Physical quantities and units of their measurement. The concept of a physical quantity and its units of measurement
The objects and phenomena of the world around us are characterized by various properties that can manifest themselves to a greater or lesser extent and, therefore, can be quantified. For a quantitative description of various properties of processes and physical bodies the concept physical quantity.
Under physical quantity understand one of the properties of a physical object ( physical system, phenomenon or process), which is qualitatively common for many physical objects, but quantitatively individual for each of them. So, all bodies have mass, temperature, but for each of them these properties are different. The same can be said about other quantities - electrical conductivity, strength, radiation flux, etc.
Usually, when speaking about measurement, they mean the measurement of physical quantities, i.e. quantities inherent in the material world. These quantities are studied in natural and technical sciences(physics, chemistry, biology, electrical engineering, heat engineering, etc.), they are the object of control and management in production (in metallurgy, mechanical engineering, instrumentation, etc.). For example, the object of measurements can be the diameter of the shaft being turned, the amount of product dispensed, the rate of liquid flow through the pipeline, the content of alloying components in the alloy, the temperature of the melt, etc.
For a more detailed study of physical quantities, they are classified into groups (Fig. 1.1). By belonging to different groups physical phenomena physical quantities are divided into space-time, mechanical, thermal, electrical and magnetic, acoustic, light, physico-chemical, etc.
Rice. 1.1. Classification of physical quantities
According to the degree of conditional independence from other quantities, physical quantities are divided into basic and derivatives. Currently in international system units use seven quantities chosen as basic (independent of one another): length, time, mass, temperature, force electric current, the amount of matter and the intensity of light. Other quantities, such as density, force, energy, power, etc., are derivatives (i.e. dependent on other quantities).
According to the presence of dimension, physical quantities are divided into dimensional ones, i.e. dimensioned and dimensionless.
The size physical quantity characterizes the quantitative content of the property in each object. Meaning a physical quantity is an expression of its size in the form of a certain number of units of measurement accepted for it. For example, 0.001km; 1m; 100 cm; 1000mm - four options for representing the same size value, in this case, the length.
Numeric value physical quantity is a number expressing the ratio of the value of the quantity to the corresponding unit of measurement.
unit of measurement represents a value of a fixed size, which is conventionally assigned a numerical value equal to 1, and is used to quantify physical quantities homogeneous with it. A unit of measurement may belong to any system of units or be non-systemic or conditional.
Obviously, the numerical value of the quantity directly depends on the chosen unit of measurement.
Units of the same quantity may differ in size, for example, meter, foot and inch, being units of length, have different sizes: 1 foot = 0.3048 m, 1 inch = 0.0254 m.
Thus, in order to measure any physical quantity, i.e. to determine its value, it is necessary to compare (compare) it with the unit of measurement of this quantity, and determine how many times it is more or less than the unit of measurement.
The following measurement definition is currently established:
measurement is a set of operations on the use of a technical means that stores a unit of a physical quantity, providing a ratio (in an explicit or implicit form) of the measured quantity with its unit and obtaining the value of this quantity.
In other words, a measurement is a physical experiment carried out with the help of measuring instruments. Without physical experience, there is no measurement. The founder of Russian metrology D.I. Mendeleev wrote: “Science begins as soon as they begin to measure; exact science is not conceivable without measure.
It is appropriate to give the definition of the concept of "measurement" given by the outstanding philosopher P.A. Florensky ("Technical Encyclopedia" 1931): "Measurement is the main cognitive process of science and technology, by means of which an unknown quantity is quantitatively compared with another, homogeneous with it and considered known."
So, if there is a certain quantity Q, the unit of measurement accepted for it, equal to [Q], then the size of the physical quantity
Q = q×[Q], (1.1)
where q is the numerical value of Q.
The expression q×[Q] is measurement result, it is composed of two parts: the numerical value q, which is the ratio of the measured value to the unit of measurement (it can be integer or fractional), and the unit of measure [Q]. Usually, a unit of physical quantity is stored by a technical device used for measurement - a measuring instrument.
Suppose, when measuring the length of a part, a measurement result of 101.6 mm is obtained. In this case, , the numerical value q = 101.6 is taken as a unit of length. If we take as a unit, then q = 10.16, if we use as a unit, then q = 40.
Equation (1.1) is called basic measurement equation, because it describes measurement as the process of comparing a physical quantity with its unit of measurement.
Different units can be chosen to measure the quantity, i.e.
Q = q 1 × [Q] 1 = q 2 × [Q] 2 (1.2)
It follows from this expression that the numerical value of the quantity is inversely proportional to the size of the unit: than larger size units, the smaller the numerical value of the quantity, and vice versa:
In addition, equation (1.3) shows that the size of the physical quantity Q does not depend on the choice of the unit of measurement.
Thus, the numerical values of the measured quantities depend on which units of measurement are used. The choice of units has great importance to ensure comparability of measurement results; to allow arbitrariness in the choice of units means to violate the unity of measurements. That is why in most countries of the world the sizes of units of measurement are fixed by law (i.e. legalized). In Russia, in accordance with the Law "On Ensuring the Uniformity of Measurements", units of the International System of Units are allowed to be used.
In the real world, units of measurement do not exist, they are the result of human activity. A unit of measurement is a certain model, according to which a certain size of a physical quantity is taken as a unit by agreement and established by law. In addition, this model is implemented in the measuring instrument, which stores it and transmits it to all other measuring instruments using this unit. Such a process of formation, storage and use of units of physical quantities has developed in the last two centuries.
A measurement is significant only when the true value of the quantity can be estimated from its result. When analyzing measurements, these two concepts should be clearly distinguished: the true value of a physical quantity and its empirical manifestation - the result of measurement.
Any measurement result contains an error due to the imperfection of the means and methods of measurement, the influence external conditions and other reasons. The true value of the measured quantity remains unknown. It can only be imagined theoretically. The result of measuring a quantity only approaches its true value to a greater or lesser extent, i.e. represents his assessment. For more information on measurement error, see Chap. 2 "Measurement errors".
Measurement scales
Measurement scale serves as the initial basis for measuring this quantity. It is an ordered set of value values.
Practical activity led to the formation various kinds scales of measurements of physical quantities, the main of which are four, considered below.
1. Scale of order (ranks) is a ranked series – an ascending or descending sequence of values that characterize the property under study. It allows you to establish an order relation in terms of increasing or decreasing values, but there is no way to judge how many times (or how much) one value is greater or less than another. In order scales, in some cases, there may be a zero (zero mark), the main thing for them is the absence of a unit of measurement, because its size cannot be determined; in these scales, mathematical operations (multiplication, summation) cannot be performed on quantities.
An example of an order scale is the Mohs scale for determining the hardness of bodies. This is a scale with reference points, which contains 10 reference (reference) minerals with different conditional hardness numbers. Examples of such scales are also the Beaufort scale for measuring the strength (speed) of the wind and the Richter earthquake scale (seismic scale).
2. Scale of intervals (differences) differs from the order scale in that for the measured quantities not only order relations are introduced, but also the summation of intervals (differences) between various quantitative manifestations of properties. Difference scales can have conditional zero-benchmarks and units of measurement established by agreement. On the scale of intervals, you can determine how much one value is greater or less than another, but you cannot say how many times. The interval scales measure time, distance (if the beginning of the journey is not known), temperature in Celsius, etc.
Spacing scales are more advanced than order scales. In these scales, additive mathematical operations (addition and subtraction) can be performed on quantities, but multiplicative ones (multiplication and division) cannot be performed.
3.Relationship scale describes the properties of quantities for which the relations of order, summation of intervals, and proportionality apply. In these scales, there is a natural zero and, by agreement, the unit of measurement is set. The ratio scale serves to represent the results of measurements obtained in accordance with the basic measurement equation (1.1) by experimental comparison of the unknown quantity Q with its unit [Q]. Examples of ratio scales are the scales of mass, length, speed, thermodynamic temperature.
The ratio scale is the most advanced and most widely used of all measurement scales. This is the only scale on which you can set the value of the measured size. Any mathematical operations are defined on the ratio scale, which allows you to make multiplicative and additive corrections to the readings printed on the scale.
4. Absolute scale has all the features of the scale of relations, but additionally it has a natural unambiguous definition of the unit of measurement. These scales are used to measure relative values(gain, attenuation, useful action, reflection, absorption, amplitude modulation, etc.). A number of such scales have boundaries between zero and one.
Scales of intervals and ratios are united by the term "metric scales". The order scale is referred to as conditional scales, i.e. to scales in which the unit of measurement is not defined and is sometimes called non-metric. Absolute and metric scales are classified as linear. The practical implementation of measurement scales is carried out by standardizing both the scales and units of measurement themselves, and, if necessary, the methods and conditions for their unambiguous reproduction.
Physical quantities. Units
Physical quantity is a property that is qualitatively common for many physical objects, but quantitatively individual for each of them.
The value of a physical quantity- this is quantification the size of a physical quantity, represented as a certain number of units accepted for it (for example, the value of the conductor resistance is 5 ohms).
Distinguish true the value of a physical quantity that ideally reflects the property of the object, and valid, found experimentally close enough to the true value to be used instead, and measured the value read by the reading device of the measuring instrument.
A set of quantities interconnected by dependencies form a system of physical quantities, in which there are basic and derived quantities.
Main a physical quantity is a quantity included in the system and conditionally accepted as independent of other quantities of this system.
Derivative a physical quantity is a quantity included in the system and determined through the basic quantities of this system.
An important characteristic of a physical quantity is its dimension (dim). Dimension- this is an expression in the form of a power monomial, made up of products of symbols of basic physical quantities and reflecting the relationship of a given physical quantity with physical quantities taken in this system of quantities as basic ones with a proportionality coefficient equal to one.
Unit of physical quantity - it is a specific physical quantity, defined and accepted by agreement, with which other quantities of the same kind are compared.
In accordance with the established procedure, units of quantities of the International System of Units (SI), adopted by the General Conference on Weights and Measures, recommended by the International Organization of Legal Metrology are allowed to be used.
There are basic, derivative, multiple, submultiple, coherent, systemic and non-systemic units.
Basic unit of the system of units- unit of the main physical quantity, chosen when building a system of units.
Meter is the length of the path traveled by light in vacuum in a time interval of 1/299792458 of a fraction of a second.
Kilogram- a unit of mass equal to the mass of the international prototype of the kilogram.
Second- time equal to 9192631770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the Cesium-133 atom.
Ampere- the strength of an unchanging current, which, when passing through two parallel rectilinear conductors of infinite length and an insignificantly small circular cross-sectional area, located in vacuum at a distance of 1 m from one another, would cause an interaction force equal to 2 ∙ 10 on each section of the conductor 1 m long -7 N.
