Gas Laws Thermodynamics - page 10
Task 29. The heat-insulated vessel is divided by a vertical porous partition into two equal parts. At the initial moment, v = 2 mol of helium is in the left side of the vessel, and the same amount of argon mol is in the right side. Helium atoms can penetrate through the partition, but the partition is impenetrable for argon atoms. Initial helium temperature T1 = 900 K, starting temperature argon T2 = 300 K. How many times has the internal energy of the gas increased in the right side of the vessel as a result of the establishment of thermodynamic equilibrium?
Solution.
As a result of the establishment of thermodynamic equilibrium in the system, 1 mol of helium = 1 mol will be in each part of the vessel, and argon will remain entirely on the right side of the vessel. Therefore, on the right side of the vessel there will be 3 mol of the mixture: mol, - and on the left side of the vessel there will be = 1 mol of helium.
In a system with a constant number of particles, according to the first law of thermodynamics, . Since the vessel is thermally insulated (Q = 0) and the volume of the vessel and its parts does not change (A = 0), it follows from the first law that .
The internal energy of a monatomic ideal gas is proportional to the temperature and the number of moles: . Therefore, initially the internal energy of the gas on the left side of the vessel (i.e. helium), the internal energy of the gas on the right side of the vessel (i.e. argon), and after the establishment of thermodynamic equilibrium, the internal energy of the gas on the left side of the vessel (i.e. helium ) ![]() , and the internal energy of the gas on the right side of the vessel (i.e. a mixture of helium and argon)
, and the internal energy of the gas on the right side of the vessel (i.e. a mixture of helium and argon) ![]() , where T is the equilibrium temperature in the system.
, where T is the equilibrium temperature in the system.
Page 1
A heat-insulated vessel with an internal volume V was evacuated to a high vacuum. At some point, the tap opens and the vessel is rapidly filled with atmospheric air.
The thermally insulated vessel is divided into two parts by a heat-impervious partition A. In the partition Ive of one of the walls B there are a large number of small holes with a total area S in each. The vessel is filled with argon and placed in an argon atmosphere. External pressure p0 and temperature T0 are kept constant.
A thermally insulated vessel contains a mixture consisting of ice and water with a mass of m 2 kg and m2 10 kg, respectively, at a total temperature (j ° C. Water vapor is supplied to the vessel at a temperature tz 100 C.
The heat-insulated vessel is divided into two parts by a non-heat-conducting piston, which can move in the vessel without friction.
A thermally insulated vessel with an ideal gas is suspended on a thread in a gravitational field.
The heat-insulated vessel is divided by a heat-conducting partition into two chambers. What will be the ratio of gas pressures in the chambers after the heat exchange is over.
A thermally insulated vessel, divided by a partition with a small opening into two sections, is filled with a rarefied gas. The gas temperatures in the sections of the vessel are not the same and are kept constant. Equilibrium is established when the fluxes of molecules through the hole are equal.
The heat-insulated vessel is divided by a heat-impervious partition into two identical sections. How does the temperature of the gas change in both cases.
The heat-insulated vessel is divided into two equal parts - by a partition, in which there is a closing opening. One half of the vessel contains t - 10 0 g of hydrogen. The second half is evacuated to high vacuum. The hole in the partition is opened, and the gas fills the entire volume.
A thermally insulated vessel with a capacity of V22 4 l is divided into two equal parts by a thin, impenetrable, heat-conducting partition. Mi 11 2 g of nitrogen at 20 C is introduced into one half of the vessel, and 8 g of nitrogen at t2 l5 C into the second half. What pressures will be established in each part of the vessel after the temperature equalizes.
A heat-insulated vessel with an internal volume V was evacuated to a high vacuum. The ambient air has a tempera - TURU T0 and pressure pa. At some point, the tap opens and the vessel is rapidly filled with atmospheric air.
gas laws. Thermodynamics
Problem 21. The pT-diagram shows the cycle of a heat engine, in which the working fluid is ideal gas(see picture). In which part of the cycle is the work done by the gas the greatest in absolute value?
Key elements of the solution
2. It is obvious that the work A 2-3 is the largest in absolute value, since the area bounded by the isotherm 2-3 and the axial lines in this cycle is the largest.
Task 22.
The pT-diagram shows the cycle of a heat engine, in which the working fluid is an ideal gas (see figure). Find the gas work ratio modulus  in sections 3-4 and 1-2.
in sections 3-4 and 1-2.
Key elements of the solution
1. Since it is convenient to compare the work of the gas in the sections of the cycle on the pV diagram, represent this cycle on the pV diagram with the obligatory observance of the scale.
2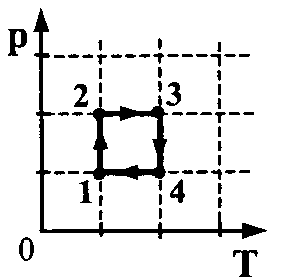 . Obviously, the modules of work in sections 3-4 and 1-2 are equal, which means that the ratio of the modules of work is 1, i.e. =1
. Obviously, the modules of work in sections 3-4 and 1-2 are equal, which means that the ratio of the modules of work is 1, i.e. =1
Task 23. The pT-diagram shows the cycle of a heat engine, in which the working fluid is an ideal gas (see figure). In what part of the cycle is the work done by the gas the smallest in absolute value?
Answer: the minimum modulo is the work A 1-2 .
W 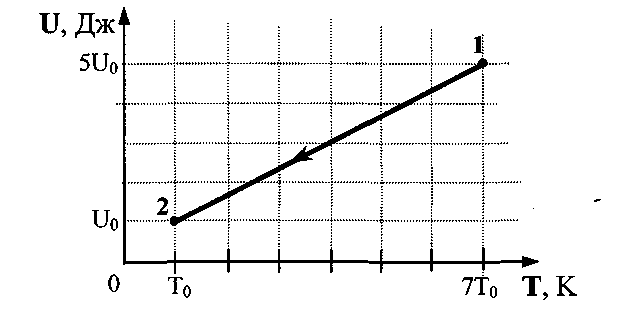 hell 24.Helium is contained in a hermetically sealed vessel. The gas has moved fromstate 1 to state 2 as shown in the dependency graphinternal energy of the gas on its temperature (see figure). Howchange the pressure exerted by the gas on the walls of the vessel? Answerjustify.
hell 24.Helium is contained in a hermetically sealed vessel. The gas has moved fromstate 1 to state 2 as shown in the dependency graphinternal energy of the gas on its temperature (see figure). Howchange the pressure exerted by the gas on the walls of the vessel? Answerjustify.
Key elements of the solution
1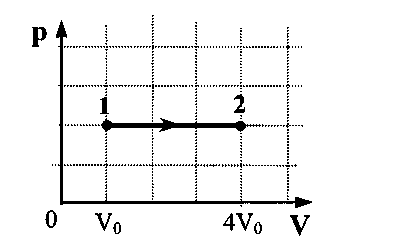 . Write the Clapeyron - Mendeleev equation for two states p 1 V 1 = νRT 1 and p 2 V 2 = νRT 2 . 2. Write down the expression for the internal energy of an ideal monatomic gas in two states: U 1 =νRT 1 ; U 2 \u003d νRT 2. 3. Perform mathematical transformations, get the answer in general form and the correct numerical answer:
. Write the Clapeyron - Mendeleev equation for two states p 1 V 1 = νRT 1 and p 2 V 2 = νRT 2 . 2. Write down the expression for the internal energy of an ideal monatomic gas in two states: U 1 =νRT 1 ; U 2 \u003d νRT 2. 3. Perform mathematical transformations, get the answer in general form and the correct numerical answer:  =
=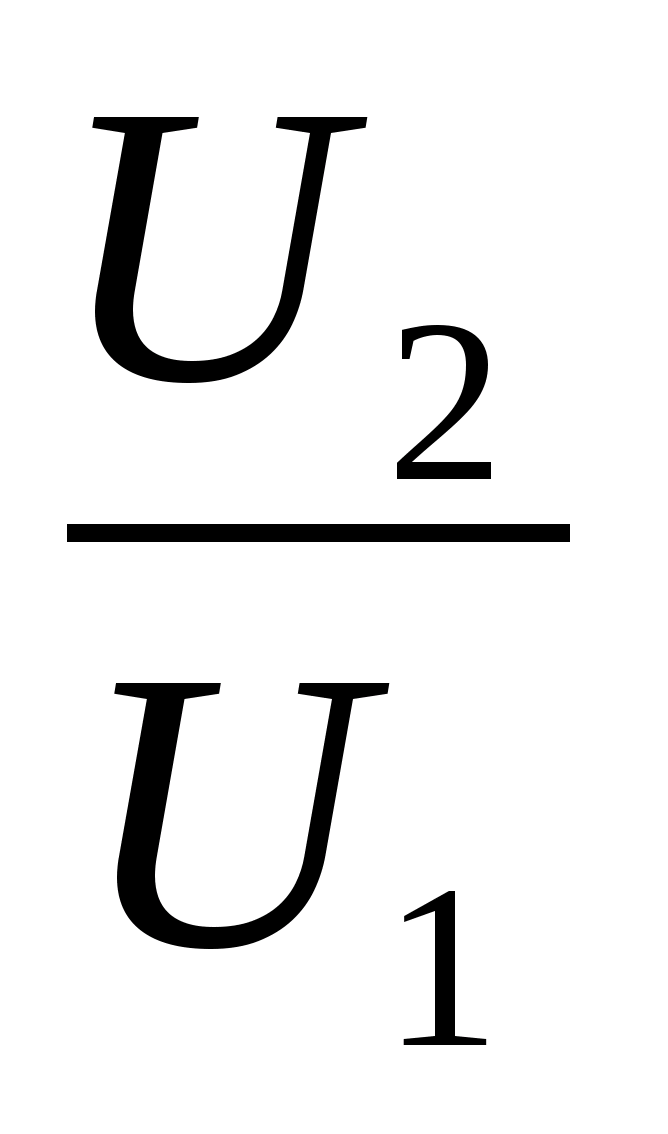 =.
=.
Task 25.An ideal monatomic gas has passed from state 1 to state 2 (see Fig.picture). How has the internal energy of the gas changed? Answer: =  =4.
=4.
Task 26.Thermally insulated vesselV= 2 m 3 separated by porouspartition into two equal parts. At the initial moment in one partthe vessel is locatedm= 1 kg of helium, and in the other -m= 1 kg of argon, and the averagethe quadratic velocity of argon and helium atoms is the same and is1000 m/s. Helium atoms can freely penetrate through the pores inpartition, while argon atoms do not. Determine the temperature of the heliumargon mixture after equilibrium has been established in the system.
Key elements of the solution
1. After equilibrium is established in the system, the temperature of both parts of the vessel will become the same and equal to T, and helium will be evenly distributed throughout the vessel.
2. The temperature in the vessel is determined from the law of conservation of energy:
ε = 2 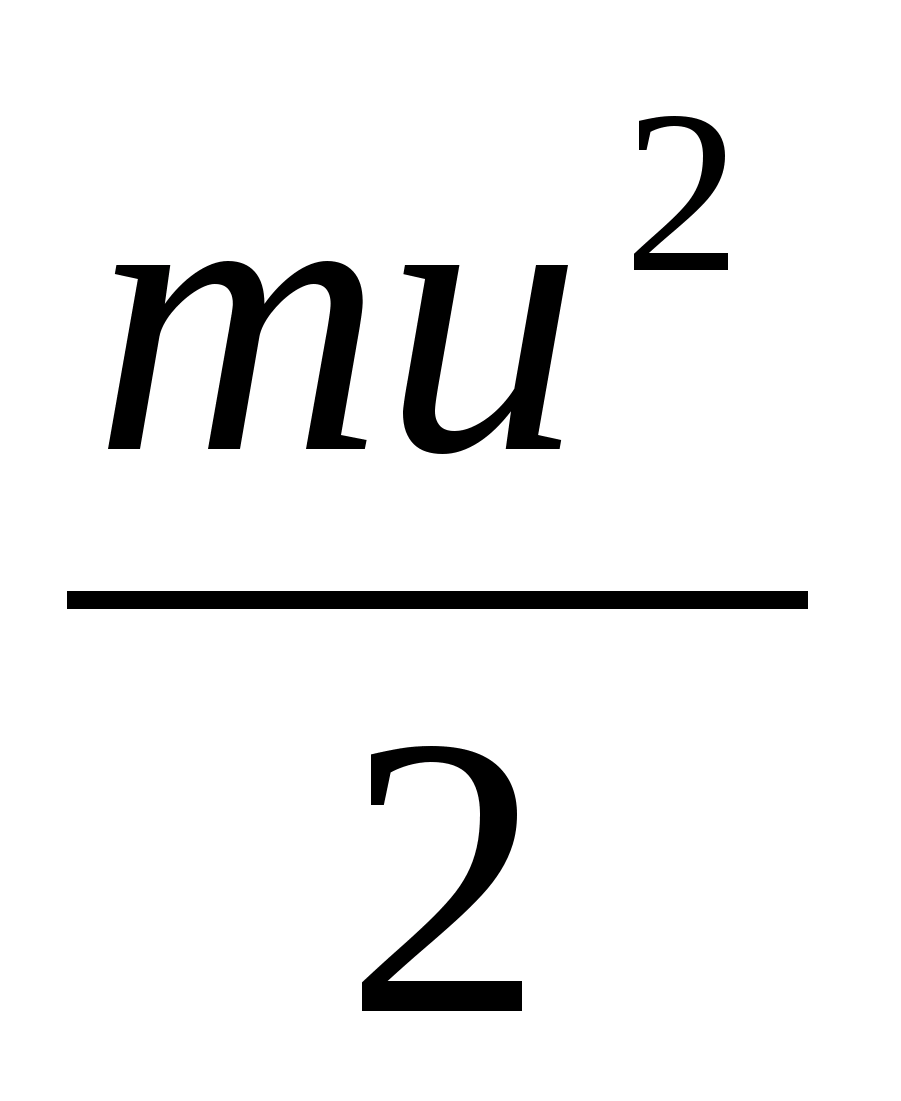 = (ν He + ν Ar) RT, where ν He = and ν Ar =
= (ν He + ν Ar) RT, where ν He = and ν Ar = 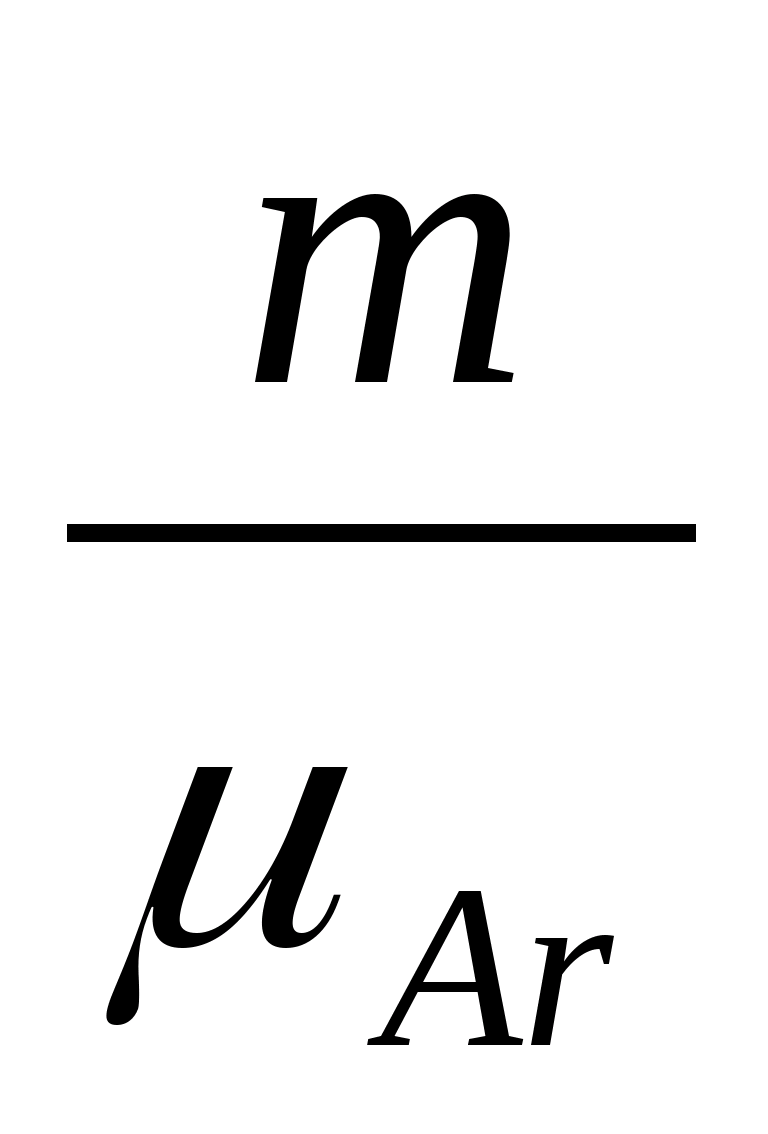 is the number of moles of helium and argon.
is the number of moles of helium and argon.
