Gas laws for an ideal gas isothermal process
What is equal to cycle efficiency conducted with an ideal monatomic gas? Give your answer as a percentage, rounded to the nearest whole number.
Solution.
The efficiency of a heat engine is defined as the ratio of the useful work and the heat transferred to the working fluid per cycle: Let us first determine the useful work per cycle, in the diagram this value corresponds to the cycle area: Calculate the heat transferred to the gas using the first law of thermodynamics: Consider successively all sections of the cycle. In section 1 - 2, the gas does no work, and the change in its internal energy (taking into account the Clapeyron-Mendeleev equation) is: Since the change in internal energy is positive, the gas receives heat in this section. In section 2 - 3, the gas does work. The change in its internal energy in this section: Therefore, in this section, the gas receives heat. In section 3 - 1, the gas does negative work, it cools, which means that it internal energy decreases, therefore, in this area it gives off heat, but does not receive it. Finally, all the heat received by the gas during the cycle is equal to Thus, the efficiency of the cycle is equal to
Answer: 10.
Answer: 10
Guest 12.01.2013 20:25
Guest
Good afternoon!
You can use any formula, depending on what is convenient for you in this particular task. In this problem, the cycle goes clockwise, therefore, the gas does positive work, so it may be more convenient to use what is used :)
Guest 22.09.2013 13:31
Alexei! Congratulations. You once again "invented" a perpetual motion machine of the second kind. Pay attention to the fact that in the condition of the problem it is indicated that the gas is monatomic.
If you do the same calculations with a diatomic gas, then the value of the efficiency will be different, which contradicts the first Carnot theorem, which says: "The efficiency of a reversible cycle does not depend on the type of substance from which the working fluid is made."
I would like to make one remark about your "theses". One of them says: "A quasi-static (slow) process is reversible." According to him, if the diesel engine is slowly turned in the opposite direction, then diesel fuel will flow into the fuel side, and purified air will come out of the air filter !!! After all, according to your thesis, everything should return to its original position. Do you really believe this nonsense?!
Alexei
Good afternoon!
I think this debate is endless. My thesis is the following, I will try to convey it again: “If a point is given on some diagram (), then the state of the system is completely given and it is in an equilibrium state (we believe that we know the equation of state). If the system is not in equilibrium, then a point in such diagrams does not make sense at all. Further, when a line is drawn on the diagram, it is a sequence of equilibrium states through which the system passes continuously, quasi-statically. The system can be moved along the line in different directions. "
As for the Carnot theorem you refer to, it seems to me that you are missing the essential fact that it is formulated for the Carnot cycle when there is a heater at one temperature and a refrigerator at another. For the Krno cycle, everything turns out as you say. But you can think of a huge bunch of convertible machines other than Carnot's machine. For example, it is possible to construct a cycle with three temperatures from adiabats and isotherms. Further generalization gives an arbitrary curve. I already told you that any line can be built from adiabats and isotherms. I hope you have no doubts about their reversibility.
Your engine example doesn't fit into this picture, of course. The process of converting fuel into heat with the ejection of combustion products cannot be reversed, no matter how hard you try.
Tasks С1–С6 are tasks, the full solution of which must be recorded in the answer form No. 2. It is recommended to carry out a preliminary solution on a draft. When making a decision in the answer form No. 2, first write down the task number ( C1 etc.), and then solving the corresponding problem.
C1. Near a small metal plate, mounted on an insulating stand, a light metal unloaded shell was hung on a silk thread. When the plate was connected to the terminal of the high voltage rectifier by applying positive charge, the sleeve is in motion. Describe the movement of the sleeve and explain it, indicating what physical phenomena and patterns it is caused by.
A complete correct solution to each of the problems С2–С6 should include laws and formulas, the application of which is necessary and sufficient to solve the problem, as well as mathematical transformations, calculations with a numerical answer, and (if necessary) a figure explaining the solution.
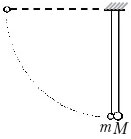
C2. Two balls, the masses of which differ by a factor of 3, hang, touching, on vertical threads (see figure). A light ball is deflected through an angle of 90° and released without initial velocity. What will be the attitude kinetic energies heavy and light balls immediately after their absolutely elastic central impact?