Kelvin- unit of thermodynamic temperature, equal to 1/273.16 of the thermodynamic temperature triple point water.
mole- the amount of substance of the system containing as many structural elements as there are atoms in carbon-12 weighing 0.012 kg.
Candela- luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540 ∙ 10 12 Hz, the energy intensity of which in this direction is 1/683 W/sr.
Two additional units are also provided.
Radian- the angle between two radii of a circle, the length of the arc between which is equal to the radius.
Steradian- a solid angle with a vertex at the center of the sphere, cutting out on the surface of the sphere an area equal to the area of a square with a side equal to the radius of the sphere.
Derived unit of the system of units- a unit of a derivative of a physical quantity of a system of units, formed in accordance with an equation connecting it with basic units or with basic and already defined derivatives. For example, the unit of power, expressed in terms of SI units, is 1W = m 2 ∙ kg ∙ s -3.
Along with SI units, the Law "On Ensuring the Uniformity of Measurements" allows the use of non-systemic units, i.e. units that are not included in any of the existing systems. It is customary to distinguish several types off-system units:
Units allowed along with SI units (minute, hour, day, liter, etc.);
Units used in special fields of science and technology
(light year, parsec, diopter, electron volt, etc.);
Disused units (millimeter of mercury,
horsepower, etc.)
Non-systemic units also include multiple and submultiple units of measurement, which sometimes have their own names, for example, the unit of mass is a ton (t). In the general case, decimal, multiple and submultiple units are formed using multipliers and prefixes.
Measuring instruments
Under measuring instrument(SI) is understood as a device intended for measurements and having normalized metrological characteristics.
According to their functional purpose, SI are divided into: measures, measuring instruments, measuring transducers, measuring installations, measuring systems.
Measure- a measuring instrument designed to reproduce and store a physical quantity of one or more dimensions with the required accuracy. A measure can be represented as a body or a device.
Measuring device(IP) - a measuring tool designed to extract measurement information and convert
into a form that can be directly perceived by the operator. Measuring instruments usually include
measure. According to the principle of operation, analog and digital IP are distinguished. According to the method of presentation of measurement information, measuring instruments are either indicating or registering.
Depending on the method of converting the measurement information signal, direct conversion devices (direct action) and balancing conversion (comparison) devices are distinguished. In direct conversion devices, the measurement information signal is converted the required number of times in one direction without the use of feedback. In balancing conversion devices, along with a direct conversion circuit, there is an inverse conversion circuit and the measured value is compared with a known value that is homogeneous with the measured value.
Depending on the degree of averaging of the measured value, devices are distinguished that give indications of instantaneous values of the measured value, and integrating devices, the readings of which are determined by the time integral of the measured value.
Measuring transducer- a measuring instrument designed to convert a measured quantity into another quantity or a measuring signal that is convenient for processing, storage, further transformations, indication or transmission.
Depending on the place in the measuring circuit, primary and intermediate transducers are distinguished. Primary transducers are those to which the measured value is supplied. If the primary transducers are placed directly on the object of study, remote from the place of processing, then they are sometimes called sensors.
Depending on the type of input signal, converters are divided into analog, analog-to-digital and digital-to-analog. Scale measuring transducers are widely used, designed to change the size of a quantity by a given number of times.
Measuring setup- this is a set of functionally integrated measuring instruments (measures, measuring instruments, measuring transducers) and auxiliary devices (interfacing, power supply, etc.) intended for one or more physical quantities and located in one place.
Measuring system- a set of functionally integrated measures, measuring transducers, computers and other technical means located in different points controlled object, in order to measure one or more physical quantities.
Types and methods of measurements
In metrology, measurement is defined as a set of operations performed with the help of a technical + - means that stores a unit of a physical quantity, which makes it possible to compare the measured quantity with its unit and obtain the value of this quantity.
The classification of types of measurements according to the main classification features is presented in Table 2.1.
Table 2.1 - Types of measurements
Direct measurement - measurement, in which the initial value of the quantity is found directly from the experimental data as a result of the measurement. For example, measuring current with an ammeter.
indirect measurement - a measurement in which the desired value of a quantity is found on the basis of a known relationship between this quantity and the quantities that are subjected to direct measurements. For example, measuring the resistance of a resistor using an ammeter and a voltmeter using a relationship that relates resistance to voltage and current.
Joint measurements are measurements of two or more dissimilar quantities to find the relationship between them. A classic example of coupled measurements is finding the temperature dependence of a resistor's resistance;
Cumulative measurements - these are measurements of several quantities of the same name, in which the desired values \u200b\u200bof the quantities are found by solving a system of equations obtained by direct measurements and various combinations of these quantities.
For example, finding the resistances of two resistors based on the results of measuring the resistances of series and parallel connections these resistors.
Absolute measurements - measurements based on direct measurements of one or more quantities and the use of physical constant values, for example, measurements of current in amperes.
relative measurements - measurements of the ratio of the value of a physical quantity to the quantity of the same name or changes in the value of the quantity in relation to the quantity of the same name taken as the initial one.
To static measurements include a measurement in which the SI operates in a static mode, i.e. when its output (for example, pointer deflection) remains unchanged during the measurement time.
To dynamic measurements include measurements performed by the SI in dynamic mode, i.e. when its readings depend on dynamic properties. The dynamic properties of the MI are manifested in the fact that the level of variable impact on it at any point in time determines the output signal of the MI at a subsequent point in time.
Measurements with the highest possible accuracy achieved at the current level of development of science and technology. Such measurements are carried out when creating standards and measuring physical constants. Typical for such measurements are the estimation of errors and the analysis of their sources.
Technical measurements are measurements carried out under specified conditions according to a certain methodology and carried out in all industries National economy except for scientific research.
The set of methods for using the principle and measuring instruments is called measurement method(fig.2.1).
Without exception, all measurement methods are based on comparing the measured value with the value reproduced by the measure (single-valued or multivalued).
The method of direct assessment is characterized by the fact that the values of the measured quantity are counted directly on the reading device measuring device direct action. The scale of the device is pre-calibrated using a multi-valued measure in units of the measured value.
Methods of comparison with a measure involve comparing the measured value and the value reproduced by the measure. The following comparison methods are most common: differential, zero, substitution, coincidence.
Figure 2.1 - Classification of measurement methods
With the zero method of measurement, the difference between the measured value and the known value is reduced to zero during the measurement process, which is recorded by a highly sensitive zero indicator.
With the differential method, the difference between the measured value and the value reproduced by the measure is counted on the scale of the measuring instrument. unknown quantity determined by the known value and the measured difference.
The substitution method provides for alternately connecting the measured and known values to the indicator input, i.e. measurements are carried out in two steps. The smallest measurement error is obtained when, as a result of selecting a known value, the indicator gives the same reading as with an unknown value.
The matching method is based on measuring the difference between the measured value and the value reproduced by the measure. When measuring, coincidences of scale marks or periodic signals are used. The method is used, for example, when measuring frequency and time using reference signals.
Measurements are performed with single or multiple observations. Here, observation is understood as an experimental operation performed in the process of measurement, as a result of which one value of a quantity is obtained, which is always random. In measurements with multiple observations, statistical processing of the observation results is required to obtain the measurement result.
2.1 Physical quantity, its qualitative and quantitative characteristics. Unit of physical quantity
In the broad sense of the word "value" is a multi-species concept. For example, such quantities as price, cost of goods, are expressed in monetary units. Another example is the value of the biological activity of medicinal substances, which is expressed in the corresponding units, denoted by the letters I.E. For example, recipes indicate the amount of many antibiotics, vitamins in these units.
Modern metrology is interested in physical quantities. Physical magnitude - this is a property that is qualitatively common for many objects (systems, their states and processes occurring in them), but quantitatively individual for each object. Individuality in quantitative terms should be understood in the sense that a property can be for one object a certain number of times more or less than for another. All electrical and radio quantities are typical examples of physical quantities.
Formalized reflection of the qualitative difference between the measured quantities is their dimension. Dimension is denoted by the symbol dim, which comes from the word dimension, which, depending on the context, can be translated as both size and dimension. The dimension of the basic physical quantities is indicated by the corresponding capital letters. For example, for length, mass and time
dim l = L; dimm = M; dim t = T. (2.1)
The dimension of derivative physical quantities can be expressed in terms of the dimensions of the basic physical quantities using a power monomial:
where dim z is the dimension of the derivative of the physical quantity z;
L, M, T, … - dimensions of the corresponding basic physical quantities;
α, β, γ, … - dimension indicators.
Each of the dimension indicators can be positive or negative, integer or fractional number, zero. If all dimensions are equal to zero, then such a quantity is called dimensionless. It can be relative if it is defined as the ratio of similar quantities (for example, the relative permittivity), and logarithmic if it is defined as the logarithm of the relative value (for example, the logarithm of the voltage ratio).
So, dimension is a qualitative characteristic of a physical quantity.
Dimension theory is widely used to quickly check the correctness of complex formulas. If the dimensions of the left and right parts of the equation do not match, then in the derivation of the formula, no matter what field of knowledge it belongs to, one should look for an error.
The quantitative characteristic of a physical quantity is its the size . Obtaining information about the size of a physical or non-physical quantity is
is the content of any dimension. The simplest way to obtain such information, which allows you to get some idea of the size of the measured value, is to compare it with another according to the principle "which is more (less)?" or "which is better (worse)?". More detailed information about how much more (less) or how many times better (worse) is sometimes not even required. In this case, the number of sizes compared with each other can be quite large. Arranged in ascending or descending order, the dimensions of the measured quantities form order scale . So, for example, in many competitions and competitions, the skill of performers and athletes is determined by their place in the final table. The latter, therefore, is a scale of order - a form of presentation of measuring information, reflecting the fact that the skill of some is higher than the skill of others, although it is not known to what extent (how much or how many times). Having built people by height, it is possible, using a scale of order, to conclude who is taller than whom, but it is impossible to say how much higher. The arrangement of dimensions in ascending or descending order in order to obtain measurement information on an order scale is called ranking .
To facilitate measurements on the order scale, some points on it can be fixed as reference points. (reference) . Knowledge, for example, is measured on a reference scale of order, which has the following form: unsatisfactory, satisfactory, good, excellent. The points of the reference scale can be assigned figures called points . For example, the intensity of earthquakes is measured on the 12-point international seismic scale MSK-64, and the strength of the wind is measured on the Beaufort scale. Reference scales also measure the strength of sea waves, the hardness of minerals, the sensitivity of photographic films, and many other quantities. Reference scales are especially widespread in the humanities, sports, and art.
