program of this course. Therefore, only stationary heat transfer processes are considered here.
THERMAL CONDUCTIVITY
Basic concepts
Thermal conductivity is the molecular transfer of heat in continuum due to the presence of a temperature difference.
This method of heat transfer takes place mainly in solids both inside one body and between two bodies when they come into contact with each other. Thermal conductivity can also be carried out through a layer of liquid or gas. However, gases and liquids, with the exception of molten metals, are very poor conductors of heat.
temperature field. The process of heat conduction, as well as other types of heat transfer, is carried out only on the condition that the temperature is not the same at different points of the body. As you know, temperature is a parameter of the state of the body and characterizes the degree of its heating. The set of temperature values at all points of the considered space in this moment time is called the temperature field. Mathematically, the temperature field is expressed as a function of coordinates
A surface at all points of which the temperature is the same is called isothermal.
Because no two people can be at the same point in space at the same time. different temperatures, then different isothermal surfaces never intersect. All of them either end on the surface of the body, or are entirely located inside it.
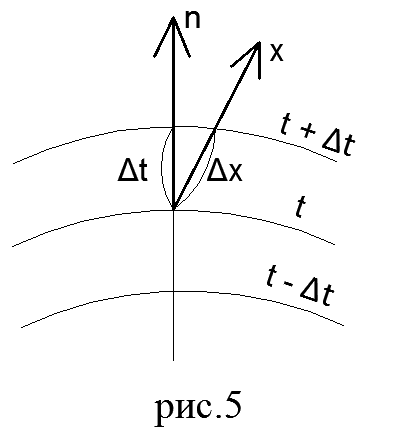 Fourier's law. Heat flow is the amount of heat Q passing per unit time through an arbitrary surface. The heat flux vector is always directed in the direction of decreasing temperature.
Fourier's law. Heat flow is the amount of heat Q passing per unit time through an arbitrary surface. The heat flux vector is always directed in the direction of decreasing temperature.
Quantitatively, the heat transfer intensity is characterized by the heat flux density q.
The heat flux density or specific heat flux is the amount of heat passing through the surface unit F per unit time τ:
Relation (51) expresses the basic law of thermal conductivity and is called the Fourier law.
The minus sign on the right side of relation (51) means that the heat flux and temperature gradient vectors are directed in opposite directions.
The coefficient of proportionality λ in expression (51) is a physical parameter of the substance, called the coefficient of thermal conductivity. It characterizes the ability of a substance to conduct heat.
The dimension of the thermal conductivity coefficient is determined from expression (51):
Consequently, the value of the thermal conductivity coefficient is numerically equal to the specific heat flux through a wall of unit thickness at a temperature difference of 1 ° C. The larger λ, the better the substance is a heat conductor.
Metals are good conductors of heat; dry, still air is a poor conductor of heat. Light porous materials do not conduct heat well, since their pores are filled with air. Materials whose thermal conductivity is less than 0.2 W / (m-deg) are called heat-insulating. Water has poor thermal conductivity, however, the thermal conductivity of a wet material increases sharply compared to its thermal conductivity in a dry state. This is due to the fact that water conducts heat 20-25 times better than air. Therefore, filling the pores of the body with water sharply reduces its thermal insulation properties.
The value of the thermal conductivity coefficient λ for each body is found experimentally. The results are summarized in tables that are used in the calculations.
Thermal conductivity of the wall
Flat single wall. Figure 6 shows a flat single-layer wall of thickness δ made of a homogeneous material (brick, metal, wood, or any other).
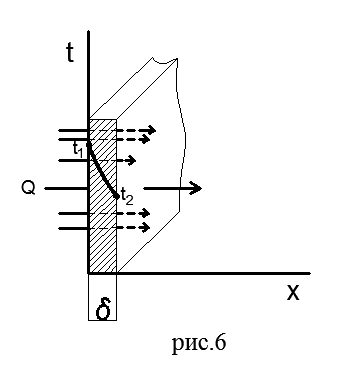 Let us assume that the coefficient of thermal conductivity of the material λ does not depend on temperature. Constant temperatures t 1 >t 2 are maintained on the outer surfaces of the wall; the temperature changes only in the direction of the x axis, which is perpendicular to the plane of the wall, i.e., the temperature field is one-dimensional, and the temperature gradient is equal to dt/dx.