Hence T = 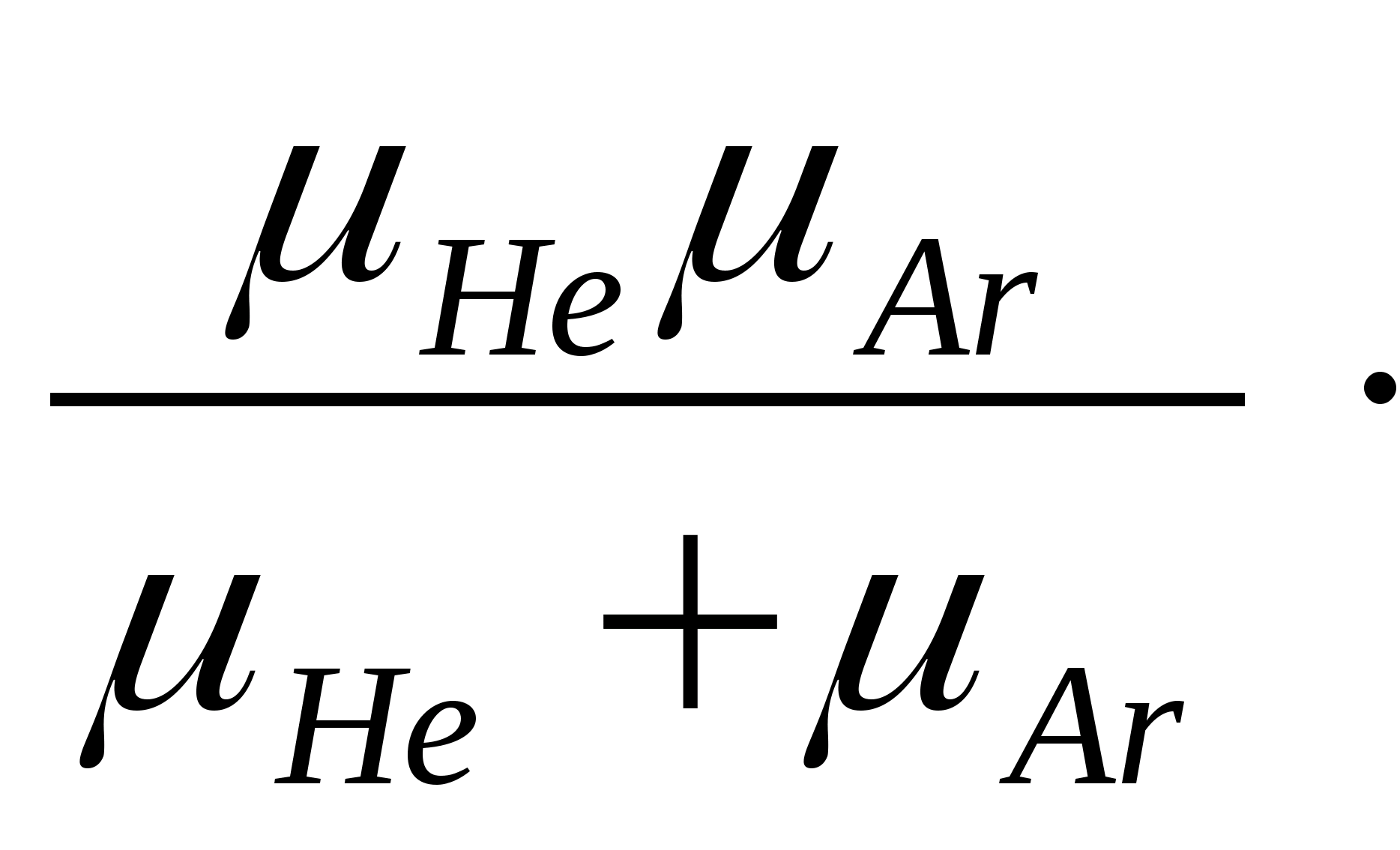 2
2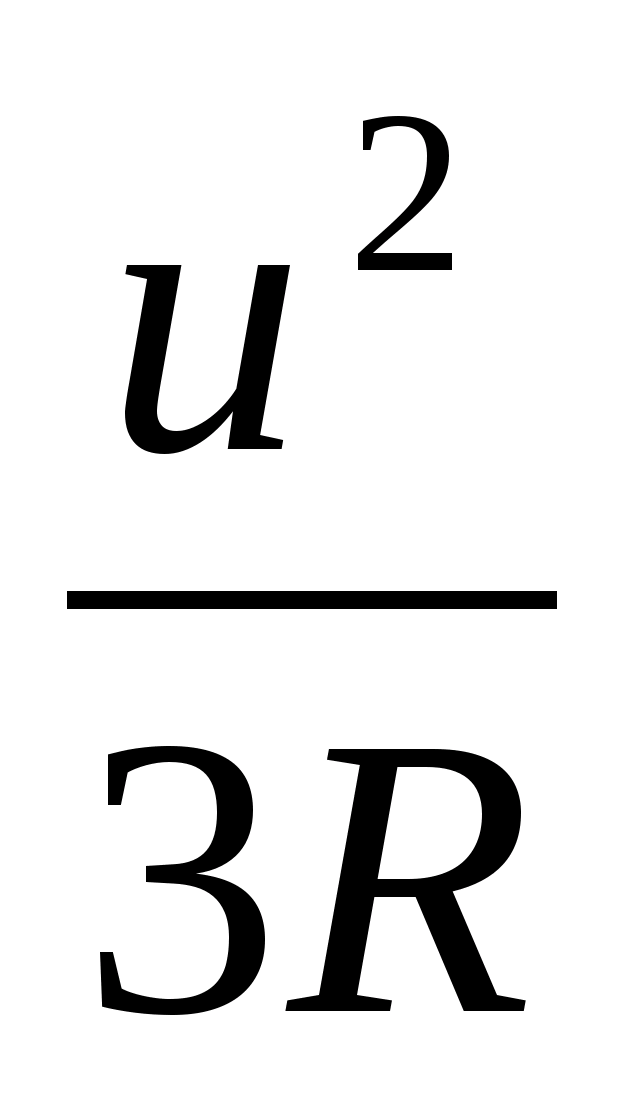
3. Substituting numerical data, we get: T = 292 K.
Task 27.VesselvolumeV= 2 m 3 divided by a porous partition into two equalparts. At the initial moment, in one part of the vessel there ism= 1 kg of helium, and in anotherm= 1 kg of argon. Initial helium temperatureequal to the temperature of argon T = 300 K. Helium atoms can freelypenetrate through the septum, but argon atoms do not. Determineinternal energy of the gas remaining in that part of the vessel where helium was originally located after equilibrium was established insystem.
O 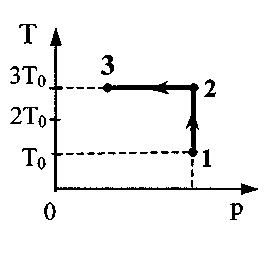 reply:ε
=
ν
1
RT=
reply:ε
=
ν
1
RT=
 mRT= 467 kJ.
mRT= 467 kJ.
Task 28.One mole of argon completes process 1-2-3. In section 2 - 3, 300 J of heat is supplied to the gas (see figure). T 0 \u003d 10 K. Find the ratio of the work done by the gas during the entire processBUT 123 , to the corresponding total amount of heat supplied to itQ 123 .
Key elements of the solution
1. Write down the formula for calculating the work done during the entire
process 1 - 2 - 3: A 123 \u003d A 12 + A 2 s. 2. Write down the formula for calculating the work A J 2 \u003d νRΔT 12 or taking into account the fact that ΔT 12 \u003d 2T 0 A 12 \u003d 2νRT 0. Write down the first law of thermodynamics for section 2-3 Q 23 \u003d ΔU 23 + A 23. Note that in isothermal processΔU 23 \u003d 0. Then Q 23 \u003d A 23 and A 123 \u003d 2νRT 0 + Q 23. Write down the first law of thermodynamics for section 1-2 Q 12 \u003d ΔU 12 + A 12. W  write down the formula for calculating the change in internal energy ΔU 12 \u003d νR ΔT 12 or taking into account the fact that ΔT 12 \u003d 2T 0: ΔU 12 \u003d 3νRT 0. 6. After transformations Q 12 = 5νRT 0 , Q 123 = 5νRT 0 + Q 23 the desired ratio is
write down the formula for calculating the change in internal energy ΔU 12 \u003d νR ΔT 12 or taking into account the fact that ΔT 12 \u003d 2T 0: ΔU 12 \u003d 3νRT 0. 6. After transformations Q 12 = 5νRT 0 , Q 123 = 5νRT 0 + Q 23 the desired ratio is 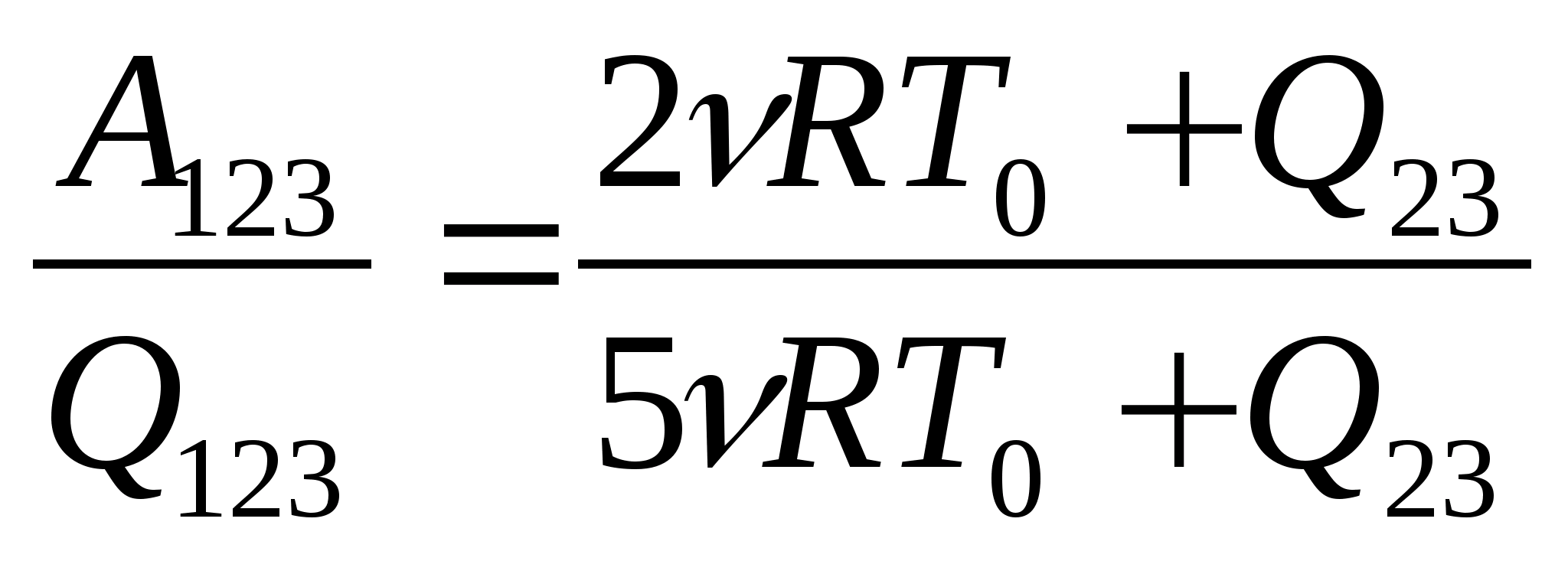 = 0,65.
= 0,65.
Task 29.One mole of an ideal monatomic gas was first heated and then cooled to an initial temperature of 300 K, reducing the pressure by a factor of 3 (see figure). How much heat is reported to the gas in section 1-2?
Answer: 5vRT=12.5 kJ
W 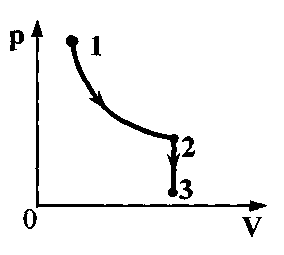 hell 30.One mole of an ideal monatomic gasfirst expanded isothermally (T 1
= 300 K).Then the gas was cooled by lowering the pressure by 3 times(see picture). How much heat is given offgas in section 2-3?
hell 30.One mole of an ideal monatomic gasfirst expanded isothermally (T 1
= 300 K).Then the gas was cooled by lowering the pressure by 3 times(see picture). How much heat is given offgas in section 2-3?
Key elements of the solution
1. Write down the first law of thermodynamics ΔU = Q + A ext. With.
Please note that in section 2-3: A 2 s \u003d 0. Then Q 23 \u003d ΔU 23.
2. Write down the formula for calculating the change in internal energy: ΔU 23 \u003d νR (Tz - T 2). Note that T 2 \u003d T 1.