The disadvantage of reference scales is the uncertainty of intervals between reference points. Therefore, points cannot be added, subtracted, multiplied, divided, etc. More perfect in this regard are scales composed of strictly defined intervals. It is generally accepted, for example, to measure time on a scale divided into intervals equal to the period of the Earth's revolution around the Sun. These intervals (years) are in turn divided into smaller ones (days), equal to the period of the Earth's revolution around its axis. The day is divided into hours, hours into minutes, minutes into seconds. Such a scale is called interval scale . According to the scale of intervals, one can already judge not only that one size is larger than the other, but also how much larger, i.e. the interval scale defines mathematical operations such as addition and subtraction. In any reckoning, a radical turning point in the course of the Second World War occurred near Stalingrad 700 years after the defeat of the German knights of the Livonian Order by Alexander Nevsky on the ice of Lake Peipus. But if we raise the question of “how many times” later this event occurred, then it turns out that according to our Gregorian style - in 1942/1242 = 1.56 times, according to the Julian calendar, counting time from the “creation of the world”, - in 7448/6748 \u003d 1.10 times, according to the Jewish, where time is counted "from the creation of Adam", - 5638/4938 \u003d 1.14 times, and according to the Mohammedan chronology, which began from the date of Mohammed's flight from Mecca to the holy city of Medina , - in 1320/620 = 2.13 times. Therefore, it is impossible to say on the scale of intervals how many times one size is larger or smaller than another. This is explained by the fact that the scale of intervals is known, and the origin can be chosen arbitrarily.
Interval scales are sometimes obtained by dividing the interval between two fiducial points proportionally. So, on the Celsius temperature scale, one degree is a hundredth of the interval between the melting temperature of ice, taken as the starting point, and the boiling point of water. On the Réaumur temperature scale, the same interval is divided into 80 degrees, and on the Fahrenheit temperature scale - into 180 degrees, and the reference point is shifted by 32 degrees Fahrenheit towards low temperatures.
If one of the two reference points is chosen as one in which the size is not taken equal to zero (which leads to the appearance of negative values), but is actually equal to zero, then on such a scale it is already possible to count the absolute value of the size and determine not only, on how much one size is more or less than the other, but also how many times it is more or less. This scale is called relationship scale. An example of this is the Kelvin temperature scale. In it, the absolute zero temperature is taken as the reference point, at which the thermal motion of molecules stops. There can be no lower temperature. The second reference point is the melting temperature of ice. On the Celsius scale, the interval between these fiducial points is approximately 273 degrees Celsius. Therefore, on the Kelvin scale, it is divided into 273 equal parts, each of which is called Kelvin and is equal to degrees Celsius, which greatly facilitates the transition from one scale to another.
The ratio scale is the most perfect of all the considered scales. It defines the largest number of mathematical operations: addition, subtraction, multiplication, division. But, unfortunately, building a scale of relationships is not always possible. Time, for example, can only be measured on a scale of intervals.
Depending on the intervals into which the scale is divided, the same size is presented in different ways. For example, 0.001 km; 1m; 10 dm; 100 cm; 1000 mm - five representations of the same size. They are called values physical quantity. Thus, the value of a physical quantity is an expression of its size in certain units of a physical quantity. The abstract number included in the expression is called numerical value eat. It shows how many units the measured size is greater than zero or how many times it is greater than the unit of measurement. Thus, the value of the physical quantity z is determined by its numerical value (z) and some size [z], taken as unit of physical quantity
z=(z)[z]. (2.3)
Equation (2.3) is called the basic measurement equation. It follows from this equation that the value of (z) depends on the size of the chosen unit [z]. The smaller the selected unit, the larger the numerical value for the measured quantity. If, when measuring the value of z, instead of the unit [z], we take another unit , then expression (2.3) will take the form
z=(z 1 ) .
Taking into account equation (2.3), we obtain
(z)[z]=(z 1 ) ,
(z 1 )=(z)·[z]/.
It follows from this formula that to go from a value (z) expressed in one unit [z] to a value (z 1 ) expressed in another unit, it is necessary to multiply (z) by the ratio of the adopted units.
2.2 Emergence, development and unification of units
physical quantities. Creating Metric Measures
Units of physical quantities began to appear from the moment when a person had the need to express something quantitatively. This "something" could be a number of items. In this case, the measurement was extremely simple, since it consisted in counting the number of objects, and one object was the unit. But then the task became more complicated, since it became necessary to determine the number of such objects (liquids, loose bodies, etc.) that could not be counted by piece. There are measures of volume. The need to measure lengths and weights gave rise to measures of length and weight. For example, the first measures of length were parts of the human body: a span, a foot, an elbow, as well as a step, etc. In addition to the quantitative determination of the properties of the body and substances, a new
the need to quantify and processes. So there was a need to measure time. The first unit of time was the day - the change of day and night.
The second stage in the development of units was associated with the development of science and the progress of the technique of scientific experiment. It was found that the properties of physical objects, which were the basis for creating measures that reproduce units of magnitude, do not have the degree of constancy and reproducibility required in science, technology and other fields of human activity. The second stage is characterized by the rejection of the units of quantities reproduced by nature, and their consolidation in "real" samples. The most characteristic for the transition from the first stage to the second is the history of the creation of metric measures. It began with precise measurements of a "natural" unit - the length of the Earth's meridian - and ended with the creation of a real standard of a unit of length - a meter.
The third stage in the development of units of physical quantities was the result of the rapid development of science and increased requirements for measurement accuracy. It turned out that real (objective) standards of units of physical quantities made by man cannot ensure the storage and transmission of these units with the accuracy that has become necessary. The discovery of new physical phenomena, the emergence and development of atomic and nuclear physics made it possible to find ways to more accurately reproduce the units of physical quantities. However, the third stage is not a return to the principles of the first stage. The difference between the third stage and the first is the detachment of units of physical quantities from the measure, from the quantitative characteristics of the properties of physical objects that serve to reproduce them. The units of measurement remained overwhelmingly the same as they were established in the second stage. A typical example is the unit of length. The discovery of the possibility of reproducing length using the wavelength of monochromatic light did not change the unit of length, the meter. The meter remained a meter, but the use of the wavelength of light made it possible to increase the accuracy of its reproduction by one decimal place.
However, now even such a definition of the meter does not allow reproducing the meter with sufficient accuracy to solve certain problems. Therefore, at the XVII General Conference of Weights and Measures (1983), a new definition of the meter was adopted, allowing the reproduction of the latter with greater accuracy.
The prospect for the development of metrology in terms of units of physical quantities is a further increase in the accuracy of reproduction of existing ones. The need to establish new units may arise when fundamentally new physical objects are discovered.
Initially, the units of physical quantities were chosen arbitrarily, without any connection with each other, which created great difficulties. A significant number of arbitrary units of the same quantity made it difficult to compare the results of measurements made by different observers. In each country, and sometimes in each city, their own units were created. Converting one unit to another was very difficult and led to a significant decrease in accuracy.
In addition to the specified variety of units, which can be called "territorial", there was a variety of units used in various areas of human activity. Within the same industry, different units of the same size were also used.
With the development of technology, as well as international relations, the difficulties in using and comparing measurement results due to differences in units increased and hampered further scientific and technological progress. For example, in the second half of the XVIII century. in Europe there were up to a hundred feet of various lengths, about fifty different miles, over 120 different pounds. In addition, the situation was further complicated by the fact that the ratio between submultiples and multiples was unusually diverse. For example, 1 foot = = 12 inches = 304.8 mm.
In 1790, France decided to create a system of new measures "based on an unchanging prototype taken from nature, so that all nations can accept it." It was proposed to consider the length of the ten-millionth part of a quarter of the meridian of the Earth passing through Paris as a unit of length. This unit is called the meter. To determine the size of the meter from 1792 to 1799, measurements were taken of the arc of the Parisian meridian. The mass of 0.001 m 3 of pure water at the temperature of the highest density (+4 °C) was taken as a mass unit; this unit was called the kilogram. With the introduction of the metric system, not only was the basic unit of length taken from nature established, but also the decimal system for the formation of multiples and submultiples was adopted, corresponding to the decimal system of numerical counting. Decimality of the metric system is one of its most important advantages.
However, as subsequent measurements showed, a quarter of the Parisian meridian contains not 10,000,000, but 10,000,856 of the originally determined meters. But even this number cannot be considered final, since even more accurate measurements give a different value. In 1872, the International Commission on Prototypes decided to move from units of length and mass based on natural standards to units based on conventional material standards (prototypes).
In 1875, a diplomatic conference was convened, at which 17 states signed the Meter Convention. According to this convention:
International prototypes of the meter and kilogram were being installed;
the International Bureau of Weights and Measures was created - a scientific institution, the funds for the maintenance of which were obliged to allocate the states that signed the convention;
an International Committee for Weights and Measures was established, consisting of scientists from different countries, one of whose functions was to manage the activities of the International Bureau of Weights and Measures;
It was established that the General Conference on Weights and Measures should be convened once every six years.
Samples of the meter and kilogram were made from an alloy of platinum and iridium. The prototype of the meter was a platinum-iridium line measure with a total length of 102 cm, at a distance of 1 cm from the ends of which strokes were applied that determined the unit of length - the meter.
In 1889, the First General Conference on Weights and Measures met in Paris, which approved international prototypes from among the newly made samples. The prototypes of the meter and kilogram were deposited with the International Bureau of Weights and Measures. The remaining samples of the meter and kilogram were distributed by the General Conference by lot among the states that signed the Metric Convention. Thus, in 1899, the establishment of metric measures was completed.
2.3 Principles of formation of a system of units of physical quantities
For the first time, the concept of a system of units of physical quantities was introduced by the German scientist K. Gauss. According to his method, when forming a system of units, several quantities are first set or chosen arbitrarily, independent of each other. The units of these quantities are called main , because they are the foundation of the system. The basic units are set in such a way that, using the mathematical relationship between quantities, it would be possible to form units of other quantities. Units expressed in terms of base units are called derivatives . The complete set of basic and derived units established in this way is the system of units of physical quantities.
We can distinguish the following features of the described method for constructing a system of units of physical quantities.
First, the method of constructing the system is not related to the specific dimensions of the basic units. For example, as one of the basic units, we can
choose a unit of length, but it does not matter which one. It can be either a meter, or an inch, or a foot. But the derived unit will depend on the choice of the base unit. For example, a derived unit of area would be a square meter, or a square inch, or a square foot.
Secondly, in principle, the construction of a system of units is possible for any quantities between which there is a relationship, expressed in mathematical form as an equation.
Thirdly, the choice of quantities, the units of which should become basic, is limited by considerations of rationality, and first of all by the fact that the optimal choice is the minimum number of basic units that would allow the formation of the maximum number of derived units.