Let us assume that the coefficient of thermal conductivity of the material λ does not depend on temperature. Constant temperatures t 1 >t 2 are maintained on the outer surfaces of the wall; the temperature changes only in the direction of the x axis, which is perpendicular to the plane of the wall, i.e., the temperature field is one-dimensional, and the temperature gradient is equal to dt/dx.
Let us find the density of the heat flux passing through a given wall and establish the nature of the temperature change along the wall thickness.
Let us select inside the wall an elementary layer of thickness dx bounded by two isothermal surfaces. The Fourier equation for this layer has the form
After integration
From this equation, one can determine the density of the heat flux passing through the considered wall. Putting in equation (53) x=δ, we get t= t2, where
| | (54) |
The heat flux density in a flat wall is directly proportional to the thermal conductivity coefficient λ, temperature difference () and inversely proportional to the wall thickness δ. It should be borne in mind that the heat flow is determined not by the absolute value of temperatures, but by their difference - the temperature difference. Equation (54) is the calculation formula for the thermal conductivity of a flat wall. It connects four quantities q, λ, δ and :
The ratio of the wall thickness to the thermal conductivity coefficient δ/λ is called the thermal resistance of the wall.
Equation (55) shows that the specific heat flux is directly proportional to the temperature difference and inversely proportional to the thermal resistance of the wall. Indeed, the larger the denominator of the fraction in equation (55), i.e., δ/λ, the lower the heat flux density q. Consequently, with an increase in wall thickness δ or with a decrease in thermal conductivity λ, the heat flux density q decreases.
Having determined the heat flux density by formula (54), we can determine the total amount of heat Q in joules transferred through the flat wall by the surface F during the time τ:
This equation is the equation of a straight line. Thus, at a constant value of the thermal conductivity coefficient, the temperature changes linearly over the thickness of a homogeneous wall. In cases where the thermal conductivity depends on temperature, it is a variable and the calculation formulas are somewhat more complicated.
Lesson #2/5 2
Topic No. 26: “Model of the structure of a liquid. Saturated and unsaturated vapors. Air humidity."
1 Fluid structure model
Liquid one of aggregate states substances. The main property of a liquid, which distinguishes it from other states of aggregation, is the ability to change its shape indefinitely under the action of tangential mechanical stresses, even arbitrarily small, while practically maintaining volume.
Fig.1
The liquid state is usually considered intermediate between solid and gas : a gas retains neither volume nor shape, but a solid retains both.
molecules liquids do not have a definite position, but at the same time they do not have complete freedom of movement. There is an attraction between them, strong enough to keep them close.
A substance in a liquid state exists in a certain interval temperatures , below which it goes intosolid state(crystallization occurs or transformation into a solid-state amorphous state glass), above into gaseous (evaporation takes place). The boundaries of this interval depend on pressure .
All liquids are usually divided into pure liquids and mixtures . Some mixtures of fluids are essential to life: blood, sea water etc. Liquids can perform the function solvents.
Fluidity is the main property of liquids. If you apply to a section of a fluid in equilibrium external force , then there is a flow of fluid particles in the direction in which this force is applied: the fluid flows. Thus, under the action of unbalanced external forces, the liquid does not retain the shape and relative arrangement of the parts, and therefore takes the form of the vessel in which it is located.
Unlike plastic solids, liquids do not haveyield strength: it is enough to apply an arbitrarily small external force for the liquid to flow.
One of the characteristic properties of a liquid is that it has certain amount ( with unchanged external conditions). Liquid is extremely difficult to compress mechanically because, unlike gas , between molecules there is very little free space. The pressure exerted on a liquid enclosed in a vessel is transmitted without change to each point of the volume of this liquid ( pascal's law , also valid for gases). This feature, along with very low compressibility, is used in hydraulic machines.