C3. A monatomic ideal gas of constant mass goes through the cyclic process shown in the figure. During the cycle, the gas receives an amount of heat from the heater Q n = 8 kJ. What is the work done by the gas per cycle?
C4. Electrical circuit consists of a current source and a rheostat. source emf= 6 V, its internal resistance r= 2 ohm. The resistance of the rheostat can be changed from 1 to 5 ohms. What is the maximum power output of the rheostat?
C5. A copper ring with a diameter of 20 cm, a ring wire diameter of 2 mm, is located in a uniform magnetic field. The ring plane is perpendicular to the magnetic induction vector. Determine the modulus of the rate of change of the magnetic induction of the field with time, if in this case a induction current 10 A. Resistivity copper ρ Cu \u003d 1.72 10 -8 Ohm m.
C6. The energy levels of an electron in a hydrogen atom are given by the formula where n= 1, 2, 3, … . When an atom passes from the state E 2 per state E 1 atom emits a photon. Once on the surface of the photocathode, a photon knocks out a photoelectron. The wavelength of light corresponding to the red border of the photoelectric effect for the surface material of the photocathode, λcr = 300 nm. What is the maximum possible speed of a photoelectron?
Instructions for checking and evaluating works, part 3
Task solutions С1–С6 part 3 (with a detailed answer) are evaluated by an expert commission. Based on the criteria presented in the tables below, for the completion of each task, depending on the completeness and correctness of the answer given by the student, from 0 to 3 points are assigned.
Attention! When assigning points for completing a task in the “Protocol for checking answers to tasks of form No. 2”, it should be borne in mind that if there is no answer (there are no records indicating that the examinee started to complete the task), then “× ", not "0".
A task C1
Sample possible solution |
|
1) The sleeve will be attracted to the plate, touch it, and then bounce and hang in a deflected state. 2) Under the action electric field plate, the distribution of electrons in the sleeve will change and it will be electrified: that side of it, which is closer to the plate (left), will have negative charge, and the opposite side (right) is positive. Since the force of interaction of charged bodies decreases with increasing distance between them, the attraction to the plate of the left side of the sleeve will be greater than the repulsion of the right side of the sleeve. The sleeve will be attracted to the plate and move until it touches it. 3) At the moment of contact, part of the electrons will pass from the sleeve to the positively charged plate, the sleeve will acquire a positive charge and repel from the now identically charged plate. |
|
4) Under the action of the repulsive force, the sleeve will deviate to the right and hang in a position where the resultant force of electrostatic repulsion, gravity and thread tension becomes equal to zero. |
|
A complete correct solution is given, including the correct answer (in this case - description of the movement of the sleeve, item 1), and a complete correct explanation (in this case - paragraphs 2–4) indicating the observed phenomena and laws (in this case - electrification in an external field and in contact with a charged body, the interaction of charged bodies). |
|
The solution is given and the correct answer is given, but there is one of the following shortcomings: – The explanation contains only general reasoning without reference to the specific situation of the problem, although all the necessary physical phenomena and laws. - The reasoning leading to the answer is not presented in full or contains logical flaws. - Not all physical phenomena and laws necessary for a complete correct solution are indicated. |
|
alone from the following cases: - Reasoning is given with an indication of physical phenomena and laws, but an incorrect or incomplete answer is given. - Reasoning is given with an indication of physical phenomena and laws, but the answer is not given. - Only the correct answer is presented without justification. |
|
A task C2
Example of a possible solution |
|
conservation law mechanical energy on impact: The law of conservation of momentum upon impact: mυ = mυ′ + MV. (2) Solving the system of equations (1), (2) taking into account the condition |
|
M = 3m, we get Answer: |
|
Criteria for assessing the performance of the assignment |
|
law of conservation of mechanical energy, law of conservation of momentum); |
|
The presented solution contains item 1 of the complete solution, but has one of the following shortcomings: - AT necessary Mathematical transformations or calculations made an error. – The necessary mathematical transformations and calculations are logically correct, do not contain errors, but are not completed. – The transformations leading to the answer are not presented, but the correct numerical answer is written or the answer is in general view. – The solution contains an error in the necessary mathematical transformations and has not been brought to a numerical answer. |
