Molecular physics. Saturated and unsaturated pairs. Steam and steam condensate facilities of the enterprise
The table shows the thermophysical properties of water vapor on the saturation line depending on the temperature. Steam properties are given in the table in the temperature range from 0.01 to 370°C.
Each temperature corresponds to the pressure at which water vapor is in a state of saturation. For example, at a water vapor temperature of 200°C, its pressure will be 1.555 MPa, or about 15.3 atm.
The specific heat capacity of steam, thermal conductivity and its increase with increasing temperature. The density of water vapor also increases. Water vapor becomes hot, heavy and viscous, with a high specific heat capacity, which has a positive effect on the choice of steam as a heat carrier in some types of heat exchangers.
For example, according to the table, specific heat water vapor Cp at a temperature of 20°C it is equal to 1877 J/(kg deg), and when heated to 370°C, the heat capacity of steam increases to a value of 56520 J/(kg deg).
The table gives the following thermophysical properties of water vapor at the saturation line:
- vapor pressure at a specified temperature p 10 -5, Pa;
- vapor density ρ″ , kg / m 3;
- specific (mass) enthalpy h″, kJ/kg;
- heat of vaporization r, kJ/kg;
- specific heat capacity of steam Cp, kJ/(kg deg);
- coefficient of thermal conductivity λ 10 2, W/(m deg);
- thermal diffusivity a 10 6, m2/s;
- dynamic viscosity μ 10 6, Pa s;
- kinematic viscosity v 10 6, m2/s;
- Prandtl number Pr.
The specific heat of vaporization, enthalpy, thermal diffusivity and kinematic viscosity of water vapor decrease with increasing temperature. The dynamic viscosity and the Prandtl number of the steam increase in this case.
Be careful! The thermal conductivity in the table is given to the power of 10 2 . Don't forget to divide by 100! For example, the thermal conductivity of steam at a temperature of 100°C is 0.02372 W/(m deg).
Thermal conductivity of water vapor at various temperatures and pressures
The table shows the values of thermal conductivity of water and steam at temperatures from 0 to 700°C and pressure from 0.1 to 500 atm. The unit of thermal conductivity is W/(m deg).
The line below the values in the table means the phase transition of water to steam, that is, the numbers below the line refer to steam, and above it, to water. According to the table, it can be seen that the value of the coefficient and water vapor increases with increasing pressure.
Note: the thermal conductivity in the table is given to the power of 10 3 . Don't forget to divide by 1000!
Thermal conductivity of water vapor at high temperatures
The table shows the thermal conductivity values of dissociated water vapor in W/(m deg) at temperatures from 1400 to 6000 K and pressures from 0.1 to 100 atm.
According to the table, the thermal conductivity of water vapor at high temperatures increases markedly in the region of 3000...5000 K. At high pressures, the maximum thermal conductivity coefficient is achieved at higher temperatures.
Be careful! The thermal conductivity in the table is given to the power of 10 3 . Don't forget to divide by 1000!
Theme “Saturated couples. Humidity ”for schoolchildren is quite complicated. Little attention is paid to it in the lessons, and the considered phenomena of liquid-vapor interfacial interaction are unusual.
The basis for solving the above problems is the idea that the liquid and its saturated vapor are in a state of dynamic equilibrium, when the rates of evaporation and condensation are the same. Pressure and concentration of molecules saturated steam depends only on its temperature. Also, to calculate the characteristics of saturated steam, you can use the Mendeleev-Claiperon equation.
Usually in the exam, such tasks are rare. However, the tasks are very interesting and allow you to feel the differences between the behavior ideal gases and saturated vapors. In our opinion, having understood their solution, you will no longer experience any difficulties in understanding this topic.
Task number 1. Pressure, number of saturated vapor molecules in a closed vessel (part A, basic level)
Solution:
A typical situation: in a closed volume, vapor and liquid are in equilibrium. Even without a direct indication in the text of the problem, it is clear that the steam is saturated. The temperature does not change, which means that the concentration of vapor molecules is constant, the number and mass of molecules are proportional to volume. If the volume decreases by 3 times, the number of molecules will also decrease by 3 times.
Answer number 4.
Task number 2. Pressure, mass, concentration of saturated vapor molecules in a closed vessel (part A, basic level)