Fourthly, they strive for the system of units to be coherent. The derived unit [z] can be expressed in terms of the basic [L], [M], [T], … using the equation
where K is the coefficient of proportionality.
coherence (consistency) of the system of units lies in the fact that in all formulas that determine derived units depending on the main ones, the proportionality coefficient is equal to one. This provides a number of significant advantages, simplifies the formation of units of various quantities, as well as performing calculations with them.
2.4 Systems of units of physical quantities. International system of units SI
Initially, systems of units based on three units were created. These systems covered a wide range of quantities conventionally called mechanical. They were built on the basis of those units of physical quantities that were accepted in one country or another. Of all these systems, preference can be given to systems built on units of length - mass - time as the main ones. One of the systems built according to this scheme for metric units is the meter - kilogram - second (MKS) system. In physics, it was convenient to use the centimeter - gram - second (CGS) system. The MKS and SGS systems are coherent in terms of units of mechanical quantities. Serious difficulties were encountered in the application of these systems for measuring electrical and magnetic quantities.
For some time, the so-called technical system of units was used, built according to the scheme length - force - time. When using metric units, the basic units of this system were the meter - kilogram-force - second (MKGSS). The convenience of this system was that the use of the unit of force as one of the main ones simplified the calculations and derivations of dependencies for many quantities used in technology. Its disadvantage was that the unit of mass in it turned out to be numerically equal to 9.81 kg, and this violates the metric principle of decimal measures. The second drawback is the similarity of the name of the unit of force - kilogram-force and the metric unit of mass - kilogram, which often leads to confusion. The third disadvantage of the MKGSS system is its inconsistency with practical electrical units.
Since the systems of mechanical units did not cover all physical quantities, for certain branches of science and technology, the systems of units were expanded by adding one more basic unit. This is how the system of thermal units meter - kilogram - second - degree temperature scale (MKSG) appeared. The system of units for electrical and magnetic measurements is obtained by adding the unit of current strength - ampere (MKSA). The system of light units contains, as the fourth basic unit, the unit of luminous intensity - the candela.
The presence of a number of systems of units of measurement of physical quantities and big number non-system units, the inconvenience that arises in practice in connection with recalculations during the transition from one system to another, necessitated the creation of a single universal system of units that would cover all branches of science and technology and would be accepted on an international scale.
In 1948, at the IX General Conference on Weights and Measures, proposals were made to adopt a unified practical system of units. The International Committee for Weights and Measures carried out an official survey of the opinions of scientific, technical and pedagogical circles in all countries, and on the basis of the answers received, recommendations were drawn up for establishing a unified practical system of units. X General Conference (1954) adopted as basic units new system the following: length - meter; mass - kilogram; time - second; current strength - ampere; thermodynamic temperature - kelvin; the power of light is the candela. Subsequently, the seventh basic unit was adopted - the amount of substance - the mole. After the conference, a list of derived units of the new system was prepared. In 1960, the XI General Conference on Weights and Measures finally adopted the new system, giving it the name International System of Units (System International) with the abbreviation "SI", in Russian transcription "SI".
The adoption of the International System of Units served as an incentive for the transition to metric units of a number of countries that retained national units (England, USA, Canada, etc.). In 1963, GOST 98567-61 "International System of Units" was introduced in the USSR, according to which the SI was recognized as preferable. Along with this, eight state standards for units were in force in the USSR. In 1981, GOST 8.417-81 "GSI. Units of physical quantities" was put into effect, covering all branches of science and technology and based on the International System of Units.
SI is the most perfect and universal of all that has existed so far. The need for a unified International System of Units is so great, and its advantages are so convincing, that this system has received wide international recognition and distribution in a short time. The International Organization for Standardization (ISO) has adopted the International System of Units in its recommendations on units. The United Nations Educational, Scientific and Cultural Organization (UNESCO) called on all member countries of the organization to adopt the International System of Units. The International Organization of Legal Metrology (OIML) recommended that the member states of the organization introduce the International System of Units by law and calibrate measuring instruments in SI units. SI was included in the recommendations on units of the International Union of Pure and Applied Physics, the International Electrotechnical Commission and other international organizations.
2.5 Basic, additional and derived units
The SI base units have the following definitions.
The unit of length is the meter (m) - the length of the path traveled by light in vacuum in 1/299792458 of a second.
The unit of mass is the kilogram (kg) - the mass equal to the mass of the international prototype of the kilogram.
The unit of time is a second (s) - the time equal to 9192631770 periods of radiation corresponding to the transition between two hyperfine levels of the ground state of the cesium-133 atom.
The unit of electric current strength - ampere (A) - is the strength of an unchanging current, which, when passing through two parallel conductors of infinite length and negligible circular cross section, located at a distance of 1 m from one another in a vacuum, would cause a force between these conductors equal to 2- 10" 7 N per meter of length.
The unit of thermodynamic temperature is the kelvin (K) - 1/273.16 of the thermodynamic temperature of the triple point of water. The International Committee for Weights and Measures allowed the expression of thermodynamic temperature in degrees Celsius: t \u003d T-273.15 K, where t is the temperature of Celsius; T is the Kelvin temperature.
The unit of luminous intensity - candela (cd) - is equal to the luminous intensity in a given direction of a source emitting monochromatic radiation with a frequency of 540-10 12 Hz, the energy intensity of which in this direction is 1/683 W / sr.
The unit of the amount of a substance is a mole - the amount of a substance of a system containing as many structural elements as there are atoms in a 12C nuclide with a mass of 0.012 kg.
The SI includes two additional units for planar and solid angles, necessary to form derived units associated with angular quantities. Angular units cannot be included in the number of basic units; at the same time, they cannot be considered derivatives, since they do not depend on the size of the basic units.
The unit of a plane angle is a radian (rad) - the angle between two radii of a circle, the length of the arc between which is equal to the radius. In degrees, a radian is 57° 17" 44.8".
The unit of the solid angle - steradian (sr) - is equal to the solid angle with the vertex in the center of the sphere, cutting out on the surface of the sphere an area equal to the area of a square with a side equal to the radius of the sphere.
Derived SI units are formed on the basis of laws that establish a relationship between physical quantities or on the basis of definitions of physical quantities. The corresponding derived SI units are derived from the equations of connection between quantities (defining equations) expressing a given physical law or definition, if all other quantities are expressed in SI units.
More detailed information about SI derived units is given in the works.
2.6 Dimension of physical quantities
The dimension of the derived SI unit of the physical quantity z is generally determined from the expression
 ,
(2.5)
,
(2.5)
where L, M, T, I, θ, N, J are the dimensions of physical quantities, the units of which are taken as the main ones;
α, β, γ, ε, η, μ, λ - exponents of the degree to which the corresponding value is included in the equation that determines the derivative value z.
Expression (2.5) determines the dimension of the physical quantity z, it reflects the relationship between the quantity z and the basic quantities of the system, in which the proportionality coefficient is taken equal to 1.
Let us give examples of the dimension of derived units in relation to SI units:
for unit area ;
for the unit of speed ;
for the unit of acceleration ;
for a unit of power ;
for the heat capacity unit ;
for the heat capacity unit ;
for the unit of illumination.
Dimensions determine the relationships between physical quantities, but they do not yet determine the nature of the quantities. One can find a number of quantities, the dimensions of the derived units of which coincide, although by their nature these quantities are different. For example, the dimensions of work (energy) and moment of force are the same and equal to L 2 M T 2 .
2.7 Multiples and submultiples
The sizes of metric units, including SI units, are inconvenient for many practical cases: they are either too large or very small. Therefore, they use multiples and submultiples, i.e. units, an integer number of times greater or less than the unit of the given system. Decimal multiples and submultiples are widely used, which are obtained by multiplying the original units by the number 10 raised to a power. To form the names of decimal multiples and submultiples, use the appropriate prefixes. In table. 2.1 is a list of currently used decimal factors and their corresponding prefixes. The designation of the prefix is written together with the designation of the unit to which it is attached. Moreover, prefixes can only be attached to simple unit names that do not contain prefixes. Connection of two or more consoles in a row is not allowed. For example, the name "micromicrofarad" cannot be used, but the name "picofarad" must be used.
When forming the name of a decimal multiple or submultiple of a unit of mass - kilogram, a new prefix is attached to the name "gram" (megagram 1 Mg \u003d 10 3 kg \u003d 10 6 kg, milligram 1 mg \u003d 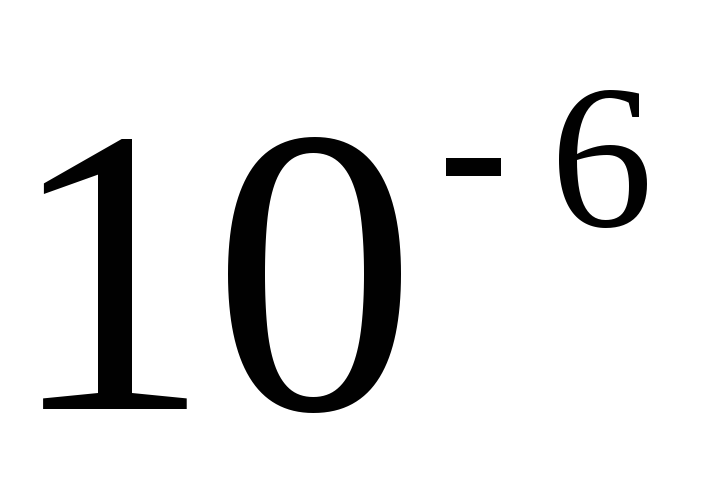 kg==
kg== 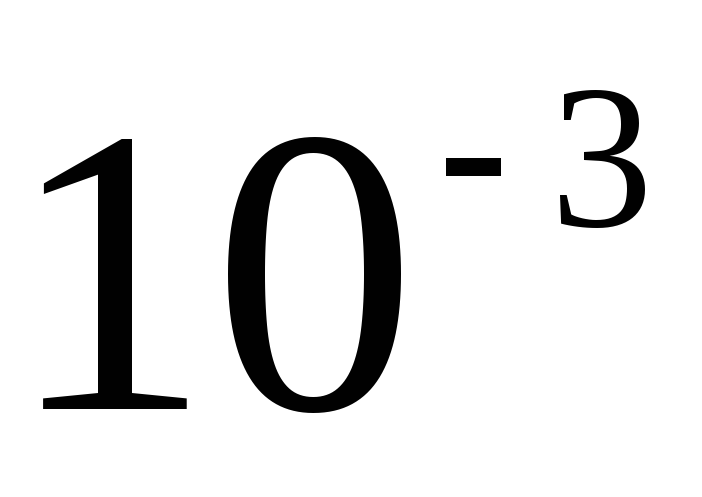 G).
G).