|
Entries corresponding to alone from the following cases: - Only provisions and formulas expressing physical laws are presented, the application of which is necessary to solve the problem, without any transformations with their use, aimed at solving the problem, and the answer. – The solution is missing ONE of the original formulas, necessary to solve the problem (or the statement underlying the solution), but there are logically correct transformations with the available formulas aimed at solving the problem. – In ONE of the initial formulas necessary for solving the problem (or the statement underlying the solution), |
|
an error, but there are logically correct transformations with the available formulas aimed at solving the problem. |
|
All cases of decision that do not meet the above criteria for scoring 1, 2, 3 points. |
|
A task C3
Example of a possible solution |
During the cycle, the amount of heat received by the gas from the heater: |
A complete correct solution is given, including the following elements: 1) formulas expressing physical laws are correctly written, the application of which is necessary to solve the problem in the chosen way (in this solution - first law of thermodynamics, Clapeyron–Mendeleev equation, formula for gas work); 2) the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed. |
A task C4
Example of a possible solution |
 Power released on the rheostat: R = IU = I (– Ir). Equation roots I (– Ir) = 0: I 1 = 0, I 2 = /r. Therefore, the maximum of the function P(I) is achieved at I = /(2r) and is equal to
Answer: Pmax= 4.5 W. |
Criteria for evaluating task completion by 3 points |
A complete correct solution is given, including the following elements: 1) formulas expressing physical laws are correctly written, the application of which is necessary to solve the problem in the chosen way (in this solution - Ohm's law for complete chain and the formula for current power); 2) the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed. |
A task C5
Example of a possible solution |
EMF induction in the ring Change magnetic flux in time ∆ t equals ΔΦ = Δ( BS), where S = π D 2/4 (ring area) is constant. On the other hand, according to Ohm's law, |
|
Criteria for evaluating task completion by 3 points |
| A complete correct solution is given, including the following elements: 1) formulas expressing physical laws are correctly written, the application of which is necessary to solve the problem in the chosen way (in this solution - Faraday's law, Ohm's law, formula for the resistance of a long thin conductor, formula for magnetic flux); 2) the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed. |
A task C6
Criteria for evaluating task completion by 3 points |
A complete correct solution is given, including the following elements: 1) formulas expressing physical laws are correctly written, the application of which is necessary to solve the problem in the chosen way (in this solution - expression for the energy of a photon emitted by a hydrogen atom during the transition from an excited state to the ground state; Einstein's equation for the photoelectric effect); 2) the necessary mathematical transformations and calculations are carried out, leading to the correct numerical answer, and the answer is presented. In this case, the solution "in parts" (with intermediate calculations) is allowed. |
Criteria for assessing the performance of tasks С3–C6 for 2, 1 and 0 points are the same as for the task C2.- Ed.
2008
Vertically located closed cylindrical vessel with height 50 cm divided by movable piston weight 110 N into two parts, each containing the same amount of hydrogen at a temperature 361 K. What mass of gas is in each part of the cylinder if the piston is at a height 20 cm from the bottom of the vessel? Ignore the thickness of the piston.
A column of mercury with a length of 15 cm, which separates the air in the tube from the atmosphere. The tube was placed vertically with the sealed end down. By how many degrees should the air in the tube be heated so that the volume occupied by the air becomes the same? Air temperature in the laboratory 300 K, and the atmospheric pressure is 750 mmHg
In the calorimeter was m 1 \u003d 1 kg ice at temperature t 1 \u003d -5 ° С. After adding to the calorimeter m 2 \u003d 25 g water in it is in thermal equilibrium at a temperature t = 0°С. What is the temperature t2 water added to the calorimeter, if only ice ended up in the calorimeter? Ignore the heat capacity of the calorimeter.
The heated vessel was covered with a piston, which was connected to the ceiling with the help of a vertical inextensible thread. By what percentage of the initial temperature will the air in the vessel decrease by the moment when the vessel comes off the surface on which it is located? Vessel weight 5 kg. The piston can slide along the walls of the vessel without friction. Vessel bottom area 125 cm2. Atmosphere pressure 10 5 Pa. Ignore the thermal expansion of the vessel and piston.