In fact, the same problem in a different formulation.
Task number 3. Psychrometer Readings (Part A, Baseline)

Solution:
The task is actually laboratory work to measure humidity with a psychrometer, you only need to predict the wet bulb reading.
To solve, we read the readings of thermometers: dry - 23, wet -16 degrees Celsius. According to the table, we determine that this corresponds to 48% humidity. If the humidity increases by 7% (up to 55%), then (we find it in the same line of the table, to the left) the difference in the readings of the thermometers will become 6 degrees. Therefore, wet will show 23-6=17 degrees. Answer number 3.
Task number 4. Saturated Vapor Pressure in a Closed Vessel (Part A, Elevated Level)

Solution:
In a closed volume, vapor and liquid are in equilibrium, therefore, vapor is saturated. As the volume increases, the water will evaporate and the steam will remain saturated for as long as possible. Obviously, all the water will evaporate and the steam will remain saturated until the volume doubles (since the mass of the liquid is initially equal to the mass of the vapor). In this case, the pressure does not change.
With a further increase in the volume of vapor will cease to be saturated, its parameters can be described in terms of isothermal process. An increase in volume by a further 2 times will lead to a twofold decrease in pressure.
In total, with an increase in volume by 4 times in this system, the pressure will decrease by 2 times.
Answer number 2.
In option No. 2 of trial testing, there was an inverse problem, but it could be solved only knowing that at 100 degrees Celsius, the vapor pressure is 100 kPa (1 atm), which is used in solving problem No. 6. This pressure value is easy to remember, knowing that the boiling of a liquid begins at a temperature at which its saturated vapor pressure becomes equal to the external pressure.
Task number 5. The dependence of pressure on volume for saturated and unsaturated vapors(part C, advanced level)

Task number 6. Water level in a sealed tube with saturated vapors (part C, elevated level)
A wide glass tube about half a meter long, sealed at one end, is completely filled with water and placed vertically with the open end down, immersing the bottom of the tube a few centimeters in a basin of water. At room temperature, the tube remains completely filled with water. The water in the basin is slowly heated. Where will the water level in the tube be set when the water in the basin begins to boil? Explain your answer using physical laws.
Solution.
At room temperature, water occupies the entire volume of the tube and does not pour out of it, because the pressure of saturated water vapor at room temperature is very low and a “Torricellian void” filled with saturated water vapor will appear above the water only if the height of the water column is about 10 meters.
As the temperature of water rises, the pressure of its saturated vapor increases until, at the boiling point, it becomes equal to the external atmospheric pressure.
When the temperature approaches the boiling point, an area filled with saturated water vapor will appear above the water. As the temperature rises further, the water level in the tube will decrease. At the boiling point, the pressure of saturated water vapor in the tube is equal and atmospheric pressure, so the water level in the tube and in the basin will be the same.
Task number 7. Mass of saturated steam (part A, baseline)