Liquids typically increase in volume (expand) when heated and decrease in volume (contract) when cooled. However, there are exceptions, for example, water shrinks when heated, at normal pressure and at temperatures between 0°C and approximately 4°C.
In addition, liquids (like gases) are characterized by viscosity . It is defined as the ability to resist the movement of one of the parts relative to the other that is, as internal friction.
When adjacent layers of a liquid move relative to each other, a collision of molecules inevitably occurs in addition to that due tothermal motion. There are forces that slow down the ordered movement. In this case, the kinetic energy of ordered motion is converted into thermal energy of the chaotic motion of molecules.
The liquid in the vessel, set in motion and left to itself, will gradually stop, but its temperature will rise.In a vapor, like a gas, one can hardly take into account the cohesion forces and consider the movement as a free flight of molecules and their collision with each other and with the surrounding bodies (walls and liquid covering the bottom of the vessel). In a liquid, molecules, as in a solid, strongly interact, holding each other. However, while in a solid body each molecule retains an indefinitely long definite position of equilibrium inside the body and its motion is reduced to oscillation around this equilibrium position, the nature of motion in a liquid is different. Liquid molecules move much more freely than solid molecules, though not as freely as gas molecules. Each molecule in a liquid moves back and forth for some time, without moving away, however, from its neighbors. This movement is reminiscent of the oscillation of a solid molecule around an equilibrium position. However, from time to time a liquid molecule breaks out of its environment and moves to another place, falling into a new environment, where again for some time it makes a movement similar to oscillation.
Thus, the movement of liquid molecules is something like a mixture of movements in a solid body and in a gas: "oscillatory" movement in one place is replaced by a "free" transition from one place to another. In accordance with this, the structure of a liquid is something in between the structure of a solid body and the structure of a gas. The higher the temperature, i.e., the greater the kinetic energy of the molecules of the liquid, the greater the role played by "free" motion: the shorter the intervals of the "oscillatory" state of the molecule and the more often "free" transitions, i.e., the more the liquid is likened to a gas. When enough high temperature characteristic of each liquid (the so-called critical temperature), the properties of the liquid do not differ from the properties of a highly compressed gas.
2 Saturated and unsaturated vapors and their properties
Above the free surface of a liquid there are always vapors of this liquid. If the vessel with the liquid is not closed, then the concentration of vapor particles at a constant temperature can vary over a wide range in the direction of decrease and increase.
Evaporation process in a closed space(closed container with liquid)can occur at a given temperature only up to a certain limit. This is due to the fact that vapor condensation occurs simultaneously with the evaporation of the liquid. First, the number of molecules emitted from the liquid in 1 s, more number molecules returning back, and the density, and hence the vapor pressure, increases. This leads to an increase in the rate of condensation. After some time, dynamic equilibrium sets in, at which the vapor density over the liquid becomes constant.
A vapor that is in dynamic equilibrium with its liquid is called saturated steam. A vapor that is not in dynamic equilibrium with its liquid is called unsaturated.
Experience shows that unsaturated vapors obey all gas laws , and the more accurate, the farther they are from saturation. For saturated vapors, the following properties are characteristic:
- density and pressure of saturated steam at a given temperature these are the maximum density and pressure that steam can have at a given temperature;
- the density and pressure of saturated vapor depend on the type of substance. The lower the specific heat of vaporization of a liquid, the faster it evaporates and the greater the pressure and density of its vapors;
- the pressure and density of saturated steam are uniquely determined by its temperature (they do not depend on how the steam reached this temperature: during heating or during cooling);
- vapor pressure and density increase rapidly with increasing temperature (Fig. 1, a, b).