In multiples and submultiples of area and volume, as well as other quantities formed by exponentiation, the exponent refers to the entire unit, taken together with the prefix, for example: 1 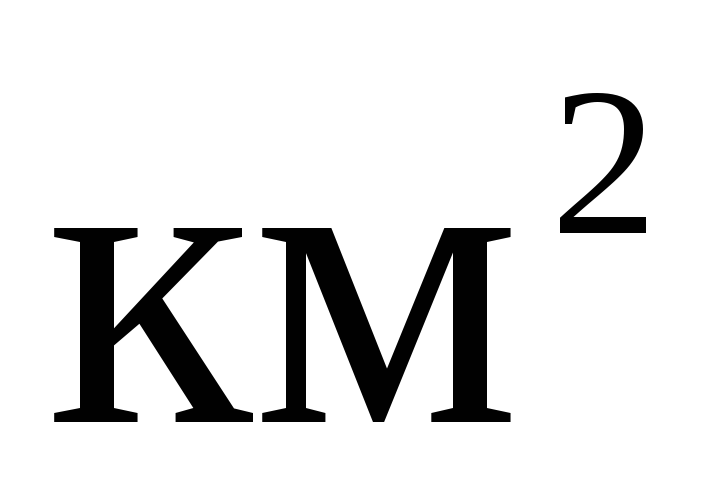 =
=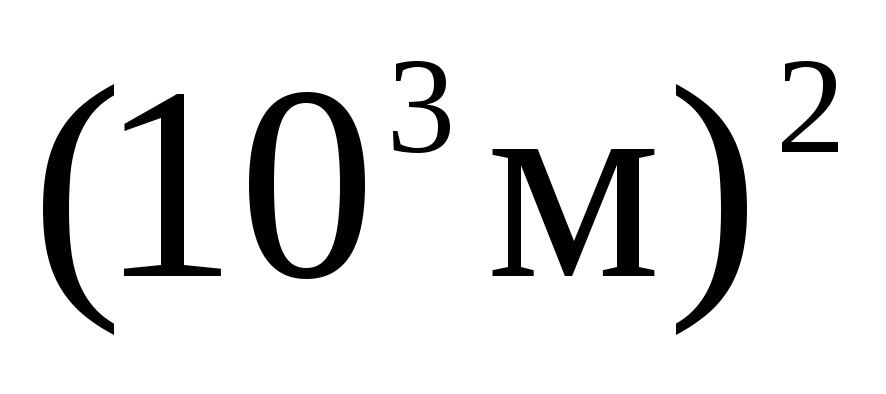 =
=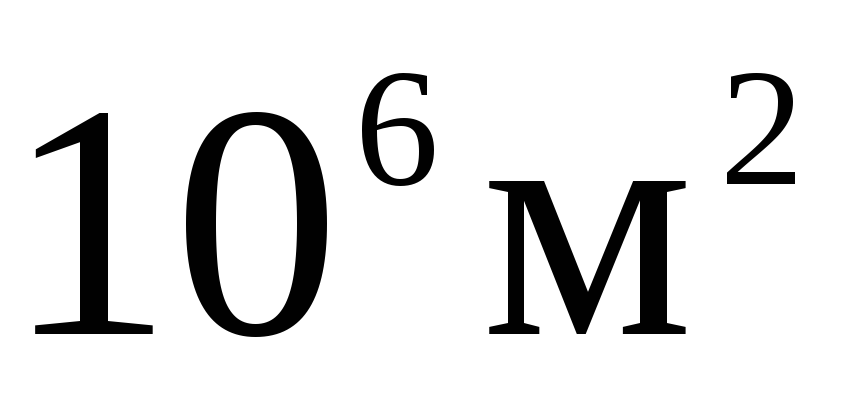 ;
;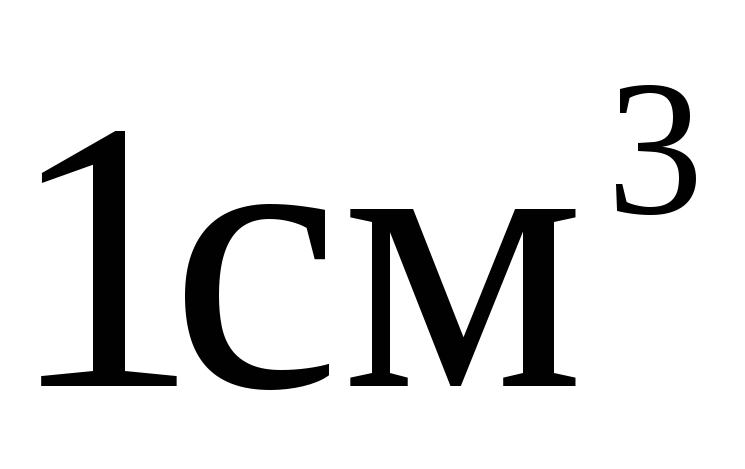 =
=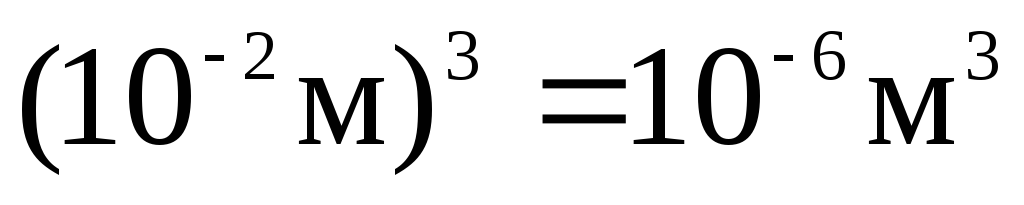 . It is incorrect to attribute the prefix to the original unit raised to a power.
. It is incorrect to attribute the prefix to the original unit raised to a power.
Decimal multiples and submultiples, whose names are formed using prefixes, are not included in the coherent system of units. Their application in relation to the system should be considered as a rational way of representing small and large numerical values. When substituting into the formula, the prefixes are replaced by their corresponding multipliers. For example, the value of 1 pF (1 picofarad) when substituting into the formula is written 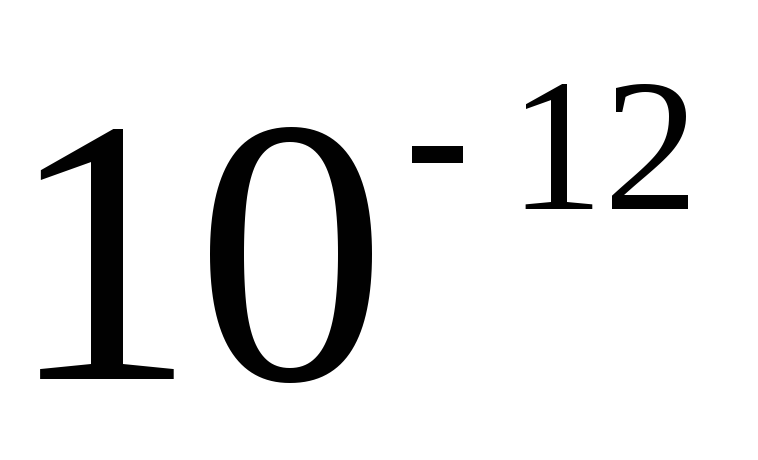 F.
F.
Table 2.1
|
Factor |
Console |
||
|
Name |
Designation |
||
|
international |
|||
|
1 000 000 000 000 000 000= 1 000 000 000 000 000= 1 000 000 000 000= 1 000 000 000= 1 000 000= 1 000= 100= 10= 0,1= 0,01= 0,001= 0,000
001= 0,000
000 001= 0,000
000 000 001= 0,000
000 000 000 001= 0,000
000 000 000 000 001= |
exa peta tera giga mega kilo hecto deca deci santi micro nano pico femto atto | ||
The prefixes deca, hecto, deci and centi are used relatively rarely, since in most cases they do not create noticeable advantages. So, the use of a unit of hectowatt when taking into account the power of electrical devices was abandoned, since it is more convenient to keep records in kilowatts, but in some cases these prefixes are very firmly rooted, for example, centimeter, hectare. The unit ar (100 m 2) is practically not used, and the hectare has found wide application everywhere. He successfully replaced the Russian tithe: 1 ha \u003d \u003d 0.9158 tithes.
When choosing prefixes to the name of a particular unit, a certain moderation should be observed. For example, the names decameter and hectometer have not been used, and only the kilometer is widely used. But further, the use of prefixes to the name of units that are multiples of a meter did not enter into practice: neither a megameter, nor a gigameter, nor a terameter are used.
The choice of a decimal multiple or submultiple of the SI unit is dictated primarily by the convenience of its use. From the variety of multiples and submultiples that can be formed with the help of prefixes, a unit is chosen, leading to numerical values of the quantity that are acceptable in practice. In most cases, multiples and submultiples are chosen so that the numerical values of the quantity are in the range from 0.1 to 1000.
Some submultiple and multiple units received special names at one time, which have survived to this day. For example, as units that are multiples of a second, not decimal multiples, but historically established units are used: 1 min \u003d 60 s; 1 h = 60 min = 3600 s; 1 day = 24 h = 86400 s; 1 week = 7 days = 604800 s. To form submultiple seconds, decimal coefficients are used with the appropriate prefixes to the name: millisecond (ms), microsecond (μs), nanosecond (not).
2.8 Relative and logarithmic quantities and
Relative and logarithmic quantities and their units are widely used in science and technology, which characterize the composition and properties of materials, the ratio of energy and force quantities, etc. Such characteristics are, for example, relative elongation, relative density, relative dielectric and magnetic permeability, amplification and weakening of capacities, etc.