3
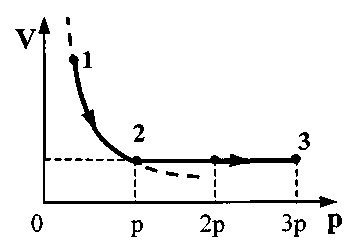 . Apply Charles' law for states 2 and 3:
. Apply Charles' law for states 2 and 3: 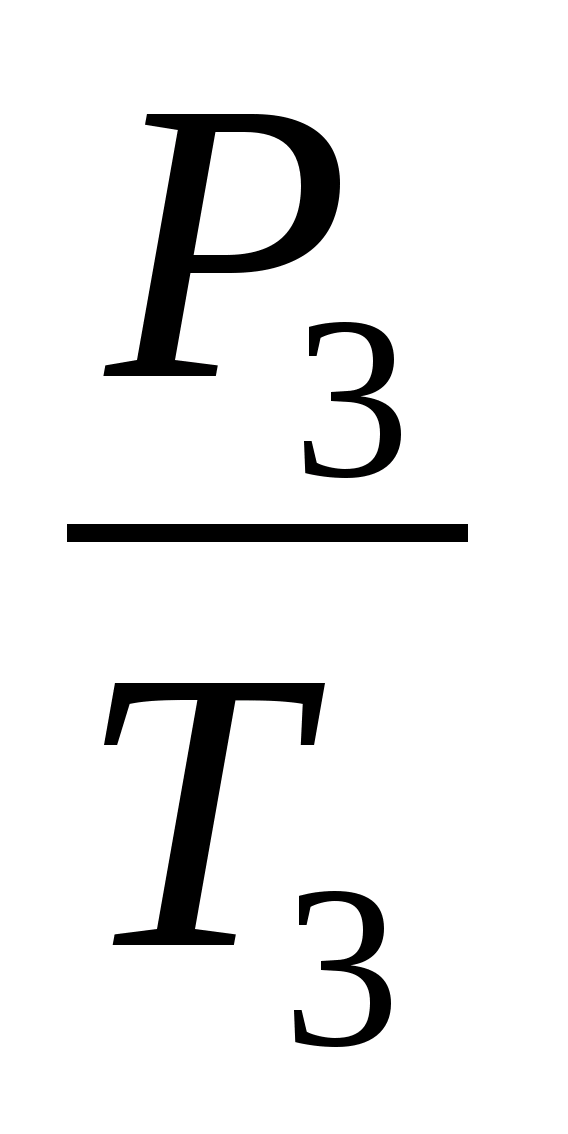 =
=
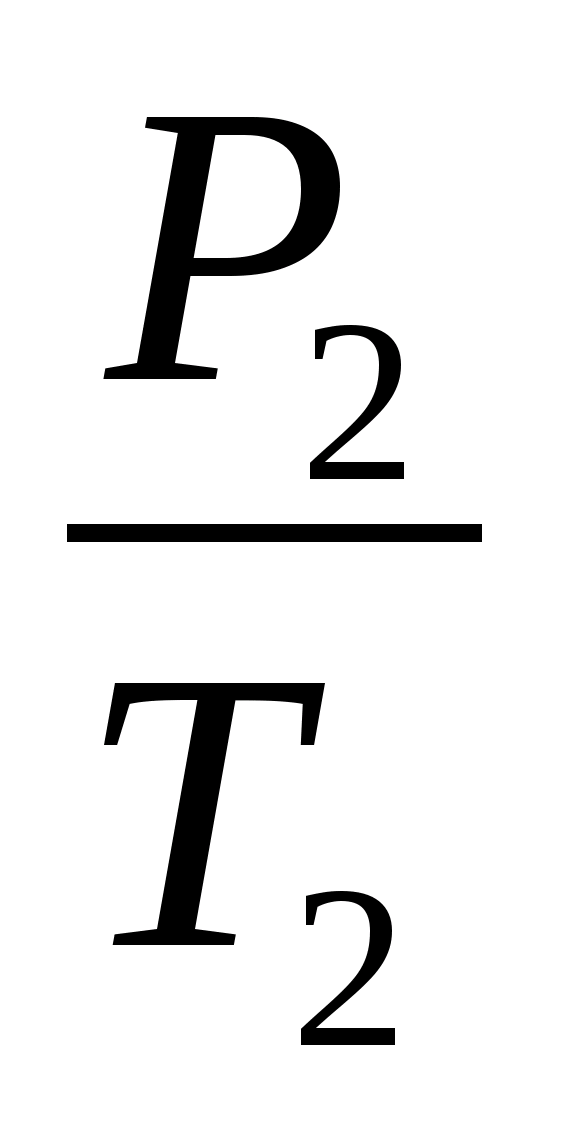 and get the ratio T 3 =
and get the ratio T 3 = 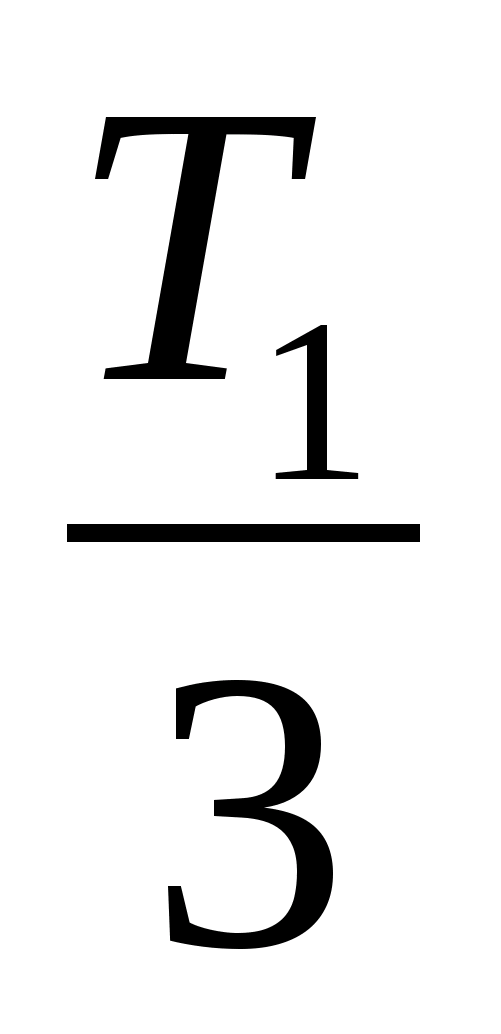 . 4. Substituting the obtained value of T 3 into the formula ΔU 23 \u003d νR (Tz - T 2) \u003d Q 23, calculate the amount of heat: Q 23 \u003d -νRT 1 \u003d 2.5 kJ.
. 4. Substituting the obtained value of T 3 into the formula ΔU 23 \u003d νR (Tz - T 2) \u003d Q 23, calculate the amount of heat: Q 23 \u003d -νRT 1 \u003d 2.5 kJ.
Task 31.One mole of an ideal monatomic gas first expanded isothermally (T 1 = 300 K). Then the gas was heated isobarically, increasing the temperature by 1.5 times (see figure). How much heat receivedgas in section 2-3?
Key elements of the solution
1. Write down the first law of thermodynamics for isobaric expansion: Q 23 \u003d ΔU 23 + A 23. 2. Write down the formulas for calculating the change in internal energy and gas work:
ΔU 23 \u003d νR (T 3 - T 2). A 23 \u003d νR (T 3 - T 2). Note that T 2 \u003d T 1 and T 3 \u003d 1.5T 2.
3. Carry out transformations and get the formula for calculating the amount of heat and its numerical value: Q 23 \u003d 1.25 νRT 1 \u003d 3.1 kJ.
Task 32.1 mole of an ideal monatomic gas firstisothermally compressed (T 1 =300 K). Then gasheated by increasing the pressure in3 times (seepicture). How much heat receivedgas in section 2-3?
Answer:Q 23 = 3 ν RT 1 = 7.5 kJ.
Task 33. One mole of helium completes the cycle shown inpV-diagram (see figure). Plot 1-2-adiabatic, 2-3 - isotherm, 3-1 - isobar. The work done on the gas per cycle is equal to A. In section 2-3, the gas gives off the amount of heatQ. What is the temperature difference between states 1 and 2?
Key elements of the solution
1. Write down the expression for the gas work per cycle:
BUT  \u003d A 12 + A 23 + A 31 or helium work in an isothermal process A 23 \u003d A - A 12 - A 31
\u003d A 12 + A 23 + A 31 or helium work in an isothermal process A 23 \u003d A - A 12 - A 31
2. Write down what work:
in an adiabatic process is A 12 = ΔU= νRΔT;
in an isothermal process, it is equal to A 23 \u003d - Q;
in an isobaric process is A 31 = νRΔT.
3. Substituting all the obtained values of work in individual sections into the formula for the work of gas per cycle, get - Q \u003d A- νRΔT - νRΔT, from where express ΔT and write down the correct answer: ΔT =  .
.
W  hell 34.
shown in the figure onpV-diagram.Whatequals ratio thermal efficiency engines
hell 34.
shown in the figure onpV-diagram.Whatequals ratio thermal efficiency engines 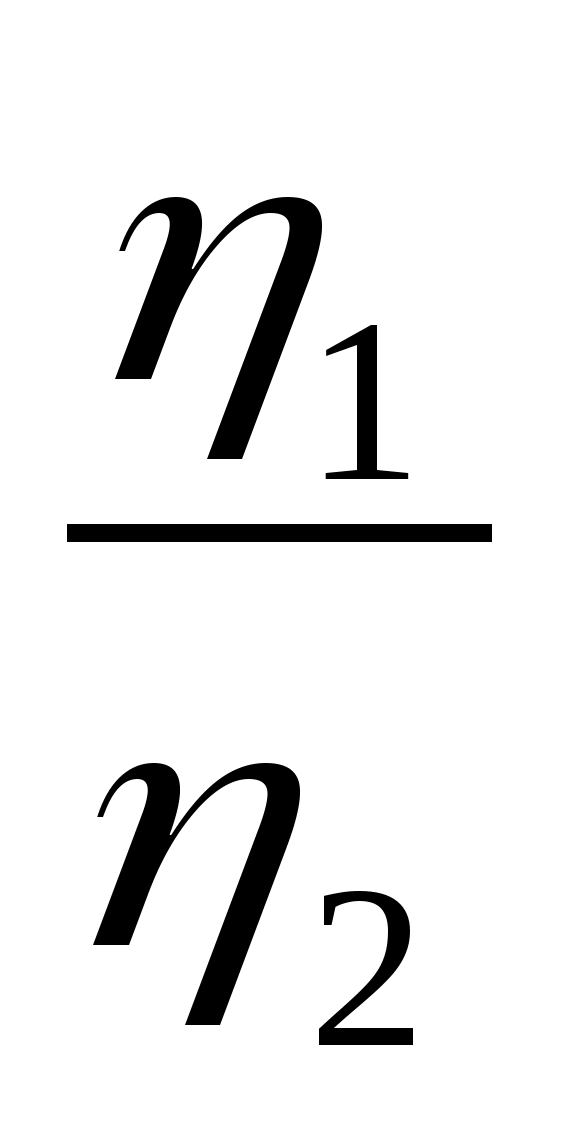 ,
based onusing these cycles?
,
based onusing these cycles?
Key elements of the solution
1. Write down the formulas for calculating the efficiency of cycles, calculating the work A of the gas per cycle and the amount of heat Q 1 received from the heater per cycle:
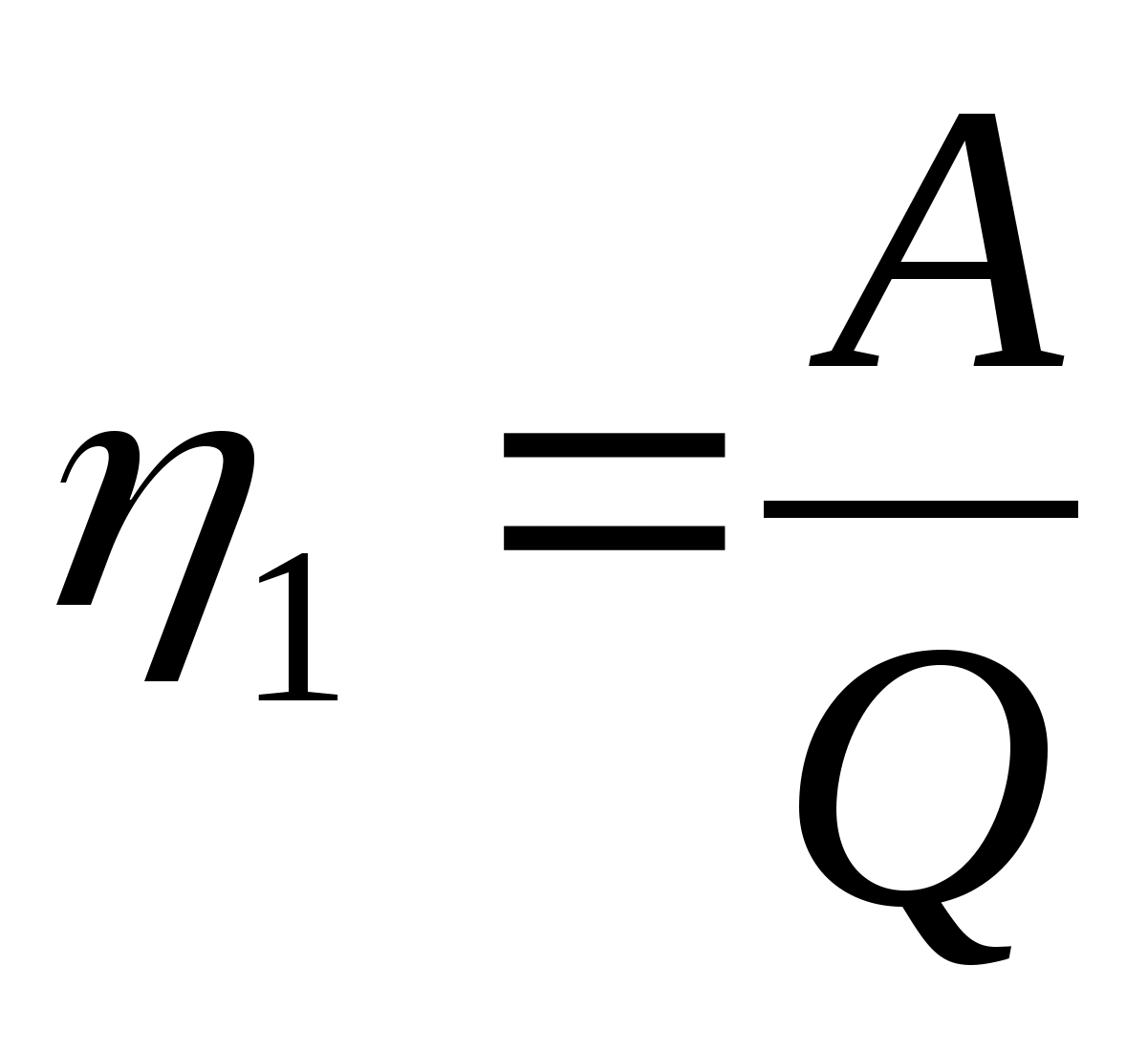 , A 1 =
, A 1 = 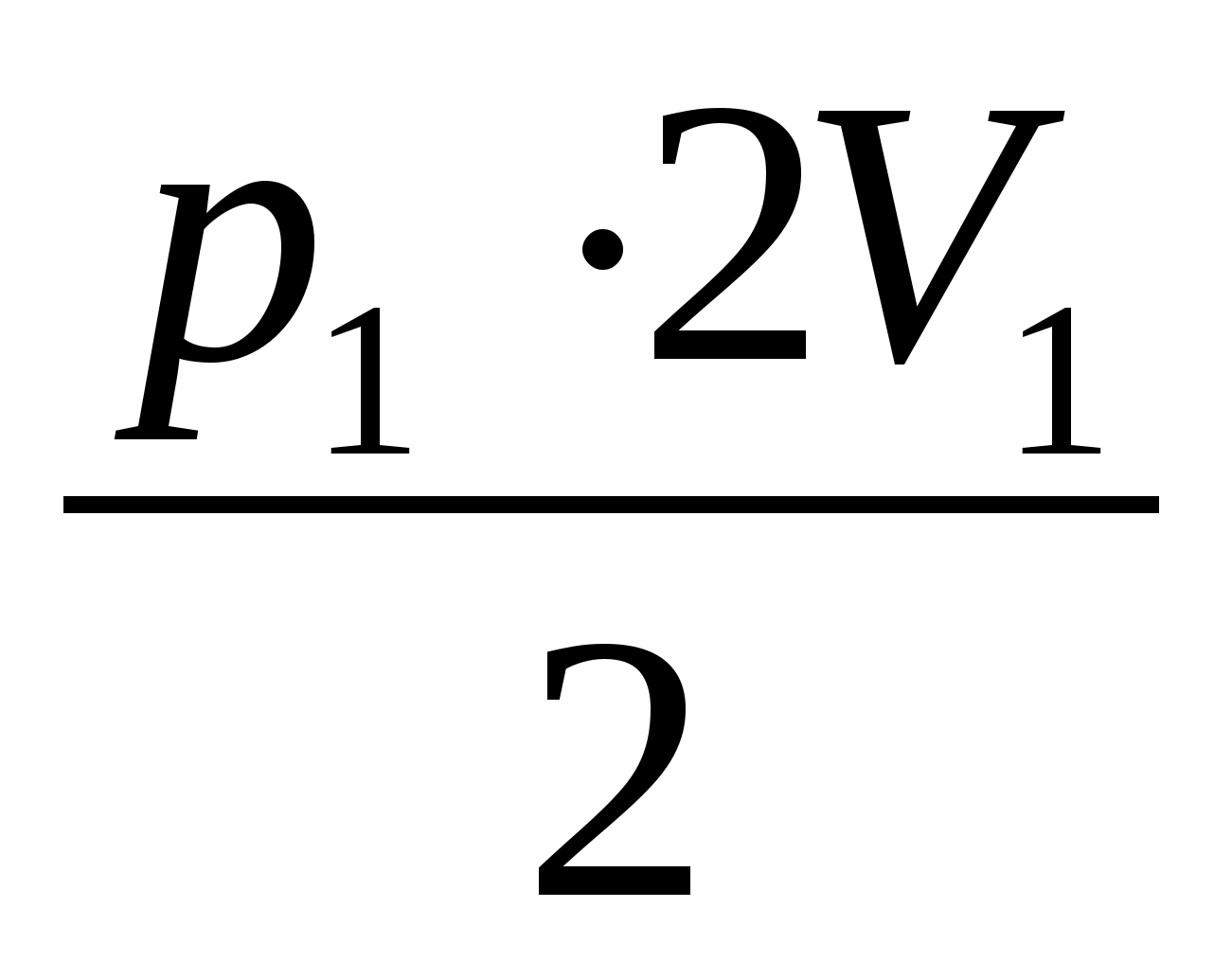 = p 1 V 1; Q 1 \u003d ΔU 12 + A 42.