Experience shows that when a liquid is heated, the level of the liquid in a closed vessel decreases. Consequently, the mass and density of the vapor increase. A stronger increase in the pressure of saturated vapor compared to an ideal gas (the Gay-Lussac law is not applicable to saturated vapor) is explained by the fact that here the pressure increases not only due to an increase in the average kinetic energy of the molecules (as in an ideal gas), but also due to increasing the concentration of molecules;
- at constant temperature, the pressure and density of saturated vapor do not depend on volume. Figure 2 shows for comparison the isotherms of ideal gas (a) and saturated steam (b).

Rice. 2
Experience shows that during isothermal expansion, the level of the liquid in the vessel decreases; the number of vapor molecules changes so that the vapor density remains constant.
3 Humidity
Air containing water vapor is called wet . To characterize the content of water vapor in the air, a number of quantities are introduced: absolute humidity, water vapor pressure and relative humidity.
absolute humidityρ air is called a value numerically equal to the mass of water vapor contained in 1 m 3 air (i.e. the density of water vapor in air under given conditions).
Water vapor pressure p is partial pressure water vapor contained in the air. The SI units for absolute moisture and elasticity are, respectively, kilogram per cubic meter (kg/m 3) and pascal (Pa).
If only absolute humidity or water vapor pressure is known, it is still impossible to judge how dry or humid the air is. To determine the degree of air humidity, it is necessary to know whether the water vapor is close or far from saturation.
relative humidity air φ called the percentage ratio of absolute humidity to densityρ 0 saturated steam at a given temperature (or the ratio of water vapor pressure to pressure p0 saturated steam at a given temperature):
The lower the relative humidity, the further the steam from saturation, the more intense the evaporation. Saturated steam pressure p0 at a given temperature tabular value. The elasticity of water vapor (and hence the absolute humidity) is determined by the dew point.
With isobaric cooling to a temperature tp the steam becomes saturated and its state is represented by a dot AT . Temperature t p at which water vapor becomes saturated is called dew point . When cooled below the dew point, vapor condensation begins: fog appears, dew falls, windows fog up.
4 Humidity measurement
Used to measure air humidity measuring instruments hygrometers. There are several types of hygrometers, but the main ones are: hair and psychrometric.
Since it is difficult to directly measure the pressure of water vapor in the air, the relative humidity of the air is measuredin an indirect way.
Operating principlehair hygrometerbased on the property of defatted hair (human or animal)change its lengthdepending on the humidity of the air in which it is located.

Hair stretched over a metal frame. The change in the length of the hair is transmitted to the arrow moving along the scale. Hair hygrometer in winter is the main instrument for measuring outdoor humidity.
A more accurate hygrometer is a psychrometric hygrometer psychrometer
(according to other Greek "psychros" means cold).
It is known that relative humidity depends evaporation rate.
The lower the air humidity, the easier it is for moisture to evaporate.

The psychrometer has two thermometers . One is ordinary, it is called dry. It measures the temperature of the surrounding air. The flask of another thermometer is wrapped in a fabric wick and lowered into a container of water. The second thermometer does not show the temperature of the air, but the temperature of the wet wick, hence the name moistened thermometer. The lower the air humidity, the more intense moisture evaporates from the wick, the greater the amount of heat per unit time is removed from the wetted thermometer, the smaller its readings, therefore, the greater the difference between the dry and wetted thermometer readings.
The dew point is determined using hygrometers. The condensation hygrometer is a metal box BUT , front wall To which is well polished (Fig. 2) An easily evaporating liquid ether is poured inside the box and a thermometer is inserted. Passing air through the box with a rubber bulb G , cause strong evaporation of the ether and rapid cooling of the box. The thermometer measures the temperature at which dew drops appear on the polished surface of the wall. To . The pressure in the area adjacent to the wall can be considered constant, since this area communicates with the atmosphere and the decrease in pressure due to cooling is compensated by an increase in the vapor concentration. The appearance of dew indicates that the water vapor has become saturated. Knowing the air temperature and dew point, you can find the partial pressure of water vapor and relative humidity.