Relative value
is a dimensionless ratio of a physical quantity to the physical quantity of the same name taken as the initial one. The relative quantities also include the relative atomic or molecular masses of chemical elements, expressed in relation to one twelfth (1/12) of the mass of carbon - 2. Relative quantities can be expressed either in dimensionless units (when the ratio of two quantities of the same name is 1), or in percentage (when the ratio is 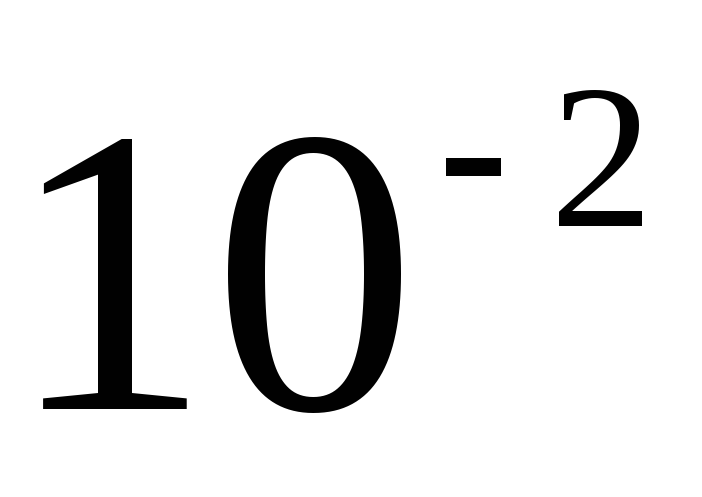 ), or in ppm (the ratio is
), or in ppm (the ratio is 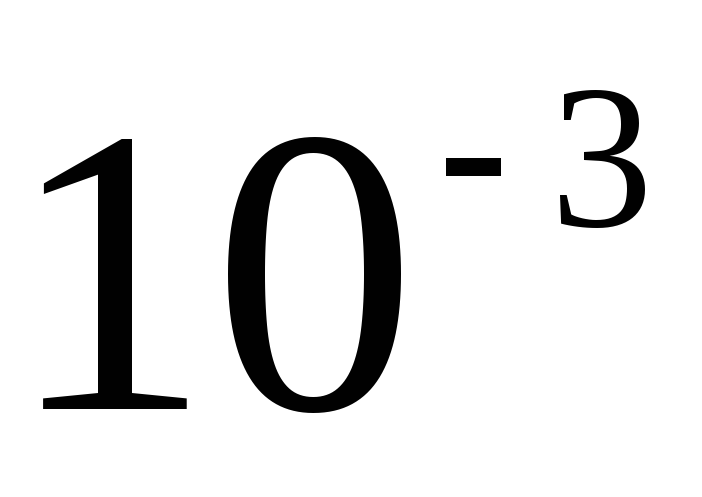 ), or in parts per million (the ratio is
), or in parts per million (the ratio is 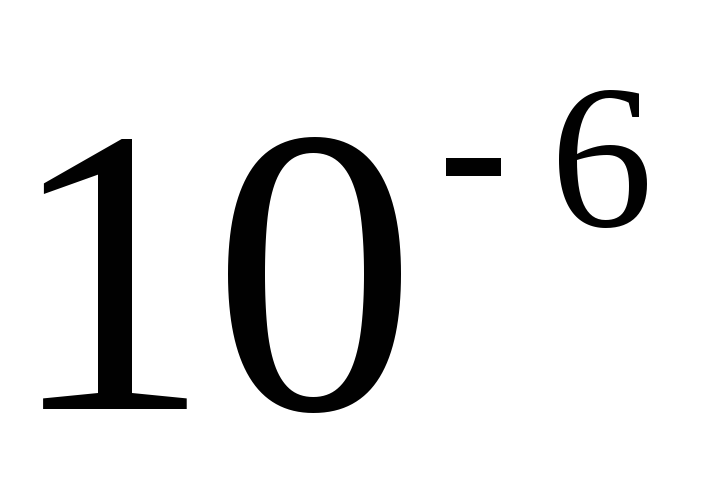 ).
).
log value
is the logarithm (decimal, natural or base 2) of the dimensionless ratio of two physical quantities of the same name. Sound pressure levels, gain, attenuation, frequency interval, etc. are expressed as logarithmic values. The unit of the logarithmic value is bel (B), defined by the following relation: 1 B = lg (P2/Pl) at P2=10 P1, where PI, P2 are energy quantities of the same name (power, energy, energy density, etc.) . If a logarithmic value is taken for the ratio of two "power" quantities of the same name (voltage, current, pressure, field strength, etc.), bel is determined by the formula 1 B = 2 lg (F2 / Fl) at F2 = 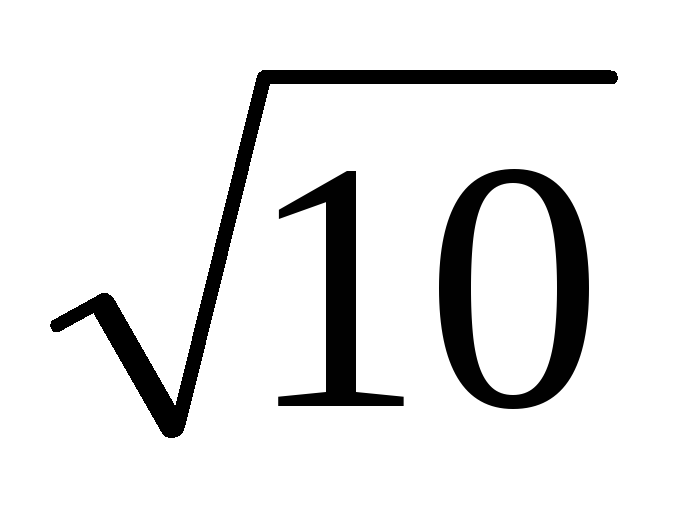 F1. The submultiple of the bela is the decibel (dB), which is equal to 0.1 B.
F1. The submultiple of the bela is the decibel (dB), which is equal to 0.1 B.
For example, in the case of an electrical power amplification characteristic with a ratio of the received power P2 to the original equal to 10, the gain will be 1 B or 10 dB, with a power change of 1000 - 3 B or 30 dB.
2.9 Units of physical quantities of the CGS system
The CGS system still retains its independent significance in theoretical physics. One basic unit of this system, the second, is the same as the basic SI unit of time, and the other two basic CGS units, the centimeter and the gram, are sub-multiples of the SI units. However, it is impossible to consider the CGS system as some kind of derivative or part of the International system. First, the proportions of the basic units are not the same (0.01; 0.001; 1). Secondly, in the formation of CGS units for electrical and magnetic quantities, as a rule, the equations of electromagnetism are used in an unrationalized form. In this regard, the sizes of the units have changed, and in cases where the CGS units had special names, the names have also changed. So, the unit of the CGS magnetomotive force - Gilbert - in SI units is 10/(4  )ampere, and the unit of magnetic field strength CGS - erstad - in SI units is 10 3 /(4
)ampere, and the unit of magnetic field strength CGS - erstad - in SI units is 10 3 /(4  ) ampere per meter.
) ampere per meter.
Some other CGS units have special names, but they are decimal fractions of SI units and therefore the transition from units of one system to units of another is not difficult. These GHS units include those shown in Table 2.2. Many GHS units do not have specific names. The most commonly used CGS units are given in the works.
Table 2.2
|
Value |
Name of SI unit |
Unit name |
Value in SI units |
|
work, energy Dynamic viscosity Kinematic viscosity magnetic flux Magnetic induction |
square meter per second |
Maxwell |
|
2.10 Non-systemic units
off-system they name those units of physical quantities that are not included in the system of units used in each particular case, either as basic or as derivatives. Non-systemic units, to one degree or another, are always some kind of obstacle to the introduction of a system of units. When carrying out calculations according to theoretical formulas, it is necessary to bring all non-systemic units to the corresponding units of the system. In some cases, this is not difficult, as, for example, with decimal multiplicity or fractions. In other cases, the conversion of units is complex and painstaking and often a source of errors. In addition, individual off-system units are very convenient in size for certain branches of science, technology, or for everyday use, and the rejection of them is associated with a number of inconveniences. Examples of such units can be: for length - astronomical unit, light year, parsec; for mass, the atomic mass unit; for the area - bari; for strength - dyna; for work - erg; for magnetic flux - maxwell; for magnetic induction - gauss.
2.11 Names and designations of units
In the names of units, several types can be distinguished. First of all, these are names that, to one degree or another, succinctly reflect the physical essence of a quantity. These names include: meter (measure), candela (candle), dyna (power), calorie (from the word heat), etc. It should be recognized that such names are the most convenient. Next come the names of derived units formed in strict accordance with physical laws. For example, joule per kilogram-kelvin [J/(kg K)] is a unit
specific heat capacity; kilogram-meter squared per second (kg m 2 / s) - unit of angular momentum, etc.
The cumbersomeness of naming derived units, and in some cases the difficulty of finding a unit name that reflects the physical essence of the quantity, led to the assignment of short and easy-to-pronounce names for many units. It was decided to assign names to such units after the names of prominent scientists. As examples, one can point to such names as kelvin, ampere, volt, watt, hertz, etc.
The names of some units are associated with the graduation of the scale. These units include: temperature degree, angular degree (minute, second), millimeter of mercury column, millimeter of water column.
The names of some units are abbreviations, i.e. initial abbreviations. For example, the unit of reactive power is called "var" from the first letters of the words "volt-ampere reactive". The unit of equivalent dose of radiation is called "rem" from the first letters of the words "biological equivalent of rad".
When designating, writing these designations and reading them, the following rules are used.
In most cases, unit abbreviations are used to designate units after a numerical expression. These abbreviations consist of one, two or three of the first letters of the unit name. The designations of derived units that do not have a special name are compiled from the designations of other units according to the formula for their formation (not necessarily from the designations of basic units).
The abbreviated designation of units, the name of which is formed by the name of the scientist, is written with a capital letter. For example: ampere - A; newton -N; pendant - Cl; joule - J, etc. In the notation of units, the dot as a sign of abbreviation is not used, except for the cases of abbreviation of words that are included in the name of the unit, but are not the names of the units themselves, for example, mm Hg. (millimeter of mercury).
In the presence of decimal fraction in the numerical value of the value, the designation of the unit should be placed after all figures, for example: 53.24 m; 8.5 s; -17.6 °C.
When specifying values of quantities with limit deviations, the numerical value with limit deviations should be enclosed in brackets and the unit designation should be placed after the brackets or the unit designation should be put down after the numerical value of the quantity and after its limit deviations, for example: (25 ± 10) ° С or 25 ° С ± 10 °С; (120±5) s or 120 s ± 5 s.
In calculations, when the equal sign is repeated, the designation of the unit is given only in the final result, for example:
 .
.