= p 1 V 1; Q 1 \u003d ΔU 12 + A 42. 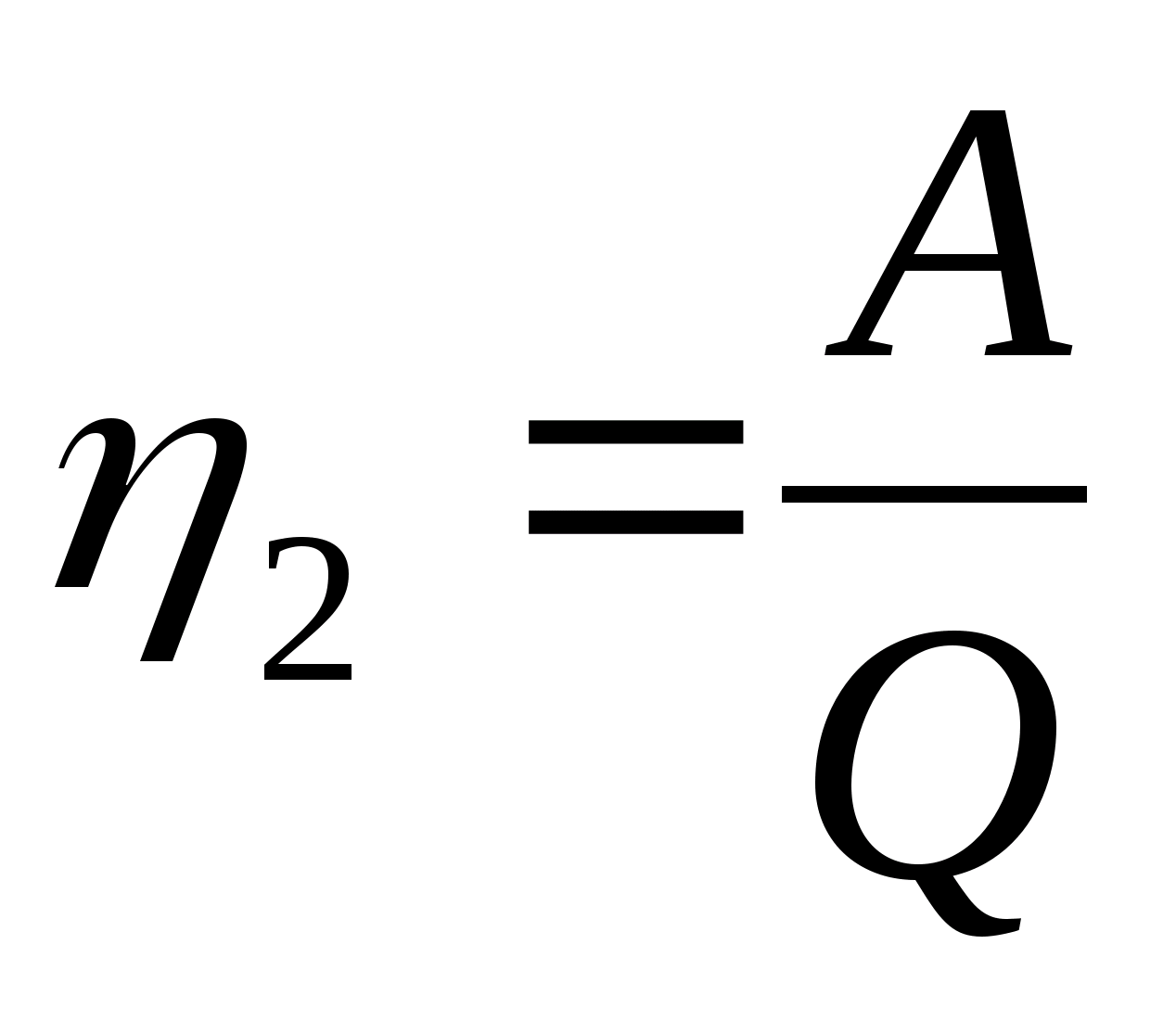 , A 2 == p 1 V 1 ; Q 2 \u003d ΔU 12 + A 12.
, A 2 == p 1 V 1 ; Q 2 \u003d ΔU 12 + A 12.
FROM  consequently, =
consequently, =  .
.
Task 35.State of a monatomic ideal gaschanges in two cycles: 1421 and 1231,presented figure on pV-diagram.What is the ratio of thermal efficiencyenginesfounded
on theusing these cycles?
Answer:
Task 36.The state of an ideal gas changes in a closed cycle. Fromstate 1 with temperature T 1 \u003d 1900 K gas, expanding adiabatically,goes into state 2 with temperature T 2 = 1260 K. From state 2the gas goes into state 3 with temperature T 3 = 360 K byisochoric cooling. The gas is transferred from state 3 to state 4 withtemperature T 4 = 540 K by adiabatic compression, from state 4 -to state 1 by isochoric heating. Calculate the efficiency for thiscycle. Answer:η ≈ 0.34
Problem 37. A vessel with a small crack contains a monatomic ideal gas. In the experiment, the gas pressure and its volume decreased by a factor of three. How many times has the internal energy of the gas in the vessel changed?
Key elements of the solution
1. Write down the Clapeyron-Mendeleev equation for two states: p 1 V 1 = ν 1 RT and p 2 V 2 = ν 2 RT. 2. Write down that the internal energy of an ideal gas is directly proportional to the amount of matter and the absolute temperature U ~ νT. 3. Analyze these expressions taking into account the condition of the problem and get the correct answer:  .
.
Problem 38.An ideal gas is contained in a vessel with a small crack. In experiencethe pressure of the gas has halved, and the volume of the vessel has decreased by 4 times atconstant temperature. How many times has the internalthe energy of the gas in the vessel?
Oreply:
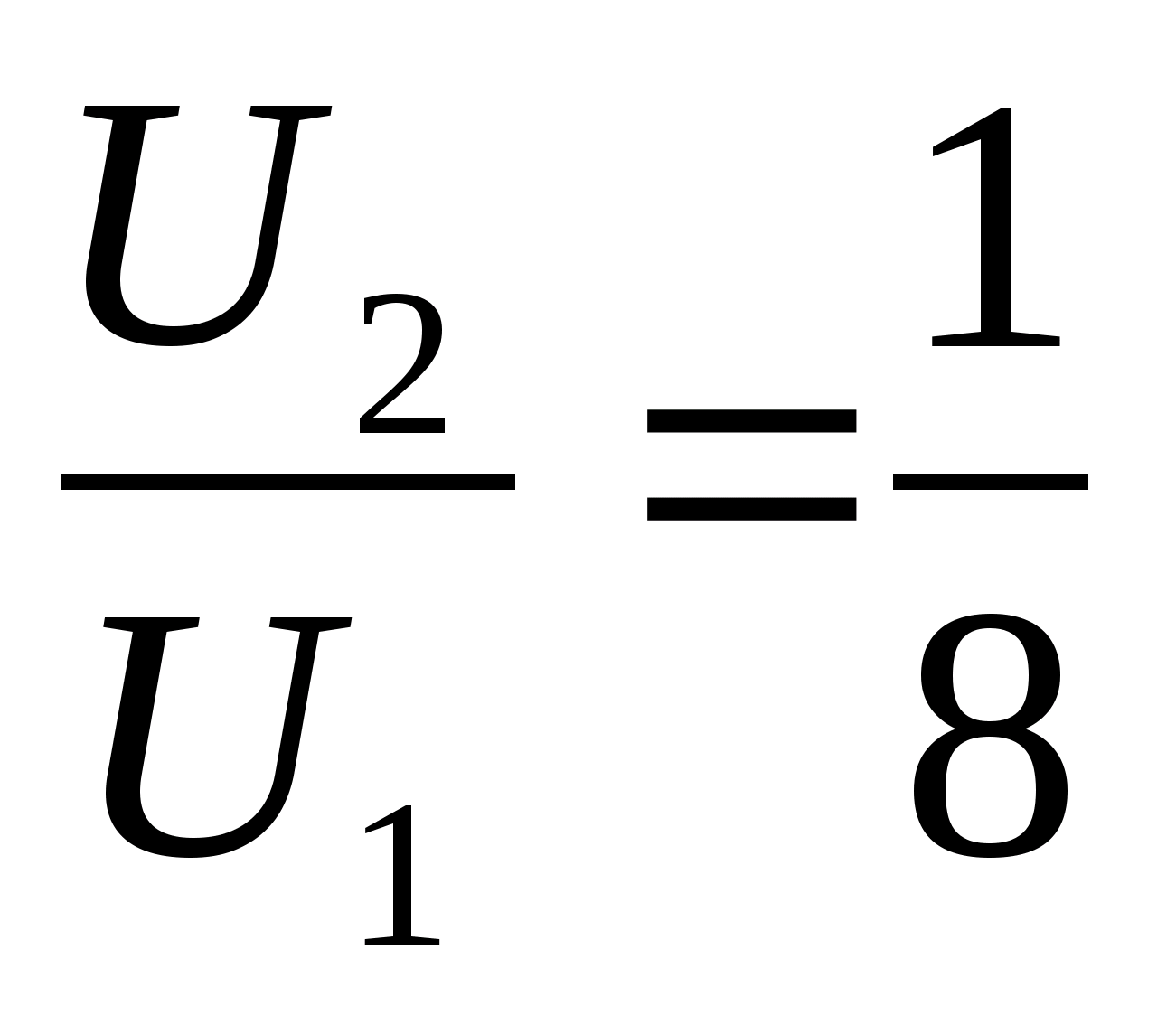 .
.
Electrostatics
Task 39. A positively charged dielectric plate, which creates a uniform electric field with intensity E=10 4 V/m, is fixed on a horizontal plane. A ball of mass m = 20 g falls on it, having positive charge q = 10 -5 Cl. With an absolutely inelastic impact, the ball transferred to the plate an impulse equal to 0.028 kg m/s. From what height did the ball fall? The initial speed of the ball is assumed to be zero.
Key elements of the solution
1. Write down the expression for the potential energy of the ball in the gravity field E p = mgh and the charge in the electric field E e = - qEh. Pay attention to the sign of the potential energy of the charge in the electric field. If we take a positively charged plane as a zero potential level, then the movement of a ball charged with the same charge as the plane, in the direction lines of force is accompanied by the performance of work positive in sign, which leads to a decrease in its potential energy. That is, relative to the plane, the energy of which is taken as 0, the potential energy of the ball becomes less than 0.
2. Write down the law of conservation of energy: (mg - qE)h =  and express the height h from it: h=.
and express the height h from it: h=.
3. Write down the expression for the momentum transferred by the ball to the plate during an absolutely inelastic impact: Δр = mv, express the velocity in terms of the momentum and mass of the ball v= 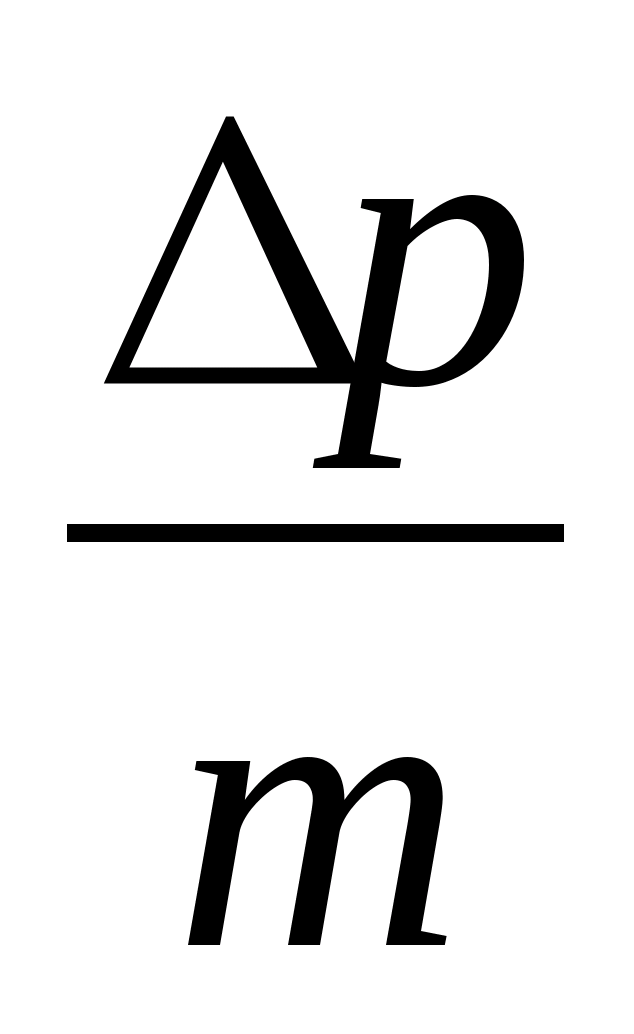 .
.
4. Substitute the value of the speed into the expression for the height of the fall and get a general answer and the correct numerical answer: h =  = 10 cm.
= 10 cm.
Task 40. A stationary horizontal positively charged dielectric plate creates a uniform electric field with a strength of E = 10 4 V/m. At her from aboveh\u003d 10 cm falls with zero initial speed a ball of massm\u003d 20 g. What is the charge of the ball if, during an absolutely inelastic impact, it transferred an impulse of 0.028 kg m / s to the plate? The ball has a positive charge. Answer:q = 10 -5 Cl.