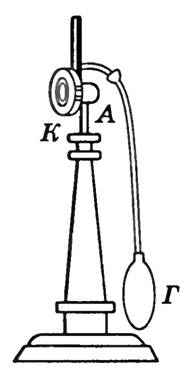
Rice. 2
5 Tasks for independent solution
Task 1
Cold autumn rain is falling outside. In which case will the laundry hung in the kitchen dry faster: when the window is open, or when it is closed? Why?
Task 2
The humidity is 78% and the dry bulb reading is 12°C. What temperature does a wet bulb thermometer show?(Answer: 10 °C.)
Task 3
The difference between dry and wet thermometer readings is 4°C. Relative air humidity 60%. What are the dry and wet bulb readings?(Answer: t c -l9 ° С, t m \u003d 10 ° С.)
The processes of evaporation and condensation are continuous and parallel to each other.
In an open vessel, the amount of liquid decreases over time, because. evaporation prevails over condensation.
Vapor that is above the surface of a liquid when evaporation prevails over condensation, or vapor in the absence of liquid, is called unsaturated.
In a hermetically sealed vessel, the liquid level does not change over time, because evaporation and condensation compensate each other: how many molecules fly out of the liquid, as many of them return to it in the same time, a dynamic (mobile) equilibrium occurs between the vapor and its liquid.
A vapor that is in dynamic equilibrium with its liquid is called saturated.
At a given temperature, the saturated vapor of a liquid has highest density ( ) and creates maximum pressure ( ) that the vapor of that liquid can have at that temperature.
The pressure and density of saturated vapor at the same temperature depends on the type of substance: more pressure creates saturated vapor of the liquid that evaporates faster. For example, and
Properties of unsaturated vapors: Unsaturated vapors obey the gas laws of Boyle - Mariotte, Gay-Lussac, Charles, and the ideal gas equation of state can be applied to them.
Saturated vapor properties:1. With a constant volume, with increasing temperature, the pressure of saturated vapor increases, but not in direct proportion (Charles' law is not fulfilled), the pressure grows faster than that of an ideal gas. , with increasing temperature ( ) , the mass of vapor increases, and therefore the concentration of vapor molecules increases () and the pressure of saturated vapor will melt for two reasons (
3 1 – unsaturated steam ( ideal gas);
2 2 - saturated steam; 3 - unsaturated steam,
1 obtained from saturated steam in the same
volume when heated.
2. The pressure of saturated vapor at a constant temperature does not depend on the volume it occupies.
With an increase in volume, the mass of the vapor increases, and the mass of the liquid decreases (part of the liquid passes into vapor), with a decrease in the volume of vapor, it becomes less, and the liquid becomes larger (part of the vapor passes into liquid), the density and concentration of saturated vapor molecules remain constant, therefore, and pressure remains constant ().
liquid
(sat. steam + liquid)
Unsaturated steam
Saturated vapors do not obey the gas laws of Boyle - Mariotte, Gay-Lussac, Charles, because the mass of steam in the processes does not remain constant, but all gas laws obtained for a constant mass. The equation of state for an ideal gas can be applied to saturated steam.
So, saturated steam can be converted to unsaturated steam, either by heating it at constant volume or increasing its volume at a constant temperature. Unsaturated steam can be converted to saturated steam either by cooling it at a constant volume or by compressing it at a constant temperature.
Critical situation
The presence of a free surface in a liquid makes it possible to indicate where the liquid phase of the substance is located, and where the gaseous one. The sharp difference between a liquid and its vapor is explained by the fact that the density of a liquid is many times greater than that of a vapor. If a liquid is heated in a hermetically sealed vessel, then due to expansion, its density will decrease, and the vapor density above it will increase. This means that the difference between a liquid and its saturated vapor is smoothed out and disappears altogether at a sufficiently high temperature. The temperature at which differences in physical properties between a liquid and its saturated vapor, and their densities become the same, is calledcritical temperature.
For the formation of liquid from gas, the average potential energy attraction of molecules must exceed their average kinetic energy.