When writing the designations of derived units, the designations of the units included in the product are separated by dots on the middle line as multiplication signs, for example: N m (newton meter); N s / m 2 (newton second per square meter). To indicate the operation of dividing one unit by another, as a rule, a slash is used, for example: m / s. A horizontal line is allowed (for example, 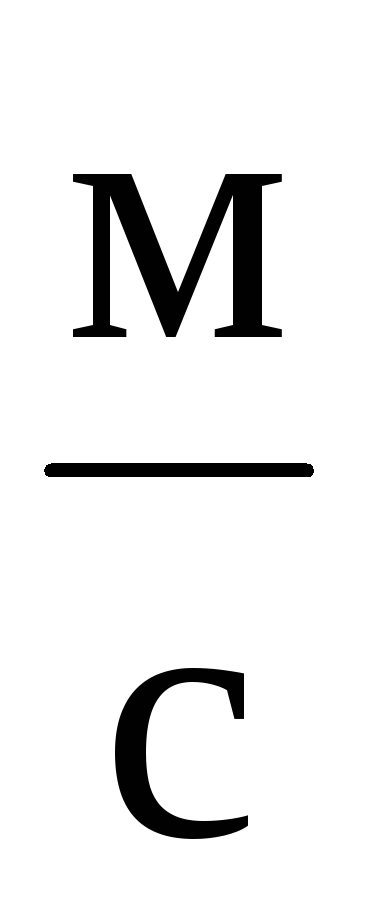 ) or representing a unit as a product of unit symbols raised to positive or negative powers (for example,
) or representing a unit as a product of unit symbols raised to positive or negative powers (for example, 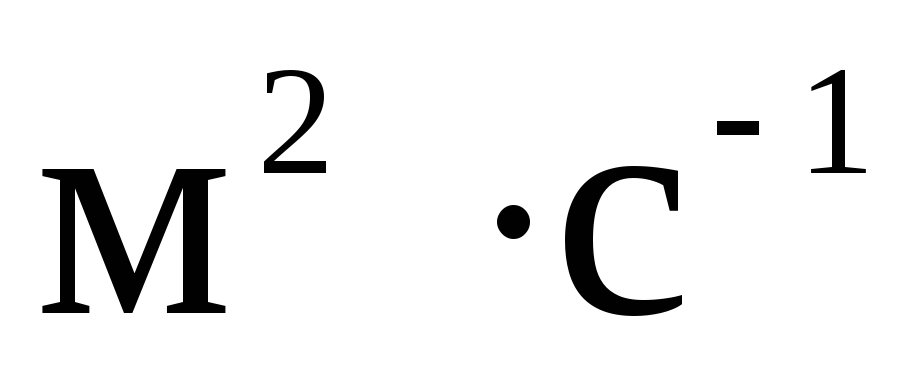 ). When using a slash, the product of units in the denominator should be enclosed in brackets, for example: W / (m K).
). When using a slash, the product of units in the denominator should be enclosed in brackets, for example: W / (m K).
It is not allowed to use more than one slash or horizontal bar in the designation of the derived unit: for example, the unit of heat transfer coefficient - watt per square meter-kelvin - should be denoted W / ( 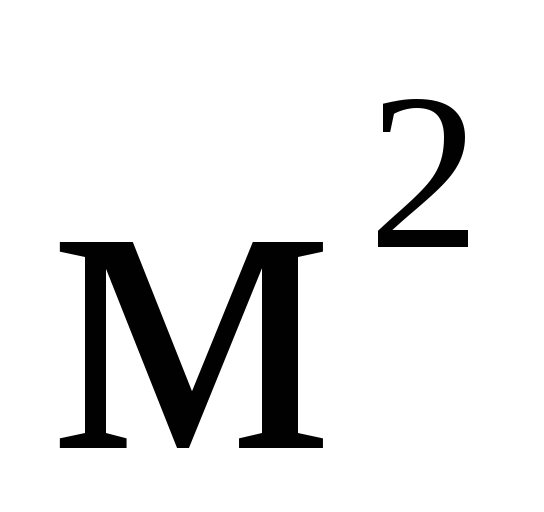 ·TO),
·TO), 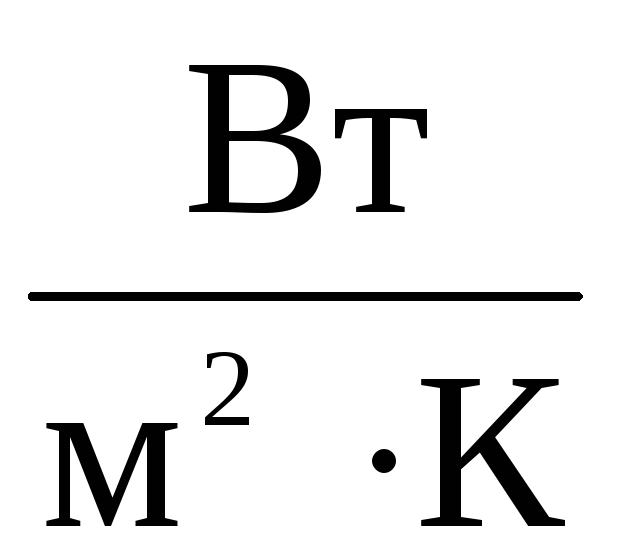 or
or 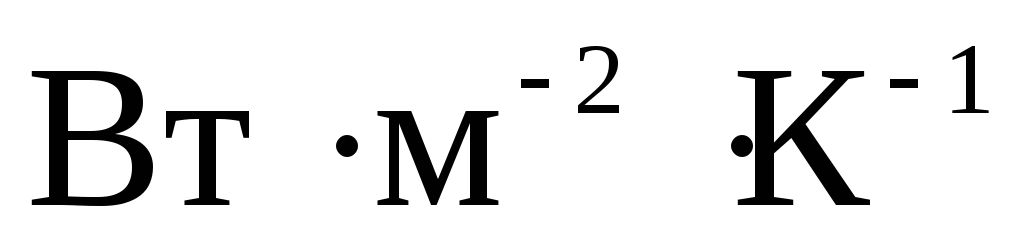 .
.
The designations of units for cases and numbers do not change, with the exception of the designation "light year", which in the genitive plural takes the form "light years".
When the name corresponds to the product of units, the prefix is attached to the name of the first unit included in the product.
For example, 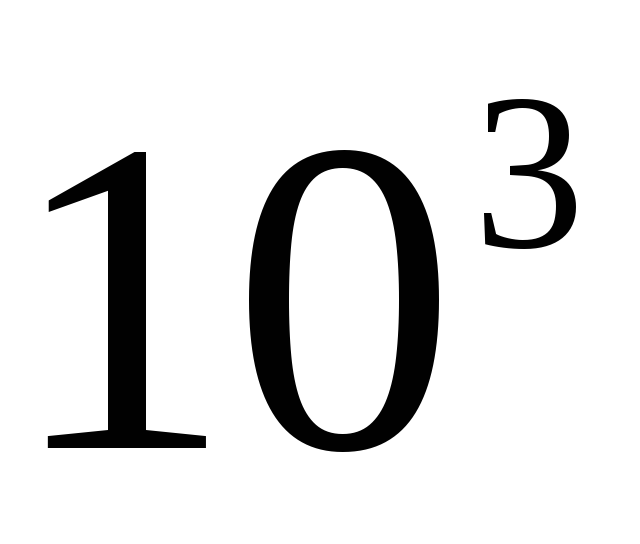 Nm should be referred to as kilonewton meter (kNm), not newton kilometer (Nkm).
Nm should be referred to as kilonewton meter (kNm), not newton kilometer (Nkm).
When the name corresponds to the ratio of units, the prefix is also attached to the name of the first unit included in the numerator. An exception to this rule is the basic SI unit - the kilogram, which can be included in the denominator without restriction.
In the names of units of area and volume, the adjectives "square" and "cubic" are used, for example, square meter, cubic centimeter. If the second or third degree of length does not represent an area or volume, then in the name of the unit, instead of the words "square" or "cubic", the expressions "squared", "third power", etc., should be used, for example, the unit of moment momentum - kilogram-meter in
square per second (kg m 2 / s).
To form the name of multiple and submultiple units from the unit, which is the degree of some original unit, the prefix is attached to the name of the original unit. For example, square meter ( 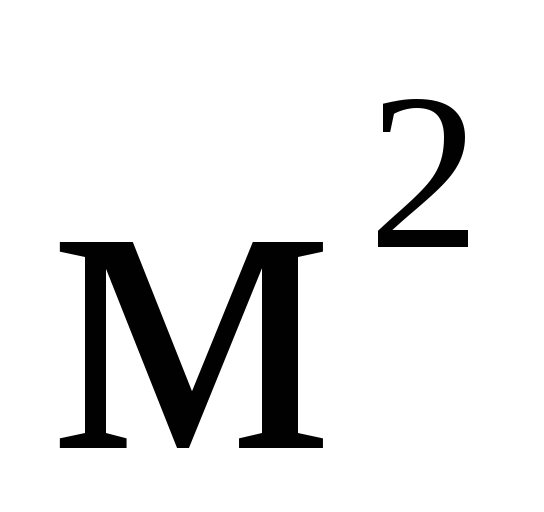 ), square kilometer (
), square kilometer ( 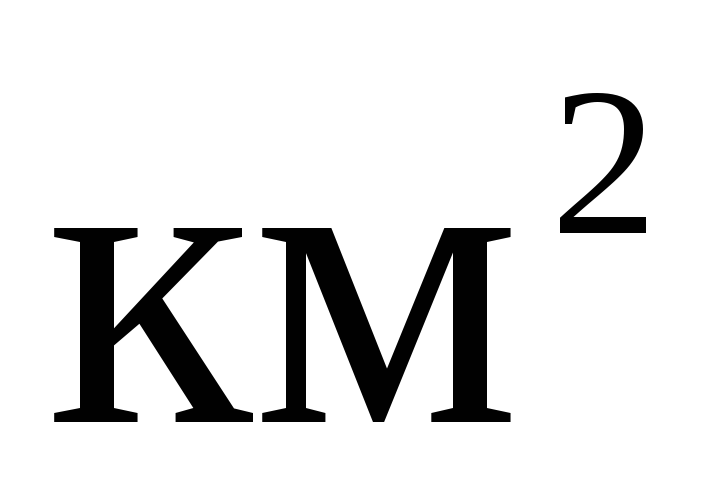 ) etc.
) etc.
In products of derived units formed as products of units, only the last name and the adjective "square" and "cubic" related to it are inflected. The names of the units in the denominator are written and read with the preposition "on", for example, a meter per second squared. The exception is units of quantities that depend on time to the first degree; in this case, the name of the unit in the denominator is written and read with the preposition "in", for example, a meter per second. When declining the names of units containing the denominator, only the part corresponding to the numerator changes.