Task 41.An electron flies into a uniform electric field with intensity E = 200 V/m at a speedv 0 = 10 7 m/s in the direction of field lines. After what time will the electron be at the same point where it flew into the field?
Key elements of the solution
1 . Specify the direction of field lines electric field, remembering that the line of force is directed from plus to minus. This means that the electron, moving in the direction of the lines of force, that is, from plus to minus, will slow down. 2. Since the acceleration of the electron does not change during the entire time of its movement, it means that the time of its deceleration in the electric field is equal to the time of the subsequent accelerated movement in the same field. Then the total time of electron motion in the electric field is t =
. Specify the direction of field lines electric field, remembering that the line of force is directed from plus to minus. This means that the electron, moving in the direction of the lines of force, that is, from plus to minus, will slow down. 2. Since the acceleration of the electron does not change during the entire time of its movement, it means that the time of its deceleration in the electric field is equal to the time of the subsequent accelerated movement in the same field. Then the total time of electron motion in the electric field is t = 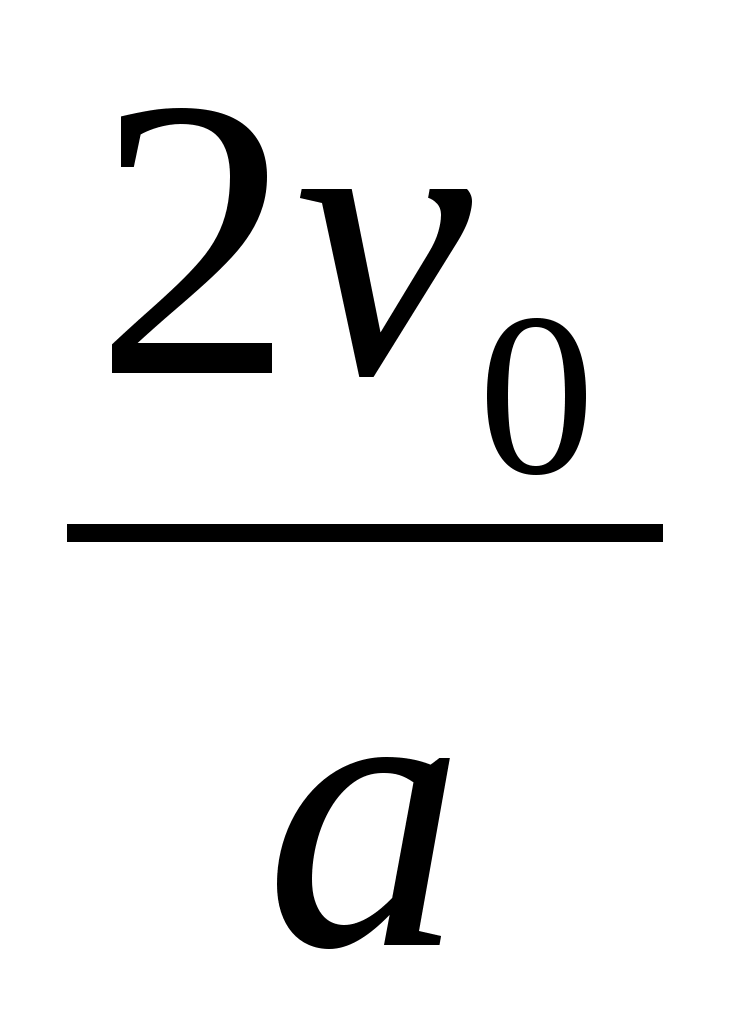 . 3. Taking into account the fact that the charge acceleration in an electric field is equal to
. 3. Taking into account the fact that the charge acceleration in an electric field is equal to 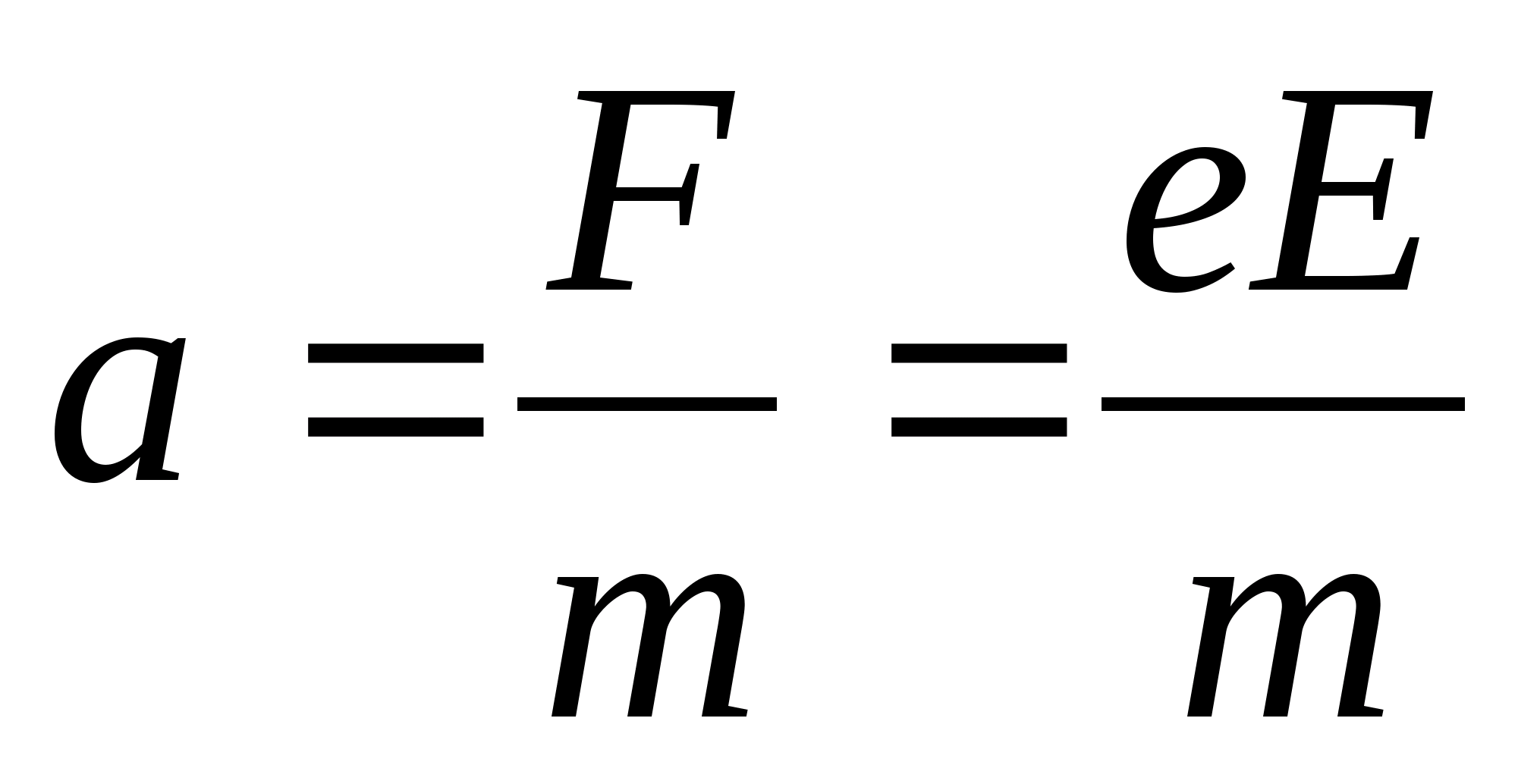 , we get
, we get 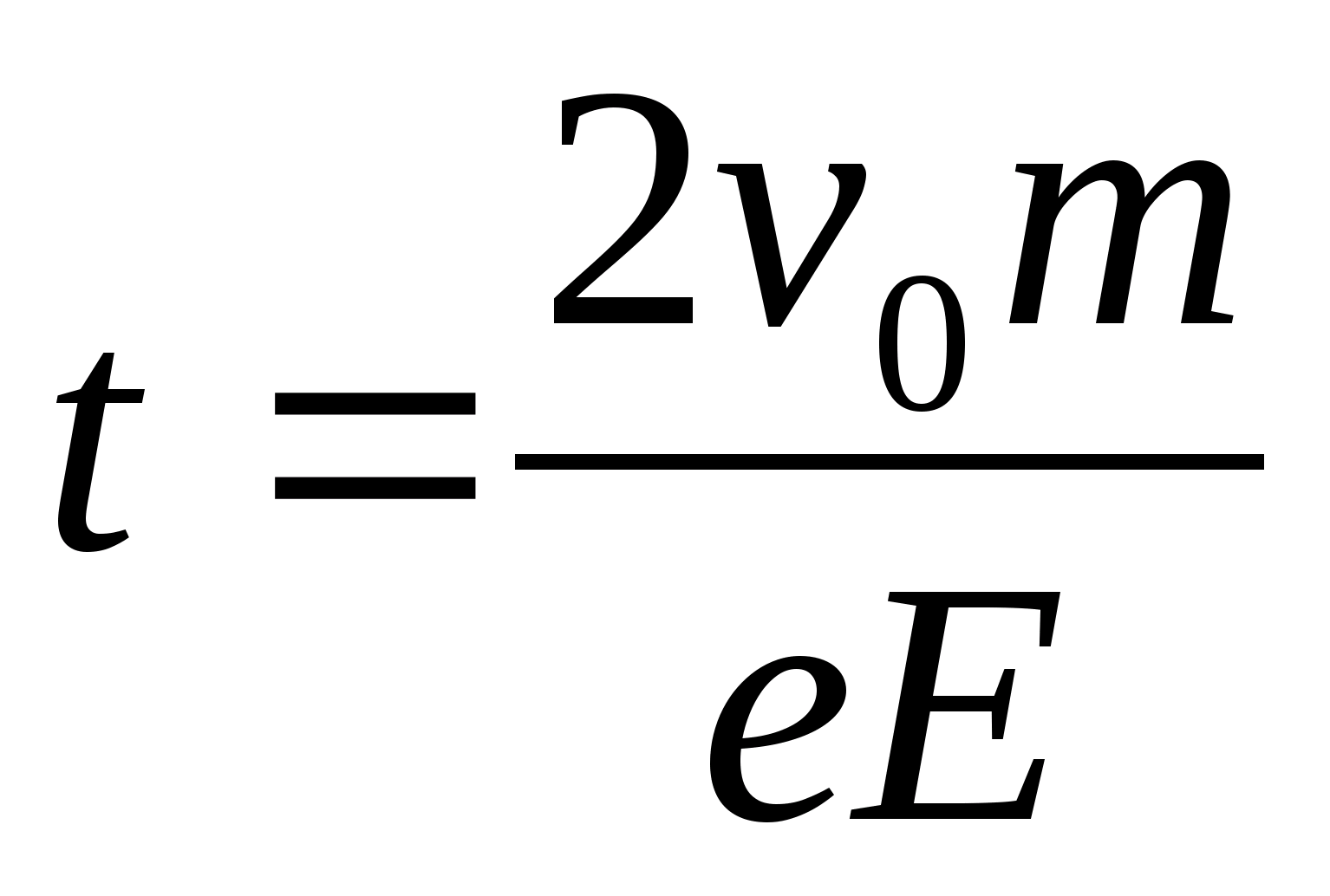 . Substituting these values, we obtain the answer in numerical form: t = 0.57 μs.
. Substituting these values, we obtain the answer in numerical form: t = 0.57 μs.
Task 42.Electron with speedv = 5-10 6 M/ cflies intothe space between the plates of a flat capacitor,between which the potential difference is maintainedU= 500 V (see figure). What is the maximum removalhan electron from the bottom plate of a capacitor?The ratio of the electron charge to its mass is γ= - 1.76 10 11 C/kg, electron incidence angle α =60°.The distance between the capacitor plates isd= 5 cm.
Key elements of the solution
1. Please note that the velocity component directed parallel to the lower plate of the capacitor, made in the form of a conductive grid, remains constant: v sinα = const, and the velocity component perpendicular to the lower plate, under the influence of the electric field force, decreases to zero when the electron reaches the height h.