Critical temperature – Maximum temperature at which the vapor is converted to liquid. The critical temperature depends on the potential energy of molecular interaction and is therefore different for different gases. Due to the strong interaction of water molecules, water vapor can be turned into water even at a temperature of . At the same time, nitrogen liquefaction occurs only at a temperature less than = -147˚, because nitrogen molecules weakly interact with each other.
Another macroscopic parameter that affects the vapor-liquid transition is pressure. With an increase in external pressure during gas compression, the average distance between particles decreases, the force of attraction between them increases and, accordingly, the average potential energy of their interaction.
Pressuresaturated steam at its critical temperature is called critical. This is the highest possible saturation vapor pressure of a given substance.
State of matter with critical parameters is called critical(critical point) . Each substance has its own critical temperature and pressure.
AT critical condition the specific heat of vaporization and the coefficient surface tension liquids. At temperatures above critical, even at very high pressures it is impossible to turn a gas into a liquid, i.e. above the critical temperature, the liquid cannot exist. At supercritical temperatures, only the vapor state of matter is possible.
Liquefaction of gases is possible only at temperatures below the critical temperature. For liquefaction, gases are cooled to a critical temperature, for example, by adiabatic expansion, and then isothermally compressed.
Boiling
Externally, the phenomenon looks like this: from the entire volume of the liquid, rapidly growing bubbles rise to the surface, they burst on the surface, and the vapor is released into the environment.
MKT explains boiling like this: there are always air bubbles in the liquid, in which evaporation from the liquid occurs. The closed volume of bubbles turns out to be filled not only with air, but also with saturated steam. The pressure of saturated vapor in them when the liquid is heated increases faster than the air pressure. When, in a sufficiently heated liquid, the pressure of saturated vapor in the bubbles becomes greater than the external pressure, they increase in volume, and a buoyant force that exceeds their gravity lifts the bubbles to the surface. Floated bubbles begin to burst when, at a certain temperature, the pressure of saturated vapor in them exceeds the pressure above the liquid. The temperature of a liquid at which the pressure of its saturated vapor in the bubbles is equal to or greater than the external pressure on the liquid is called boiling point.
The boiling point of different liquids is different, because the pressure of saturated vapor in their bubbles is compared with the same external pressure at different temperatures. For example, the saturation vapor pressure in the bubbles is equal to the normal atmospheric pressure for water at 100°C, for mercury at 357°C, for alcohol at 78°C, for ether at 35°C.
The boiling point remains constant during the boiling process, because all the heat that is supplied to the heated liquid is spent on vaporization.
The boiling point depends on the external pressure on the liquid: with increasing pressure, the temperature rises; as the pressure decreases, the temperature decreases. For example, at an altitude of 5 km above sea level, where the pressure is 2 times lower than atmospheric pressure, the boiling point of water is 83 ° C, in the boilers of steam engines, where the steam pressure is 15 atm. (), the water temperature is about 200˚С.
Air humidity
There is always water vapor in the air, so we can talk about air humidity, which is characterized by the following values:
1.Absolute humidity is the density of water vapor in the air (or the pressure that this vapor creates ( .
Absolute humidity does not give an idea of the degree of saturation of the air with water vapor. The same amount of water vapor different temperature creates a different feeling of moisture.
2.Relative Humidity is the ratio of the density (pressure) of water vapor contained in air at a given temperature to the density (pressure) of saturated vapor at the same temperature : or
is the absolute humidity at a given temperature; - density, saturated vapor pressure at the same temperature. The density and pressure of saturated water vapor at any temperature can be found in the table. The table shows that the higher the air temperature, the greater the density and pressure of water vapor in the air must be in order for it to be saturated.
Knowing the relative humidity, you can understand how many percent of the water vapor in the air at a given temperature is far from saturation. If the vapor in the air is saturated, then . If a , then there is not enough vapor in the air to a state of saturation.
The fact that the vapor in the air becomes saturated is judged by the appearance of moisture in the form of fog, dew. The temperature at which water vapor in the air becomes saturated is called dew point.