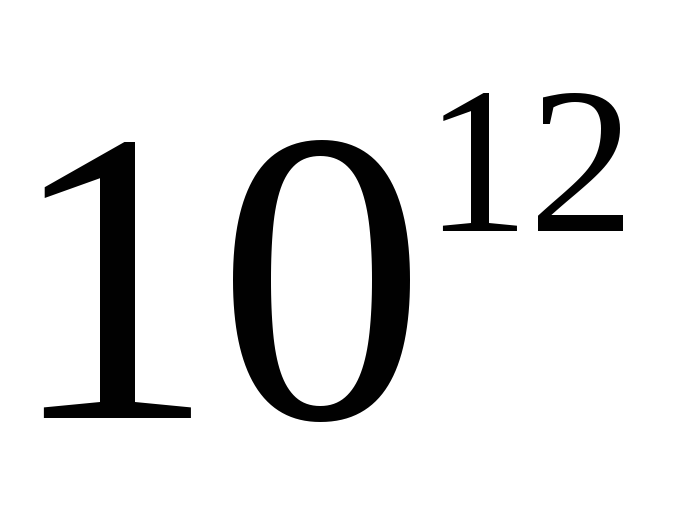
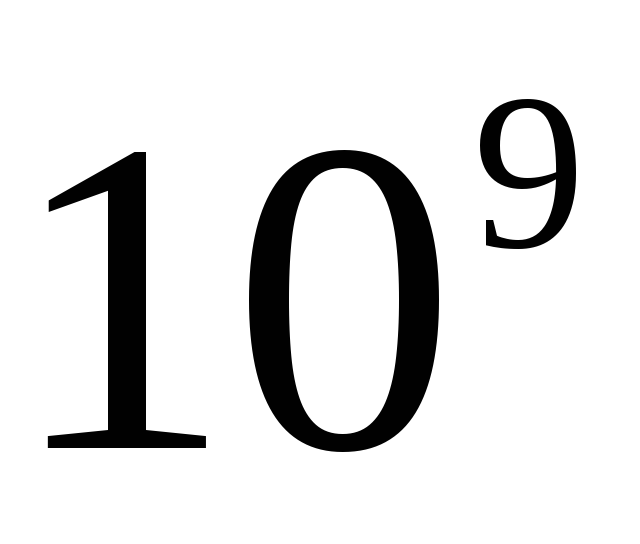
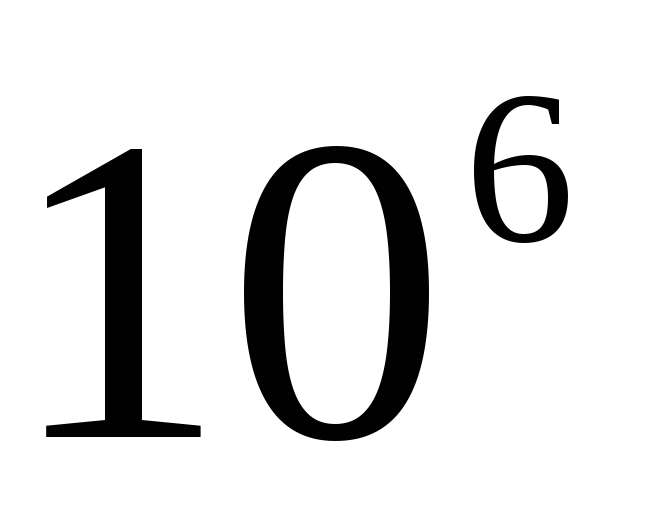
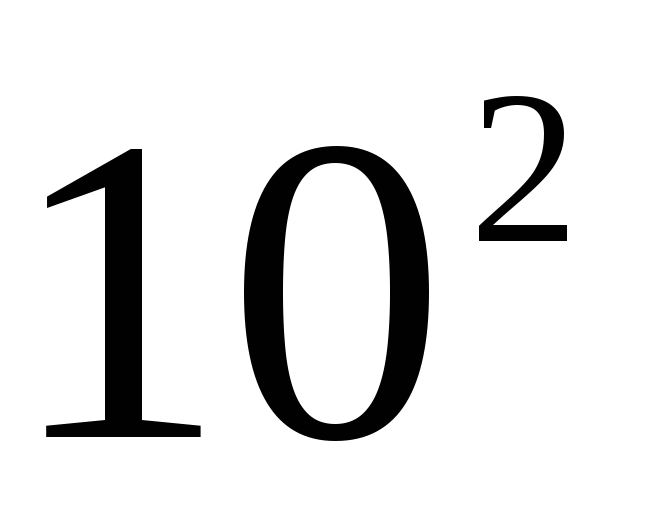
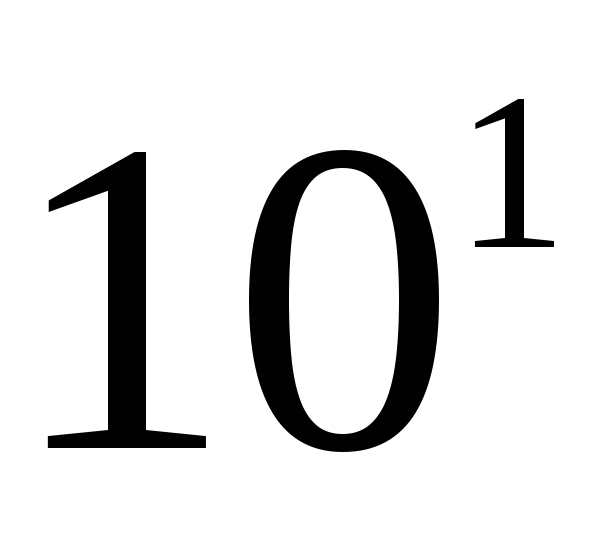
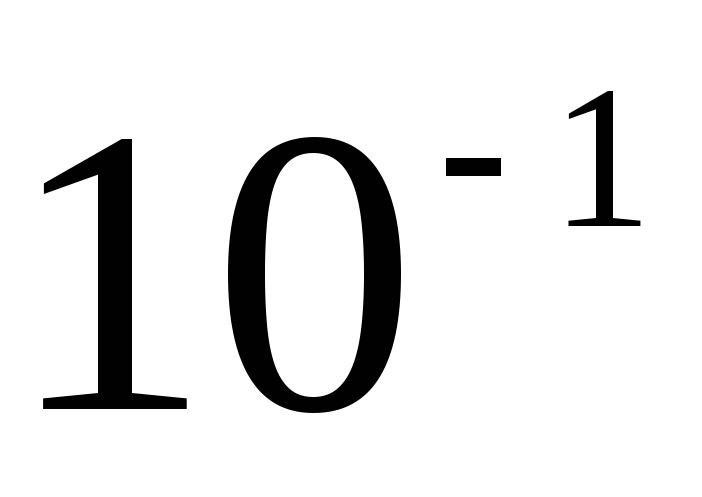
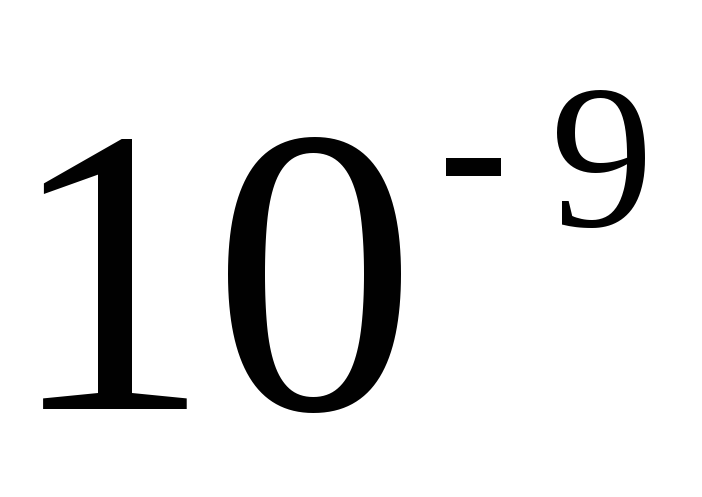
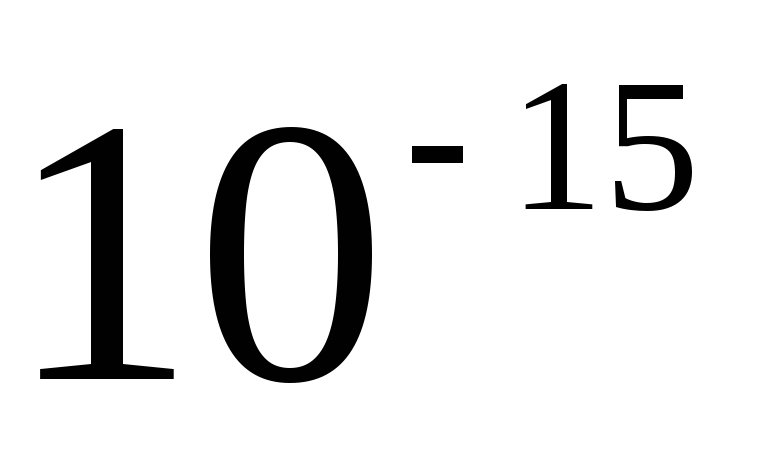
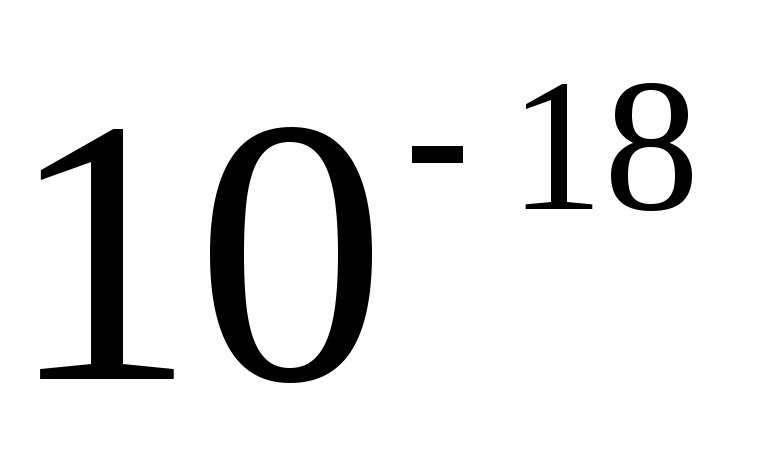
 H
H J
J
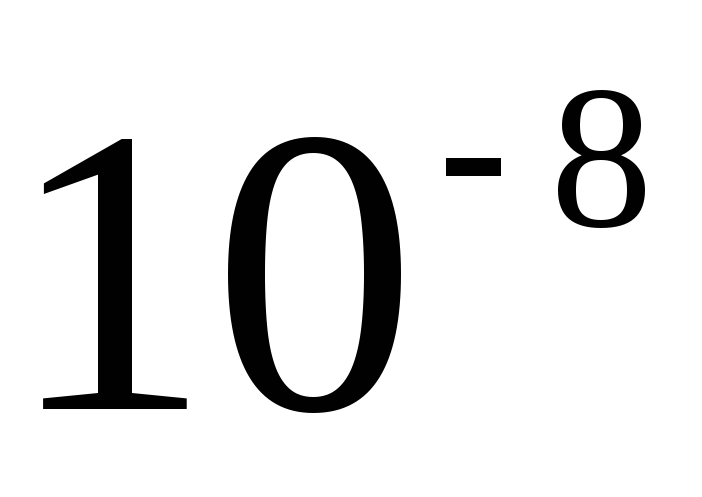 wb
wb