kinetic energy electrons can pass throughgrids?Electric field strength between platescondenser is co-directed with the horizontalcomponent of the electron velocity.Key elements of the solution
1. Decompose the electron velocity into two components: horizontal and vertical. 2. Write down the theorem about kinetic energy in the form A = ΔE or eU = 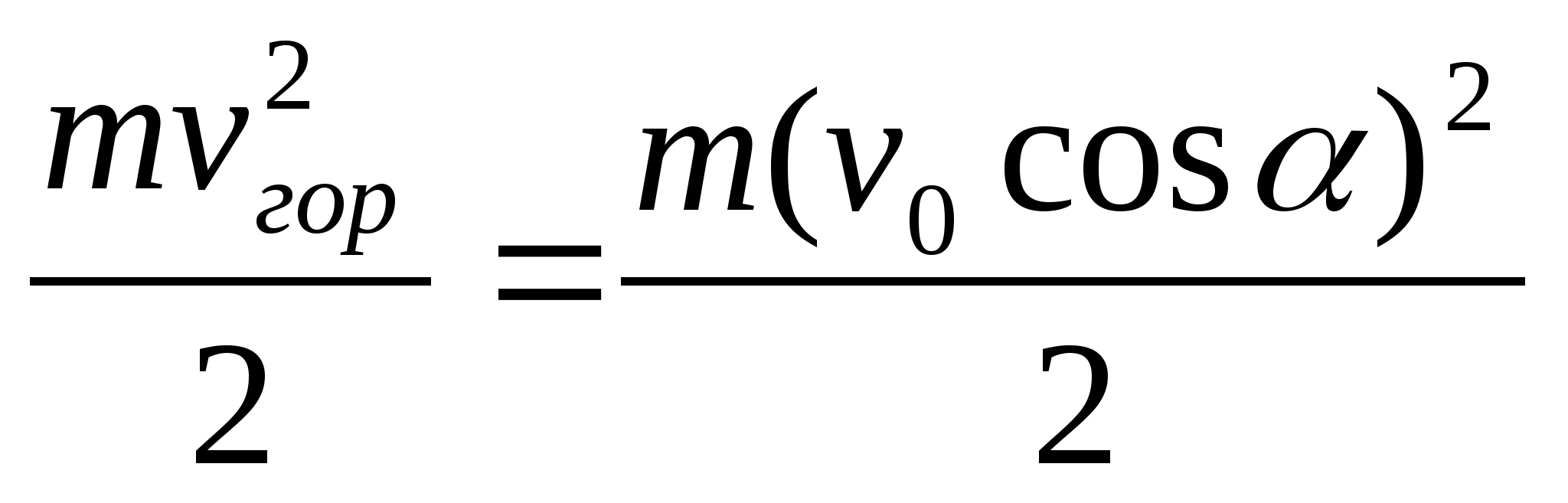 . 3. Get the calculation formula: E k \u003d
. 3. Get the calculation formula: E k \u003d 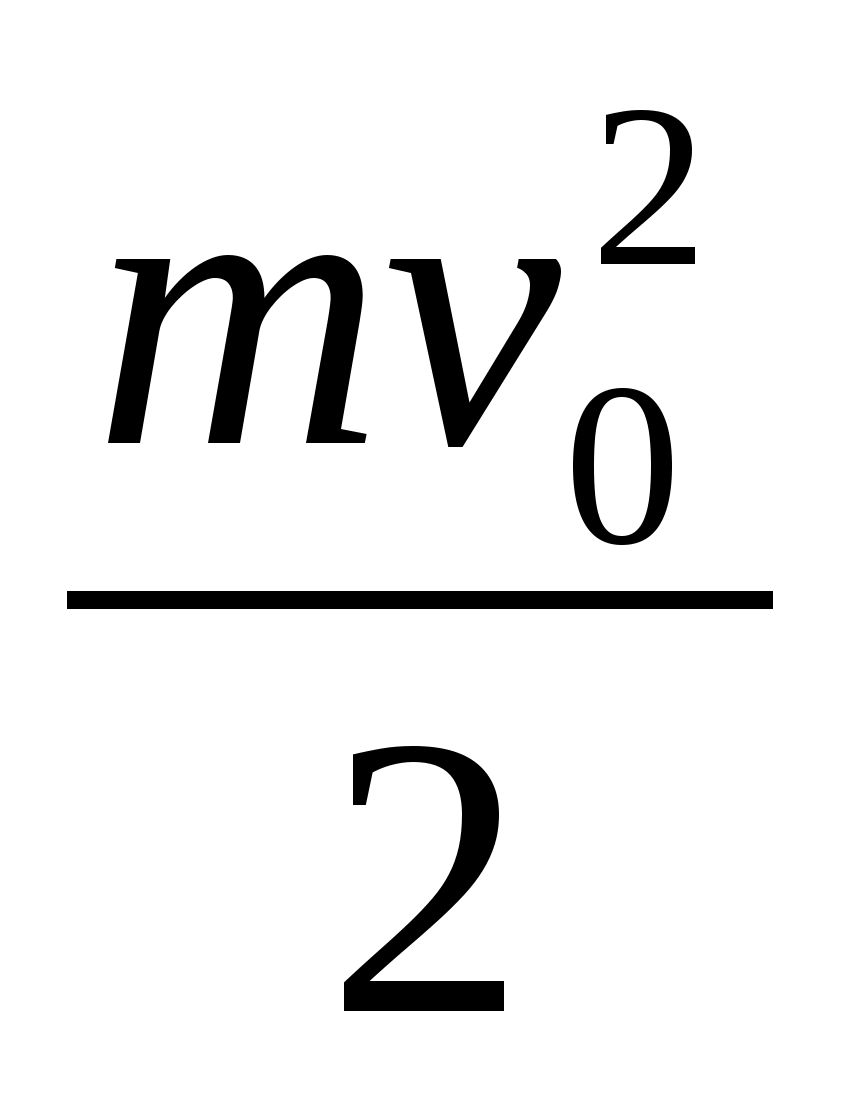 =
=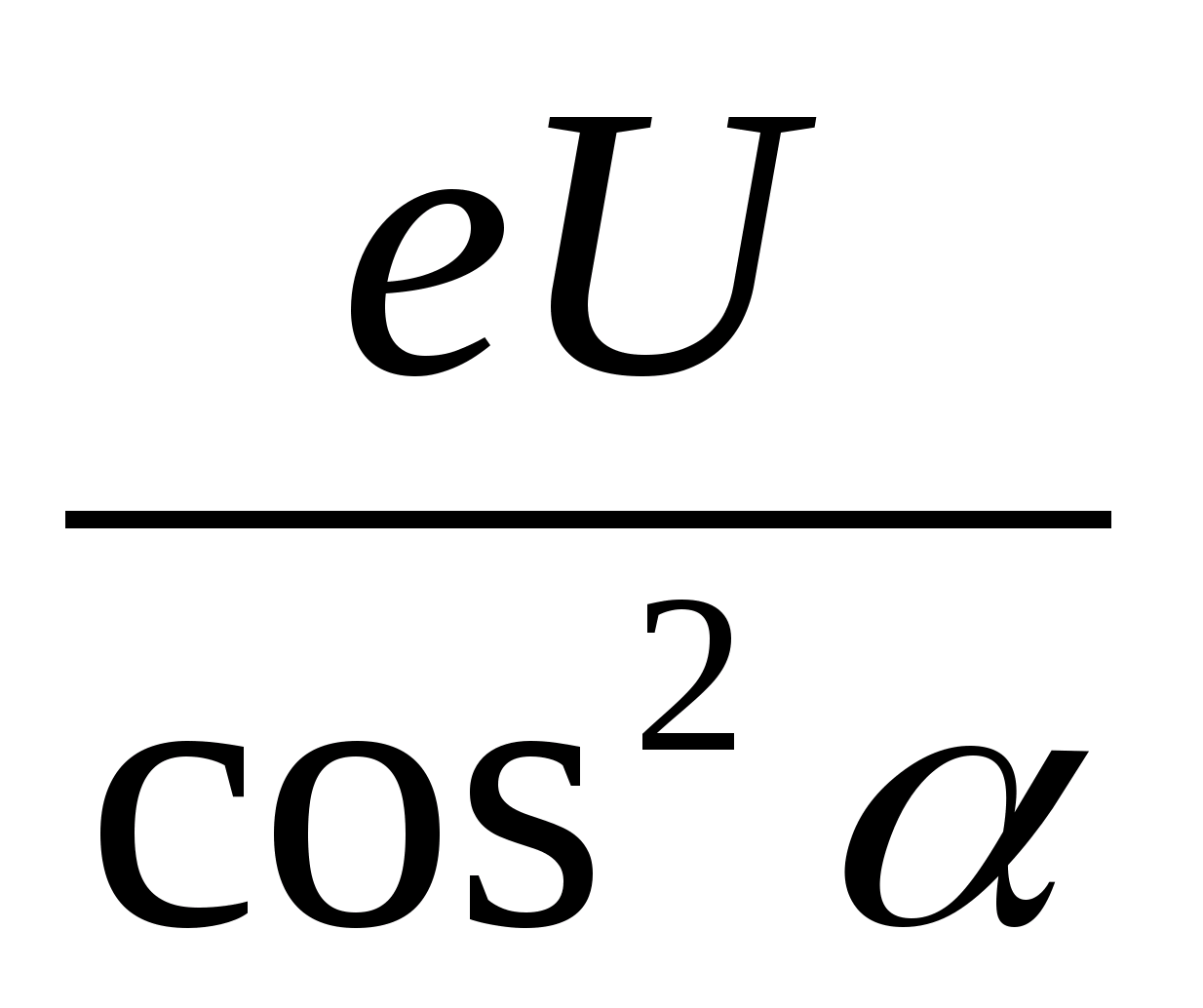 \u003d 1.28 10 -16 J.
\u003d 1.28 10 -16 J.
Task 44. d= 50 cm apart.The field strength between them is equal to E=10 5 V/m. Betweenplates at an equal distance from them, a ball with a charge is placedq= 10 –5 cl and massm= 10g. After the ball is released, itstarts to fall. What speedvthe ball had before hitting aboutplate?
Key elements of the solution
1. Please note that the plates are vertical, not horizontal. That is, the ball moves in a vertical gravitational field with acceleration a at =
g and in horizontal electric with acceleration a X
=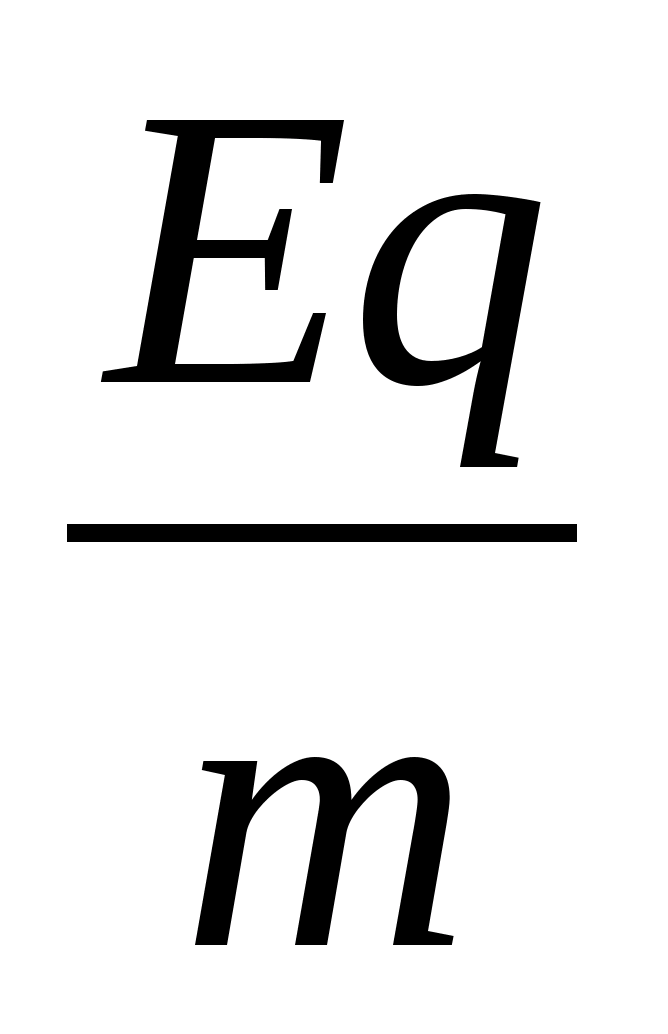 . 2. Then the horizontal component of the ball velocity before the impact is v r =
. 2. Then the horizontal component of the ball velocity before the impact is v r = 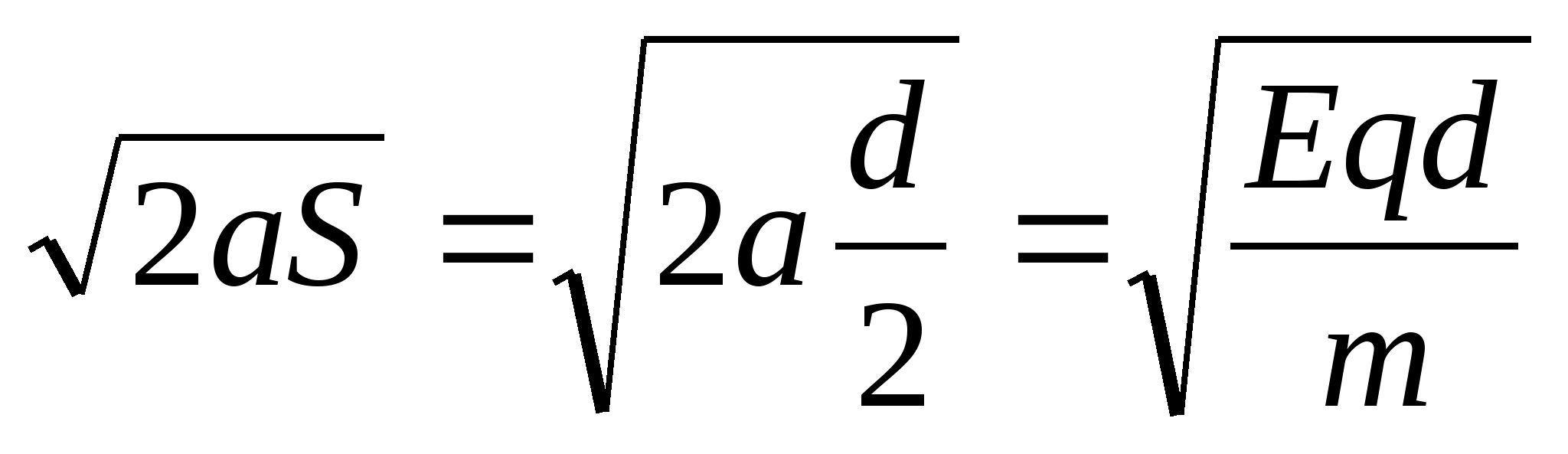 , and the flight time of the ball until it hits the plate
, and the flight time of the ball until it hits the plate  . 3. The vertical component of the ball velocity before the impact is v B = gΔt. This means that the modulus of the ball's velocity at the moment of impact is determined by the equation v 2 = v r 2 + v B 2 , whence v =
. 3. The vertical component of the ball velocity before the impact is v B = gΔt. This means that the modulus of the ball's velocity at the moment of impact is determined by the equation v 2 = v r 2 + v B 2 , whence v =  = 1 m/s.
= 1 m/s.
Task 45.Two non-conductive vertically arranged parallelcharged plates are at a distanced= 5 cm apart.Field strength between them E= 10 4 V/m. Between the platesa ball with a charge of 10 is placed at an equal distance from them. -5 cland massm= 20 g. After the ball was released, it began to fall.After what time Δtthe ball hit one of the plates? Answer: 1 c.
Task 46.A negatively charged body of small size with a chargeq = - 10 5 cland weightm= 20 g begins to slide without friction on an unchargednon-conductive inclined plane, the angle of inclination of which is φ=30°.The initial velocity of the body is zero. Horizontal non-conductivesurface under inclined plane positively charged and creates a vertically directed uniform field with intensityE = 10 4 V/m. How long does it take for the body to slide off the topinclined plane, the height of whichh= 50 cm, to the base?