Solution:
The problem is easily solved using the Mendeleev-Claiperon equation, given that the saturated vapor pressure at a given temperature is equal to atmospheric
During evaporation, simultaneously with the transition of molecules from liquid to vapor, the reverse process also occurs. Randomly moving above the surface of the liquid, some of the molecules that left it return to the liquid again.
Saturated steam pressure.
When saturated vapor is compressed, the temperature of which is maintained constant, the equilibrium will first begin to be disturbed: the vapor density will increase, and as a result, more molecules will pass from gas to liquid than from liquid to gas; this will continue until the vapor concentration in the new volume becomes the same, corresponding to the concentration of saturated vapor at a given temperature (and the equilibrium is restored). This is explained by the fact that the number of molecules leaving the liquid per unit time depends only on temperature.
So, the concentration of saturated vapor molecules at constant temperature does not depend on its volume.
Since the pressure of a gas is proportional to the concentration of its molecules, the pressure of a saturated vapor does not depend on the volume it occupies. Pressure p 0, at which the liquid is in equilibrium with its vapor, is called saturated steam pressure.
When saturated steam is compressed, most of it is converted into liquid state. A liquid occupies a smaller volume than a vapor of the same mass. As a result, the volume of vapor at a constant density decreases.
Dependence of pressure of saturated vapor on temperature.
For an ideal gas, linear dependence pressure versus temperature at constant volume. Applied to saturated steam with pressure p 0 this dependence is expressed by the equality:
p 0 =nkT.
Since the pressure of saturated vapor does not depend on volume, then, therefore, it depends only on temperature.
Experimentally determined dependence p 0 (T) different from dependence ( p 0 =nkT) for ideal gas.
With increasing temperature, the pressure of saturated vapor increases faster than the pressure of an ideal gas (section of the curve AB on the image). This becomes especially obvious if we draw an isochore through the point A(dashed line). This happens because when the liquid is heated, part of it turns into vapor, and the vapor density increases. Therefore, according to the formula ( p 0 =nkT), the pressure of saturated vapor increases not only as a result of an increase in the temperature of the liquid, but also due to an increase in the concentration of molecules (density) of the vapor. The main difference in the behavior of an ideal gas and saturated steam is the change in the mass of steam with a change in temperature at a constant volume (in a closed vessel) or with a change in volume at a constant temperature. Nothing like this can happen with an ideal gas (the molecular-kinetic theory of an ideal gas does not provide for phase transition gas to liquid).
After the evaporation of all the liquid, the behavior of the vapor will correspond to the behavior of an ideal gas (section sun curve in the figure above).
unsaturated steam.
If in a space containing the vapor of a liquid, further evaporation of this liquid can occur, then the vapor in this space is unsaturated.
A vapor that is not in equilibrium with its liquid is called unsaturated.
Unsaturated vapor can be converted into a liquid by simple compression. Once this transformation has begun, the vapor in equilibrium with the liquid becomes saturated.
Page 1 of 5
7.1. Table 8 gives the pressure of water vapor saturating the space at different temperatures. How to make a table from this data m masses of water vapor in volume V \u003d 1 m 3 air saturated with water vapor at different temperatures For example, solve the problem at a temperature t\u003d 50 ° C.
7.2. Find the density p and saturated water vapor at temperature t=50°C.

7.3. How many times the density p and saturated water vapor at a temperature t= 16°C less density p water.

7.4. How many different density p and 1 of saturated water vapor at a temperature t 1 \u003d 200 ° C is greater than the density p n2 saturated water vapor at a temperature t 1 \u003d 100 ° C?

7.5. What is the mass m water vapor contained in the volume V\u003d 1m 3 of air on a summer day at a temperature of t \u003d 30 ° C and relative humidity w = 0,75 ?

7.6. In a closed space V= 1 m 3 relative humidity air w = 0.6 at temperature t\u003d 20 ° C. What is the mass d m water must still be evaporated from this volume in order for the water vapor to become saturated?

7.7. Room temperature / g = 18 ° C, relative humidity co = 0.5 . Cold water was poured into a metal kettle, what is the temperature tz water, at which the kettle will stop fogging?


7.8. Find a number n molecules of saturated water vapor contained in a unit volume at a temperature t 1 = 30 ° C.

7.9. Mass m=0.5 g of water vapor occupies the volume V 1\u003d 10 l at a temperature of t \u003d 50 ° C, what is the relative humidity w? What mass dm of vapor will be condensed if the volume is isothermally reduced from V 1 to V 2 \u003d V 1 / 2?