The vapor in the air can be made saturated by adding vapor due to additional evaporation of the liquid without changing the temperature of the air, or by lowering its temperature with the amount of vapor in the air.
Normal relative humidity, the most favorable for humans, is 40 - 60%. Great importance has knowledge of humidity in meteorology for weather forecasting. In weaving, confectionery production, a certain humidity is necessary for the normal course of the process. Storing works of art and books requires maintaining the humidity at the required level.
Humidity instruments:
1. Condensation hygrometer (allows you to determine the dew point).
2. The hair hygrometer (based on the length of the fat-free hair versus humidity) measures the relative humidity in percent.
3. The psychrometer consists of two dry and wet thermometers. The wet bulb bulb is wrapped in a cloth dipped in water. Due to evaporation from the fabric, the temperature of the moistened is lower than that of the dry. The difference in thermometer readings depends on the humidity of the surrounding air: the drier the air, the more intense the evaporation from the fabric, the greater the difference in thermometer readings and vice versa. If the air humidity is 100%, then the readings of the thermometers are the same, i.e. the difference in readings is 0. To determine the humidity using a psychrometer, a psychrometric table is used.
Melting and crystallization
When melting solid body the distance between the particles forming the crystal lattice increases, and the lattice itself is destroyed. The melting process requires energy. When a solid body is heated, the kinetic energy of vibrating molecules increases and, accordingly, the amplitude of their oscillations. At a certain temperature, called melting point, the order in the arrangement of particles in crystals is disturbed, the crystals lose their shape. A substance melts, changing from a solid state to a liquid state.
During crystallization there is a convergence of molecules that form a crystal lattice. Crystallization can only occur when the liquid releases energy. When the molten substance is cooled, the average kinetic energy and the speed of the molecules decrease. Attractive forces can keep particles near the equilibrium position. At a certain temperature, called solidification (crystallization) temperature, all molecules are in a position of stable equilibrium, their arrangement becomes ordered - a crystal is formed.
The melting of a solid occurs at the same temperature at which the substance solidifies.
Each substance has its own melting point. For example, the melting points for helium are -269.6˚С, for mercury -38.9˚С, for copper 1083˚С.
During the melting process, the temperature remains constant. The amount of heat supplied from outside goes to the destruction of the crystal lattice.
During the curing process, although heat is removed, the temperature does not change. The energy released during crystallization is used to maintain a constant temperature.
Until all the substance melts or all the substance solidifies, i.e. as long as the solid and liquid phases of a substance exist together, the temperature does not change.
TV+liquid liquid + tv
, where - the amount of heat, - the amount of heat required to melt the substance released during the crystallization of the substance mass mass
- specific heat of fusion– the amount of heat required to melt a 1 kg substance at its melting point.
What amount of heat is spent during the melting of a certain mass of a substance, the same amount of heat is released during the crystallization of this mass.
Also called specific heat crystallization.
At the melting point, the internal energy of a substance in a liquid state is greater internal energy the same mass of matter in the solid state.
At a large number When a substance melts, its volume increases and its density decreases. On hardening, on the contrary, the volume decreases, and the density increases. For example, solid naphthalene crystals sink in liquid naphthalene.
Some substances, for example, bismuth, ice, gallium, cast iron, etc., shrink when melted, and expand when solidified. These deviations from general rule explained by the structural features of crystal lattices. Therefore, water is denser than ice, ice floats in water. The expansion of water during freezing leads to the destruction of rocks.
The change in the volume of metals during melting and solidification is essential in foundry business.
Experience shows that change in external pressure on solid affects the melting point of the substance. For those substances that expand during melting, an increase in external pressure leads to an increase in the melting point, because. hinders the melting process. If substances are compressed during melting, then for them an increase in external pressure leads to a decrease in the melting temperature, because helps the melting process. Only a very large increase in pressure noticeably changes the melting point. For example, to lower the melting point of ice by 1˚C, the pressure must be increased by 130 atm. The melting point of a substance at normal atmospheric pressure called the melting point of the substance.