Key elements of the solution
1. Make a schematic drawing, indicating on it all the forces acting on the body. Note that the force of gravity is directed vertically downwards, and the Coulomb force from the side of a positively charged horizontal plane is also vertically downwards. That is, the projection of the acceleration of a charged body on an axis directed along an inclined plane has the form  . 2. Write down the formulas for the distance traveled and time for uniformly accelerated motion, taking into account the fact that the angle at the base of the plane is 30 0, that is, S = 2h: S =
. 2. Write down the formulas for the distance traveled and time for uniformly accelerated motion, taking into account the fact that the angle at the base of the plane is 30 0, that is, S = 2h: S = 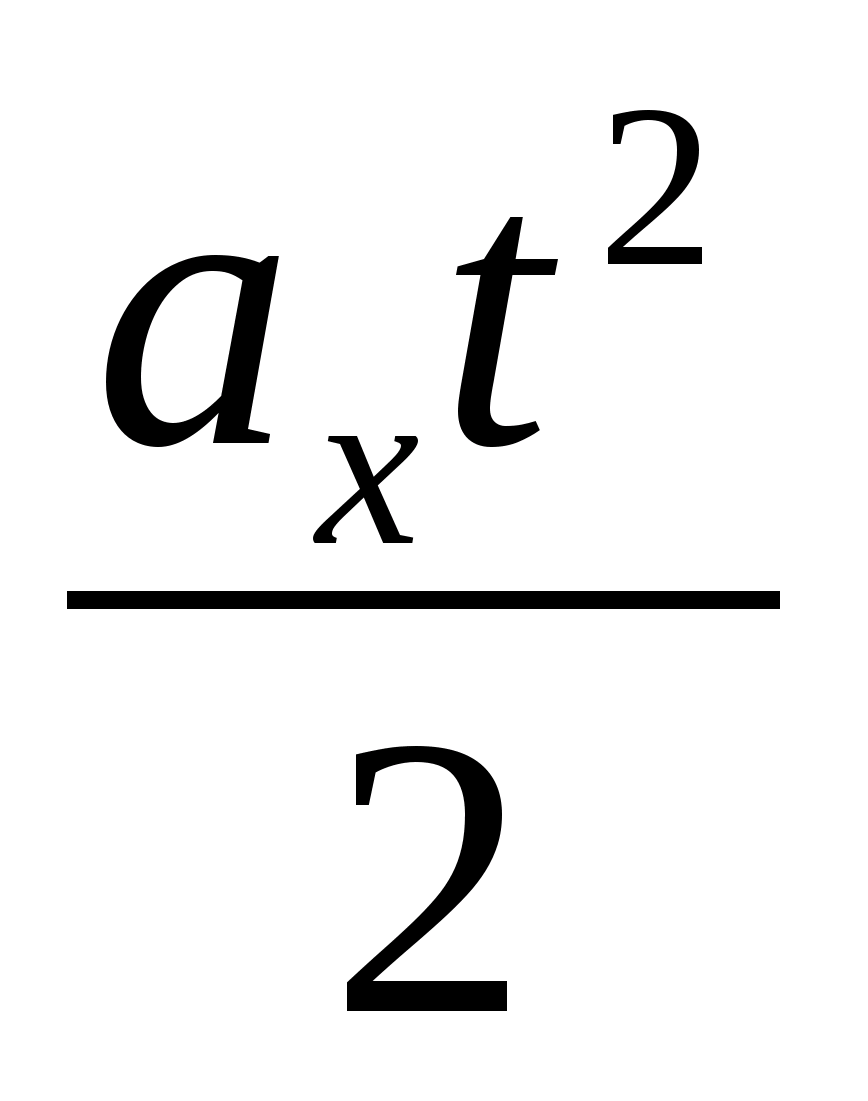 = 2h, t =
= 2h, t = 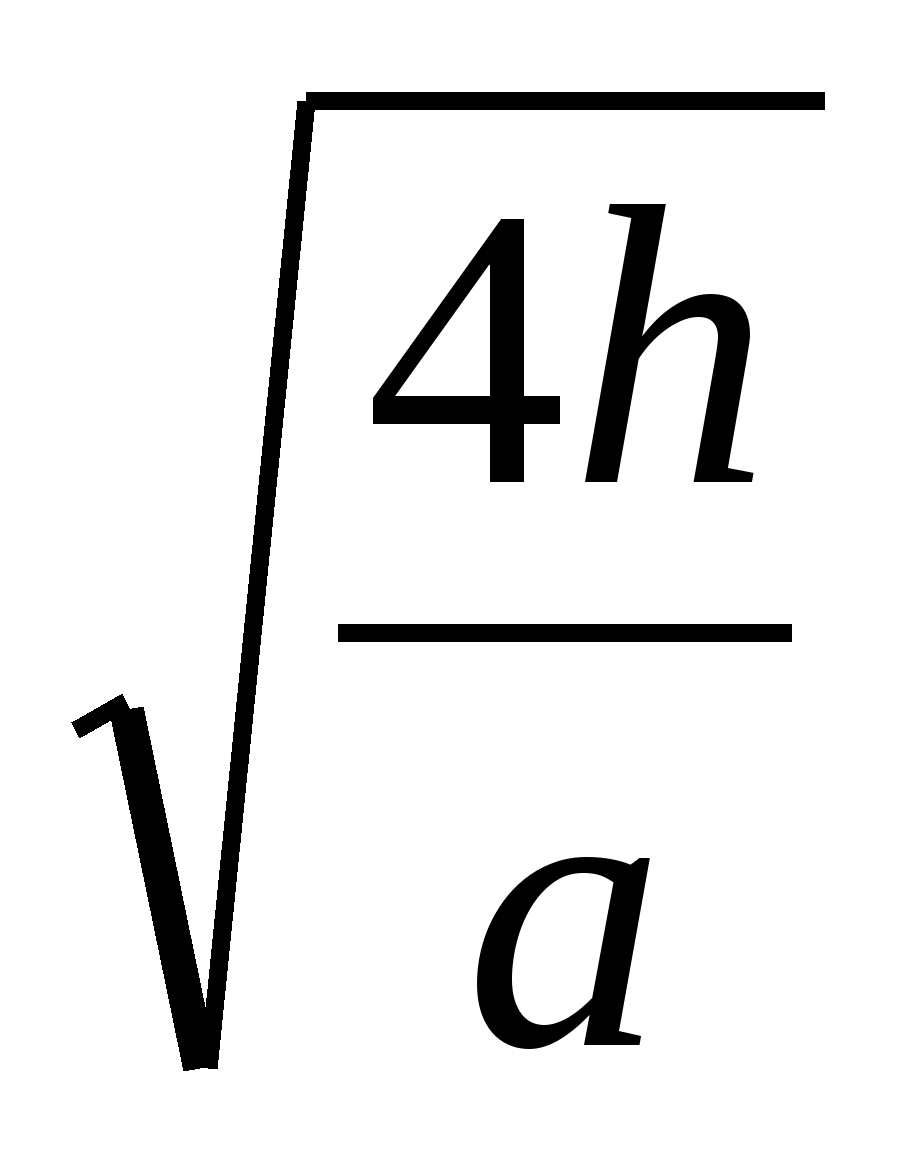 . 3. Perform mathematical transformations, get the answer in general form and, substituting these values - in numerical terms: t =
. 3. Perform mathematical transformations, get the answer in general form and, substituting these values - in numerical terms: t =  = 0.52 s.
= 0.52 s.
Task 47. A small, negatively charged body that has a chargeq = 10 -5 cl and massm= 20 g, begins to slide without friction on a non-conductive uncharged inclined plane, the angle of inclination of which is φ=30. The initial velocity of the body is zero. A horizontal non-conductive surface located under an inclined plane is positively charged and creates a vertically directed uniform field with intensity E = 10 4 V/m. Sliding time of the body from the top to the bottom of the planet= 0.4 s. What is the height of the inclined plane?
Answer:h=0.31 m
W 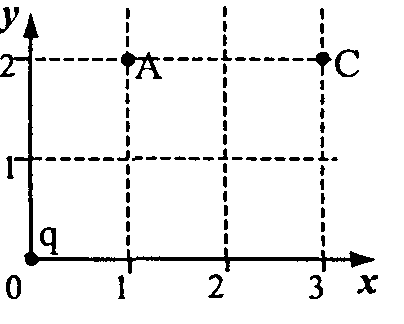 hell 48.point chargeq, placed at the origin, creates at point A (see figure) an electrostatic field with intensityE 1
= 65 V/m. What is the magnitude of the field strengthE 2
at point C?
hell 48.point chargeq, placed at the origin, creates at point A (see figure) an electrostatic field with intensityE 1
= 65 V/m. What is the magnitude of the field strengthE 2
at point C?
Key elements of the solution
1. Write down the formula for the modulus of the field strength of a point charge 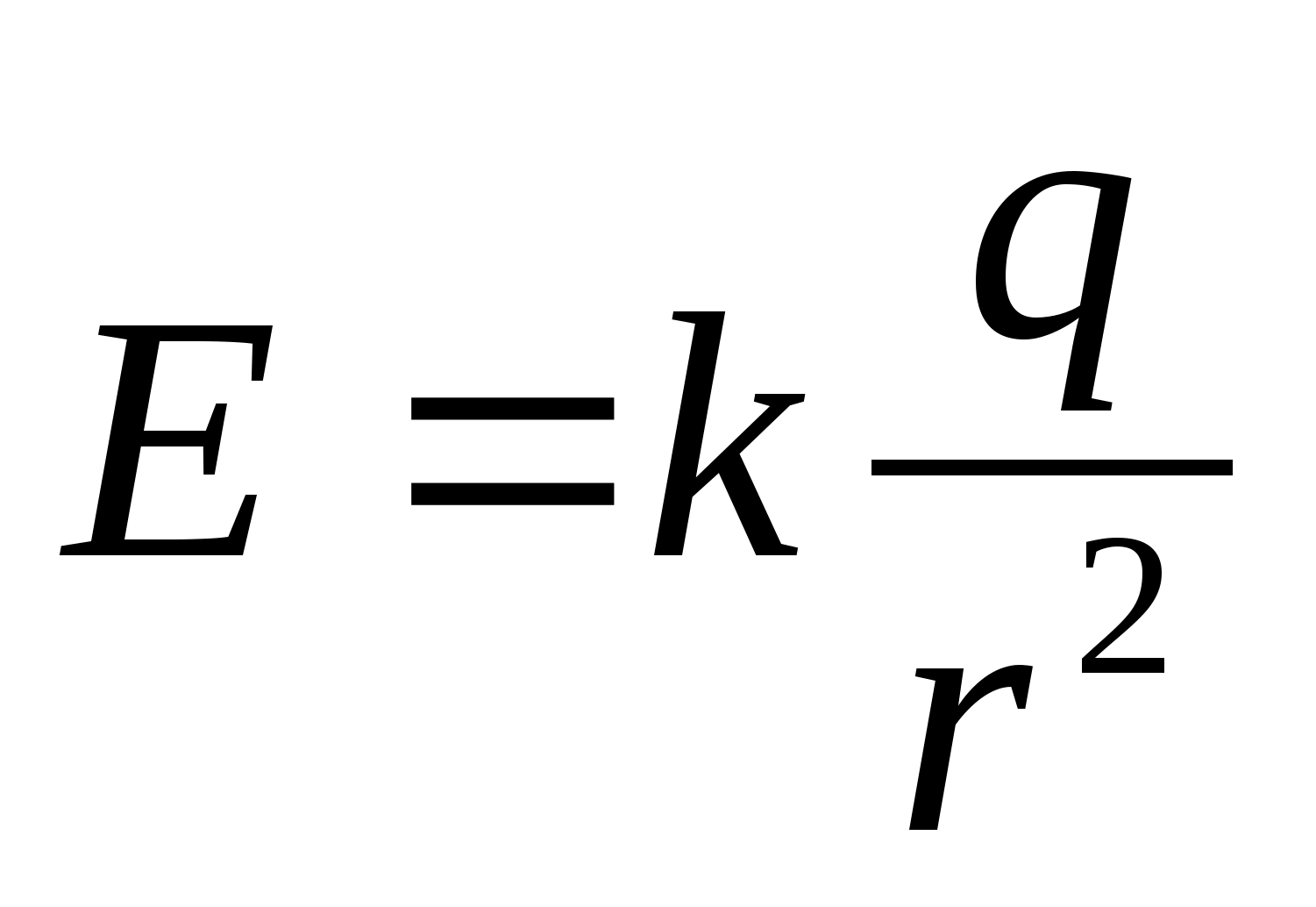 and the expression for E 2 in general form:
and the expression for E 2 in general form: 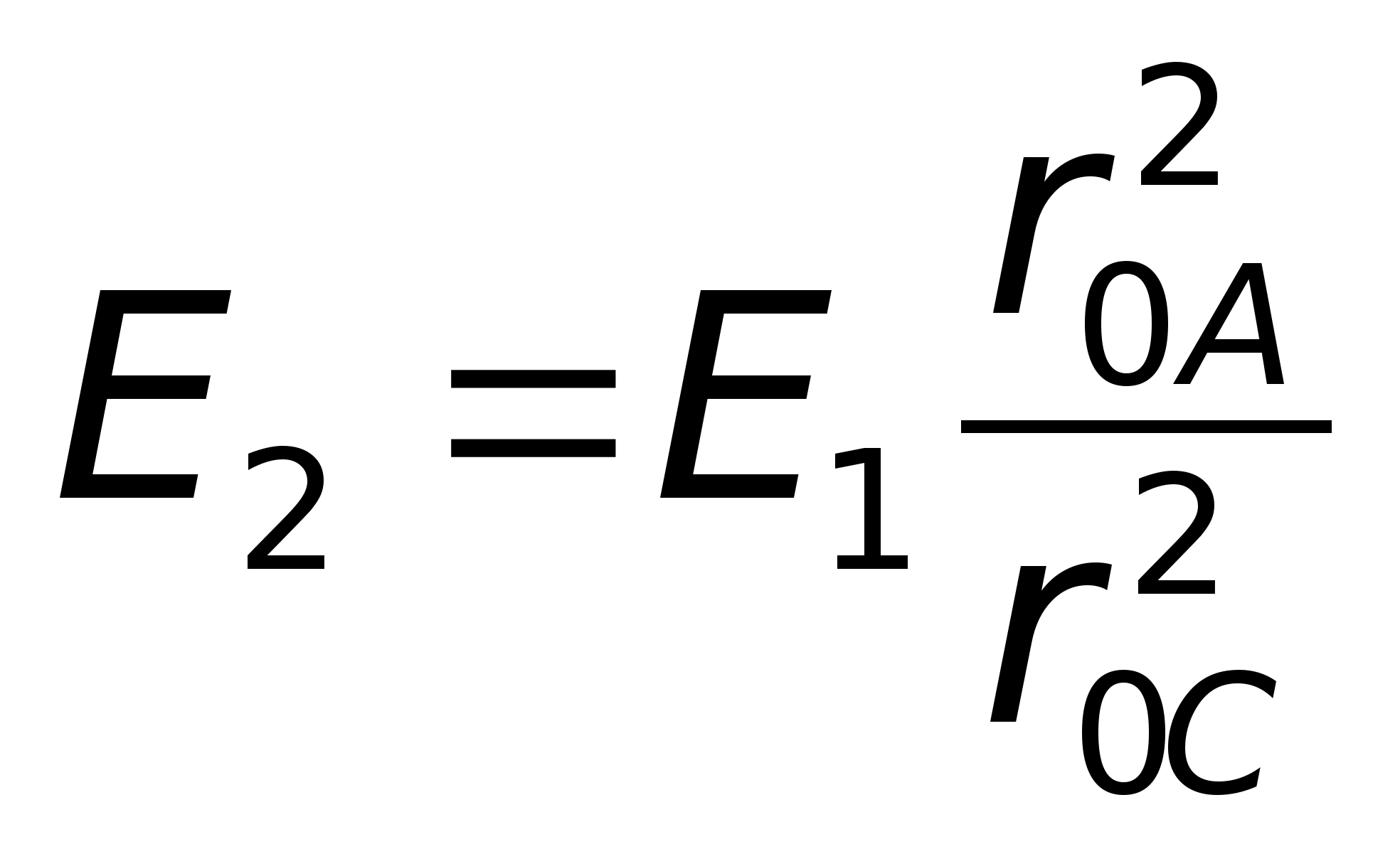 .
.
Calculate the squares of relative distances according to the scale given in the problem:
r 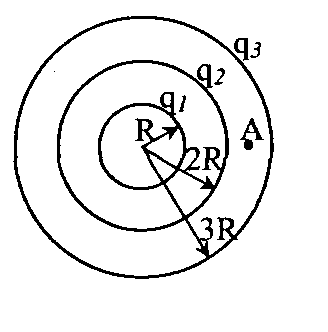 0 A 2 \u003d l l +2 2 \u003d 5 and r 0 C 2 \u003d 3 2 +2 2 \u003d 13, get a numerical answer: E 2 \u003d 25 V / m.
0 A 2 \u003d l l +2 2 \u003d 5 and r 0 C 2 \u003d 3 2 +2 2 \u003d 13, get a numerical answer: E 2 \u003d 25 V / m.
Problem 49. T point chargeqcreates at a distance R from it an electric field with potential 1 =10 V. Three concentric spheres with radiiR, 2 Rand 3Rhave charges evenly distributed over their surfacesq 1 = + 2 q, q 2 = - q andq 3 = + qrespectively (see figure). What is the potential of the field at point A, which is 2.5 R away from the center of the spheres?