7.10. A cloud chamber with a volume of K = 1 liter contains air saturated with water vapor. Initial temperature chamber r = 20 ° C. When the piston moved, the volume of the chamber increased to
V 2 = 1.25k, . The expansion is considered adiabatic, and the adiabatic exponent is 1.4. Find: a) water vapor pressure before expansion; b) weight m 1 water vapor in the chamber before expansion; c) density p 1 of water vapor before expansion; d) temperature t2 steam after expansion (neglect temperature changes due to heat release during steam condensation); e) mass dm condensed steam; f) density p2 water vapor
after condensation; g) the degree of supersaturation, i.e. the ratio of the density of water vapor after expansion (but before condensation) to the density of water vapor saturating the space at the temperature established after condensation


7.11. Find the specific volume v of water in the liquid and vapor states under normal conditions.
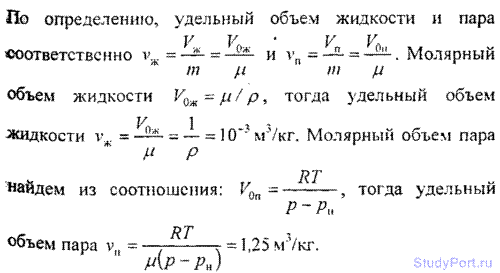
7.12. Using the first law of thermodynamics and the data in Table 7 and 8, find the specific heat of vaporization r water at t = 200 ° C. For water, the critical temperature T to= 647 K, critical pressure p= 22 MPa. Check the correctness of the result obtained according to table 9.
7.13. What part of the heat of vaporization of water at a temperature of t \u003d 100 ° C goes to increase internal energy systems?

7.14. The specific heat of vaporization of benzene (C 6 H 6) at a temperature t \u003d 77 ° C is equal to r = 398 kJ/kg. Find the change in internal energy d W during evaporation of the mass dm = 20 g of benzene.

7.15. Using the Clausius-Clapeyron equation and the data in Table 8, find the specific heat of vaporization r
water at a temperature t = 5 ° C. Check the correctness of the result obtained according to table 9.


7.16. Saturated mercury vapor pressure at temperatures t 1 \u003d 100 ° C and t 2 = 120 ° C are p 1 \u003d 37.3 Pa and p 2 \u003d 101.3 Pa.
Find the mean specific heat vaporization r mercury in the specified temperature range.


7.17. The boiling point of benzene (C 6 H 6) at a pressure p \u003d 0.1 MPa is t k \u003d 80.2 ° C. Find the pressure p saturated benzene vapor at a temperature t = 15.6° C. The average value of the specific heat of vaporization of benzene in this temperature range is taken equal to r = 0.4 MJ/kg.

7.18. The pressure of saturated vapor of ethyl alcohol (C 2 H 5 OH) at temperatures t 1 \u003d 40 ° C and t 2 \u003d 60 ° C are equal
p 1 =17.7 kPa and p 2 =67.9 kPa. Find the change in entropy dS during the evaporation of mass d m = 1 G ethyl alcohol at a temperature t\u003d 50 ° C.

7.19. The change in entropy during the evaporation of the amount dv \u003d 1 mol of some liquid at a temperature of t 1 \u003d 50 ° C is dS \u003d 133J / K. Saturated vapor pressure at a temperature t 1 = 50 ° C is p 1 = 12.33 kPa. By how much does the saturated vapor pressure of a liquid change with a change in temperature from t1 = 50° C to t1\u003d 51 0 C?