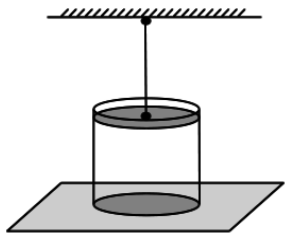
A balloon whose shell has mass M= 145 kg and volume V\u003d 230 m 3, filled with hot air at normal atmospheric pressure and ambient temperature t o \u003d 0 o C. What is the minimum temperature t must have air inside the shell for the balloon to start rising? The shell of the ball is inextensible and has a small hole at the bottom.
10 mol monoatomic ideal gas first cooled, reducing the pressure by 3 times, and then heated to the initial temperature of 300 K (see figure). How much heat was received by the gas in section 2 - 3?
year 2009
The constant mass of a monatomic ideal gas performs the cyclic process shown in the figure. For a cycle from the heater, the gas receives the amount of heat Q H = 8 kJ. What work is done external forces during the transition of the gas from state 2 to state 3?

2010
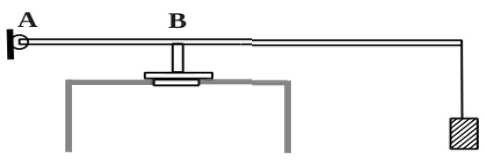
Air is pumped into the cylinder at a speed 0.002 kg/s. At the top end of the cylinder there is a hole with area 5 10 -4 m 2 closed by a safety valve. The valve is held closed by a weightless rod of length 0.5 m, which can freely rotate around an axis at a point BUT(see picture). Distance AB equals 0.1 m. A mass of mass is suspended from the free end of the rod 2 kg. The valve opens through 580 s operation of the pump, if at the initial moment of time the air pressure in the cylinder was equal to atmospheric. The air temperature in the cylinder and outside does not change and is equal to 300 K. Determine the volume of the cylinder.
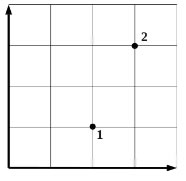
In the physics classroom, experiments were carried out with a rarefied gas of constant mass. Due to inattention, the student, having marked the initial and final states of the gas on the graph (see figure), did not indicate which two of the three quantities (pressure p, volume V, temperature T) are plotted along the axes. There was an entry in the journal, according to which the named values changed as follows: p 1< р 2 , V 1 >V2, T1< Ί 2 . Пользуясь этими данными, определите, какие величины были отложены на горизонтальной и vertical axes. Explain your answer by indicating what physical laws you used.
A horizontal cylinder with a piston is fixed in a vacuum. The cylinder contains 0.1 mole of helium. The piston is held by stops and can slide to the left along the walls of the cylinder without friction. A bullet of mass 10 g, flying horizontally at a speed of 400 m/s, hits the piston and gets stuck in it. The helium temperature at the moment the piston stops in the extreme left position increases by 64 K. What is the mass of the piston? Assume that during the movement of the piston, the gas does not have time to exchange heat with the piston and cylinder.

2011
One mole of an ideal monatomic gas is transferred from state 1 with a temperature T 1 \u003d 300 K to state 2 in such a way that during the entire process the gas pressure increases in direct proportion to its volume. During this process, the gas receives an amount of heat Q = 14958 J. How many times n does the density of the gas decrease as a result of this process?
A bottle with a volume of V = 1 liter contains helium at normal atmospheric pressure. The neck of a bottle with an area of \u200b\u200bS \u003d 2 cm 2 is plugged with a short cork having a mass m \u003d 20 g. If the bottle lies horizontally, then in order to slowly pull the cork out of its neck, a horizontally directed force F \u003d 1 N must be applied to the cork. on the table vertically with the neck up. How much heat must be imparted to the helium in the bottle in order for it to squeeze the cork out of the neck?
A monatomic ideal gas is contained in a horizontal cylindrical vessel closed by a piston. Initial gas pressure p 1 = 4 10 5 Pa. The distance from the bottom of the vessel to the piston is L. Piston cross-sectional area S\u003d 25 cm 2. As a result of slow heating, the gas received an amount of heat Q= 1.65 kJ, and the piston has moved a distance x\u003d 10 cm. When the piston moves, a friction force of magnitude F tr \u003d 3 10 3 N. Find L. Assume that the vessel is in a vacuum.