Key elements of the solution
1. Consider that the contributions of the two inner spheres to the potential of the electric field at point A are equal, respectively  ,
,
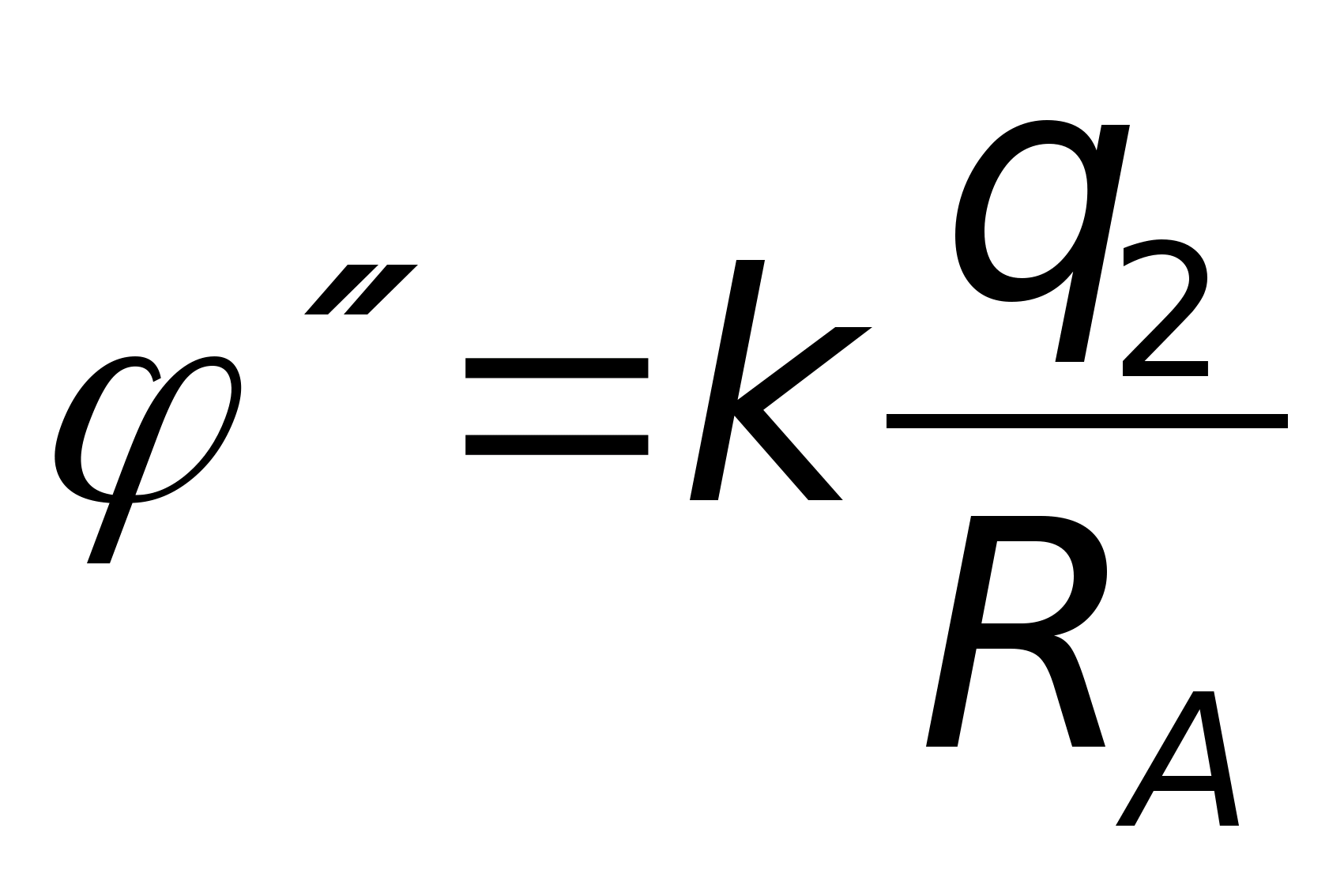 , where, according to the condition,
, where, according to the condition, 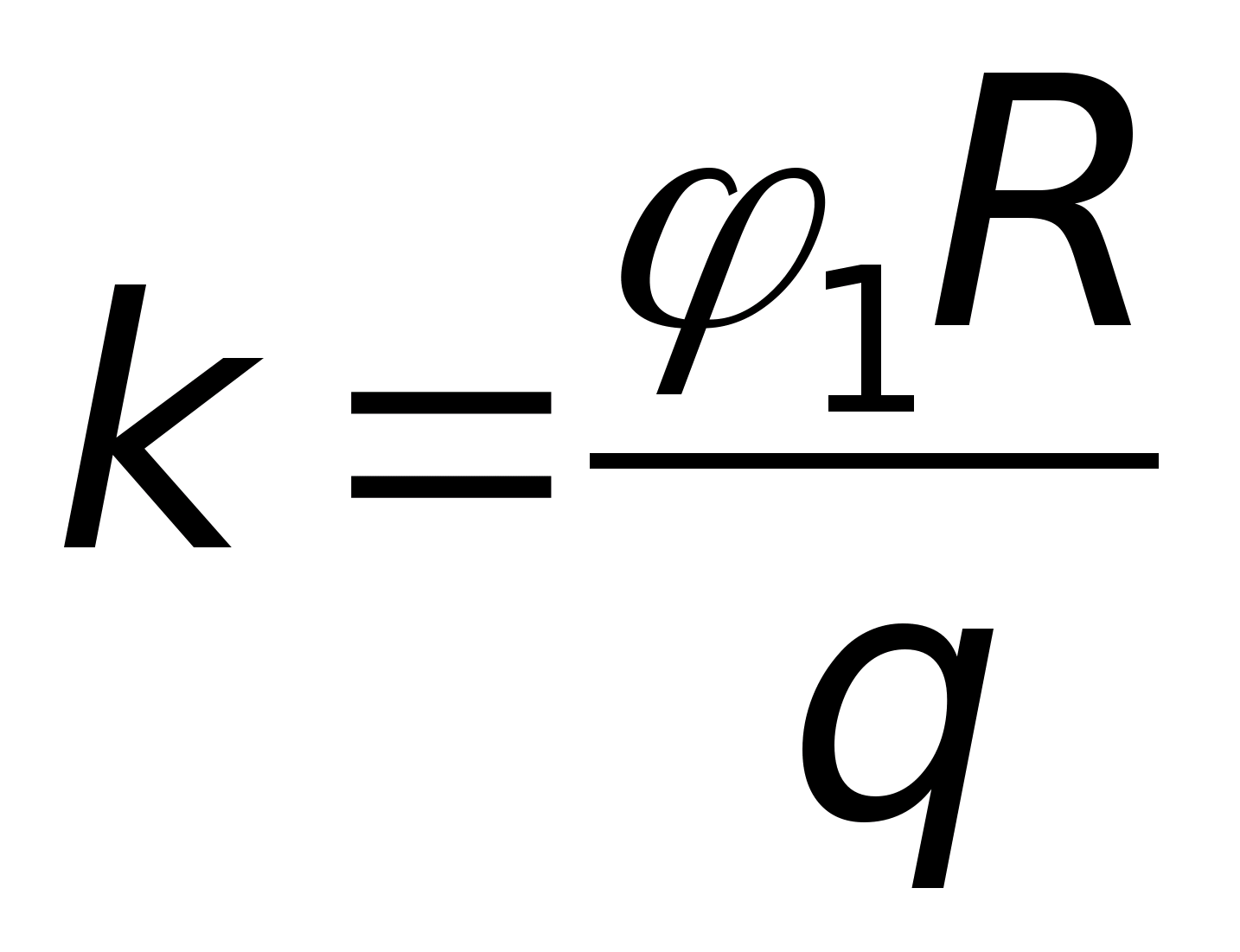 . 2. The contribution of the outer sphere to the potential of the electric field at point A is
. 2. The contribution of the outer sphere to the potential of the electric field at point A is 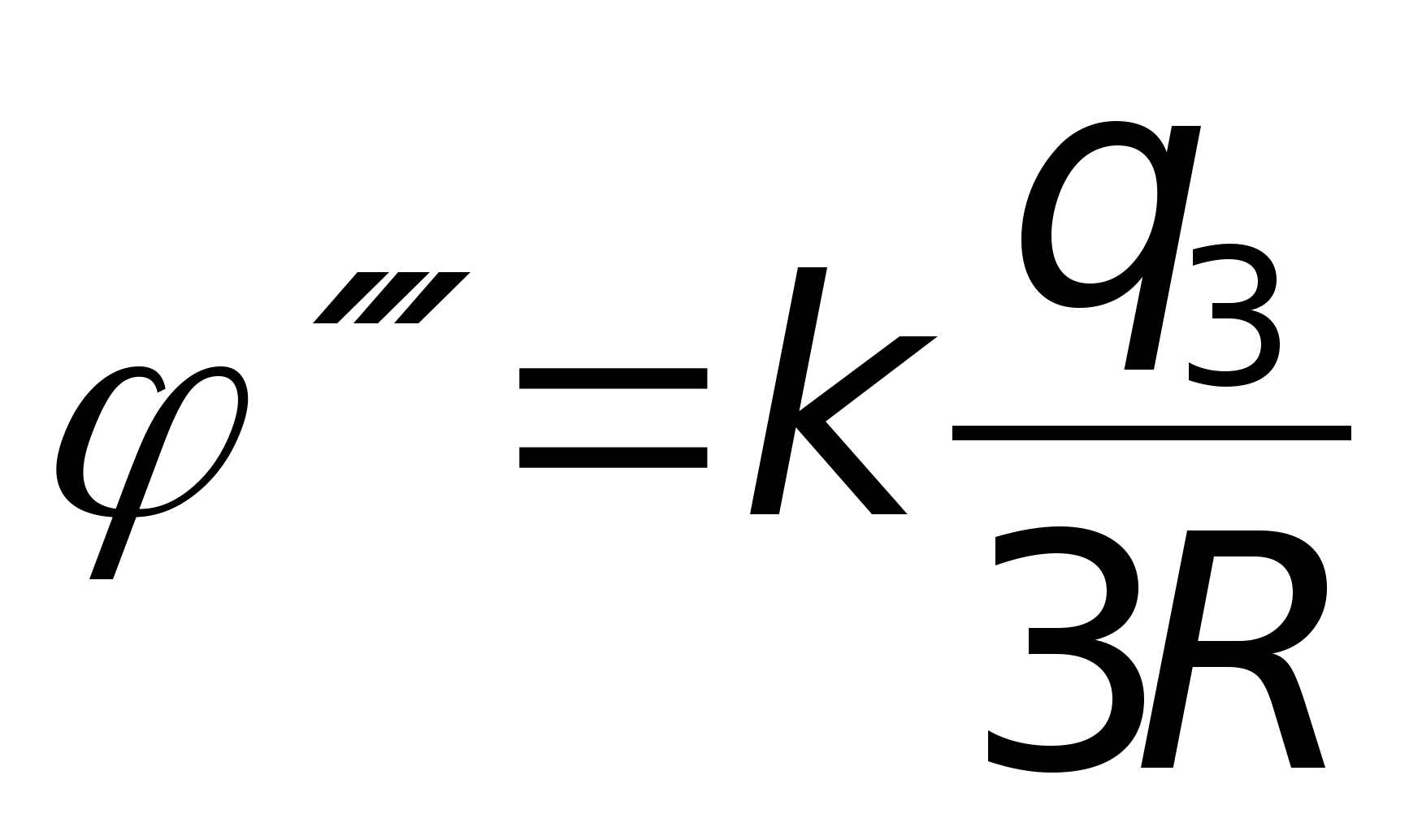 . Then, according to the principle of superposition, the potential of the field at point A is equal to
. Then, according to the principle of superposition, the potential of the field at point A is equal to 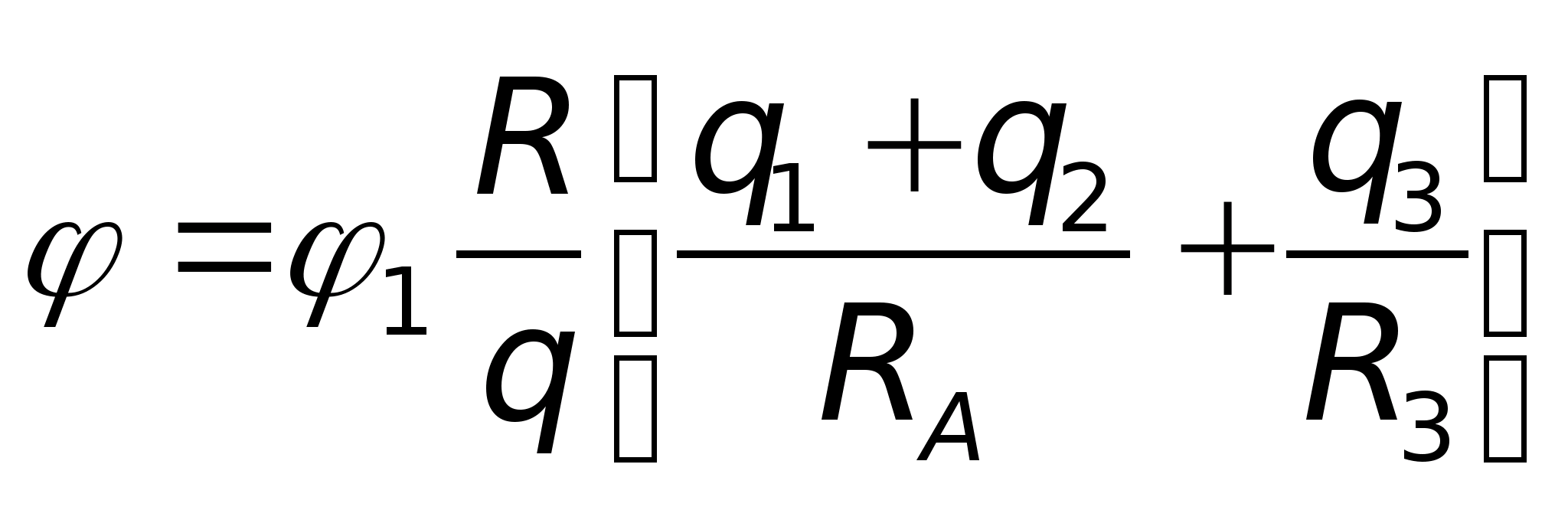 and taking into account these values A = 7.3 V. Answer:
BUT = 7.3 V.
and taking into account these values A = 7.3 V. Answer:
BUT = 7.3 V.
Task 50.A dielectric plate is placed in a uniform electric fieldnegligible thickness. The dielectric constantplate substance is equal to 2. The normal to the plate surfacemakes an angle of 60° with the electric field lines. question Document
Questions semester control work by ... Explain why for the manufacture of the core, anchors and yokes use magnetically soft ferromagnets. ... the relay system uses hard magnetic ferromagnets. 7 questions 2 points each 1 2 3 4 | 5 6 7 | 8 9 ...
A task. A heat-insulated vessel with a volume of V = 2 m 3 is divided by a porous partition into two equal parts. At the initial moment, in one part of the vessel there is He = 2 mol of helium, and in the other - Ar = 1 mol of argon. The helium temperature T He = 300 K, and the argon temperature T Ar = 600 K. Helium atoms can freely penetrate through the pores in the partition, but argon atoms cannot. Determine the helium temperature after thermal equilibrium has been established in the system. Solution.1. The vessel is thermally insulated, which means that the helium-argon system does not receive or give off heat. Q syst = 0 The walls of the vessel do not move, which means that the system does not perform work on the surrounding bodies (work = displacement force, no displacement - no work) A syst = 0 According to the first law of thermodynamics Q syst = U + A syst It turns out that for our system U = 0, i.e., the internal energy of the system does not change: U initial = U final 2. Internal energy of our system at the initial moment U initial is the sum of the energy of helium (3/2) He R T He and the energy of argon (3/2) Ar R T Ar (both gases are monatomic) U initial = (3/2) He R T He + (3/2) Ar R Т Ar 3. When thermal equilibrium is established, both gases will have the same temperature T (as a result of heat transfer, a single temperature will necessarily be established in the system) U final = (3/2) (He + Ar)R Т