7.20. To what pressure limit p can the vessel be evacuated using a mercury diffusion pump operating without a mercury trap if the temperature of the water jacket of the pump is t = 15°C? The pressure of saturated mercury vapor at a temperature t 0 \u003d 0 ° C is p0\u003d 0.021 Pa, the average value of the specific heat of vaporization of mercury in this temperature range is taken equal to r = 10.08 MJ/kt.
DEFINITION
water vapor- this is gaseous state water.
It has no color, no taste, no smell. Water is the most common substance in nature. In addition to existing as a gas, it can also be in a liquid or solid (ice) state, each of which is determined by temperature and pressure (Fig. 1).
Rice. 1. Diagram of the state of water.
The AO curve corresponds to equilibrium in the ice-steam system, DO - to the equilibrium in the supercooled water-steam system, the OC curve - to the equilibrium in the water-steam system, and the OB curve - to the equilibrium in the ice-water system. At point O, all curves intersect. This point is called triple point and corresponds to equilibrium in the ice-water-steam system.
The empirical formula of water vapor coincides with the empirical formula of water and has the form 2 O. As is known, the molecular weight of a molecule is equal to the sum of the relative atomic masses atoms that make up the molecule (the values of relative atomic masses taken from the Periodic Table of D.I. Mendeleev are rounded to integers).
Mr(H 2 O) = 2×Ar(H) + Ar(O);
Mr(H 2 O) \u003d 2 × 1 + 16 \u003d 2 + 16 \u003d 18.
Molar mass (M) is the mass of 1 mole of a substance. It is easy to show that the numerical values of the molar mass M and the relative molecular mass M r are equal, however, the first value has the dimension [M] = g/mol, and the second is dimensionless:
M = N A × m (1 molecules) = N A × M r × 1 a.m.u. = (N A ×1 amu) × M r = × M r .
It means that molar mass water vapor is 18 g/mol.
Examples of problem solving
EXAMPLE 1
| Exercise | How many grams of zinc reacted with hydrochloric acid if 0.5 g of hydrogen gas was formed? |
| Solution | We write the reaction equation for the interaction of zinc with hydrochloric acid: Zn + 2HCl \u003d ZnCl 2 + H 2. Let's find the molar mass of molecular hydrogen (the value of the relative atomic mass, taken from the Periodic Table of D.I. Mendeleev, will be rounded up to an integer). It is known that M \u003d Mr, which means (H 2) \u003d 2 × Ar (H) \u003d 2 × 1 \u003d 2 g / mol. Calculate the amount of hydrogen substance: n(H)=m(H)/M(H); n (H) \u003d 0.5 / 2 \u003d 1 mol. According to the reaction equation n (H 2) : n (Zn) \u003d 1: 1, which means that n (Zn) \u003d 1 mol. Let's find the molar mass of zinc (the value of the relative atomic mass, taken from the Periodic Table of D.I. Mendeleev, rounded up to an integer). It is known that M = Mr, which means (Zn) = 65 g/mol. Determine the mass of zinc: m(Zn) = n (Zn) × M (Zn); m(Zn) = 1 × 65 = 65 g. |
| Answer | The mass of zinc is 65 g. |
EXAMPLE 2
| Exercise | What mass of copper (II) oxide is needed to obtain 128 g of copper from it when reduced with hydrogen? |
| Solution | We write the equation for the reduction of copper (II) oxide with hydrogen: CuO + H 2 \u003d Cu + H 2 O. Let's find the molar mass of copper (the value of the relative atomic mass, taken from the Periodic Table of D.I. Mendeleev, rounded up to an integer). It is known that M = Mr, which means (Cu) = 64 g/mol. Calculate the amount of copper substance: n(Cu)= m(Cu)/M(Cu); n (Cu) \u003d 128/ 64 \u003d 2 mol. According to the reaction equation n(Cu) :n(CuO) = 1: 1, so n(CuO) = 2 mol. Let's find the molar mass of copper (II) oxide (the values of relative atomic masses taken from the Periodic Table of D.I. Mendeleev are rounded to integers). As is known, the molar mass of a molecule is equal to the sum of the relative atomic masses of the atoms that make up the molecule (M = Mr): M(CuO) = Ar(Cu) + Ar(O) = 64 + 16 = 80 g/mol. Let's determine the mass of copper oxide (II): m(CuO) = n(CuO) × M(CuO); m(CuO) \u003d 2 × 80 \u003d 160 g. |
| Answer | The mass of copper (II) oxide is 160 g |






