Specific heat of fusion of mercury. Specific heat capacity of naphthalene
Specific heat of fusion of metals
Specific heat melting |
Specific heat of fusion |
||||
| Aluminum | 393 | 94 | Platinum | 113 | 27 |
| Tungsten | 184 | 44 | Mercury | 12 | 2,8 |
| Iron | 270 | 64,5 | Lead | 24,3 | 5,8 |
| Gold | 67 | 16 | Silver | 87 | 21 |
| Magnesium | 370 | 89 | Steel | 84 | 20 |
| Copper | 213 | 51 | Tantalum | 174 | 41 |
| Sodium | 113 | 27 | Zinc | 112,2 | 26,8 |
| Tin | 59 | 14 | Cast iron | 96-140 | 23-33 |
Specific heat of fusion of certain substances (at normal atmospheric pressure)
Substance |
Specific heat of fusion |
Substance |
Specific heat of fusion |
||
| Nitrogen | 25,7 | 6,2 | Naphthalene | 151 | 36 |
| Hydrogen | 59 | 14 | Paraffin | 150 | 35 |
| Wax | 176 | 42 | Alcohol | 105 | 25 |
| Glycerol | 199 | 47,5 | Stearin | 201 | 48 |
| Oxygen | 13,8 | 3,3 | Chlorine | 188 | 45 |
| Ice | 330 | 80 | Ether | 113 | 27 |
Change in the volumes of substances during their melting
The table shows the volume of liquid V l, formed during the melting of solids from various substances volume 1000 cm 3
Most substances in the transition from solid state into liquid increases its volume. The exceptions are ice, bismuth and some other substances.
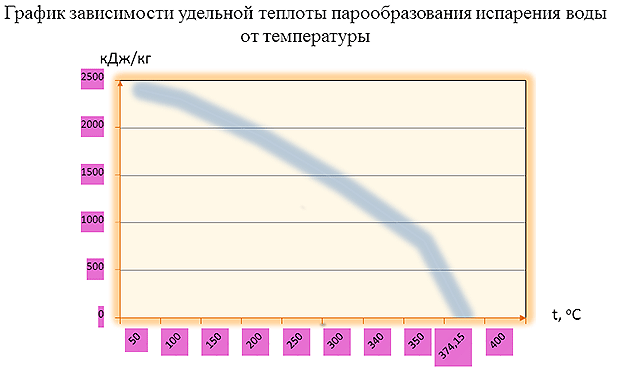
Specific heat of evaporation (vaporization) of water at different temperatures
and normal atmospheric pressure
Specific heat of vaporization |
Specific heat of vaporization |
||||
| 0 | 2501 | 597 | 80 | 2308 | 551 |
| 5 | 2489 | 594 | 100 | 2256 | 539 |
| 10 | 2477 | 592 | 160 | 2083 | 497 |
| 15 | 2466 | 589 | 200 | 1941 | 464 |
| 18 | 2458 | 587 | 300 | 1404 | 335 |
| 20 | 2453 | 586 | 370 | 438 | 105 |
| 30 | 2430 | 580 | 374 | 115 | 27 |
| 50 | 2382 | 569 | 374,15* | 0 | 0 |
* At a temperature of 374.15 o C and a pressure of 22.13 Pa (225.64 atm), water is in critical condition. In this state, the liquid and its saturated steam have the same properties - the difference between water and its saturated steam disappears.
Change in the volume of liquids during evaporation and gases (vapors) during condensation
The table shows the volume of gas (steam) formed during the evaporation of 1 liter of liquid taken at a temperature of 20 o C and normal atmospheric pressure, as well as the volume of liquid formed during the condensation of 1 m 3 of gas (steam).
Specific heat of vaporization of liquids and molten metals
(at boiling point and normal atmospheric pressure)Liquid |
Specific heat of vaporization |
Liquid |
Specific heat of vaporization |
||
| liquid nitrogen | 201 | 48 | Liquid hydrogen | 450 | 108 |
| Aluminum | 9200 | 2200 | Air | 197 | 47 |
| Petrol | 230-310 | 55-75 | Helium liquid | 23 | 5,5 |
| Bismuth | 840 | 200 | Iron | 6300 | 1500 |
| Water (at t=0 o C) | 2500 | 597 | Kerosene | 209-230 | 50-55 |
| Water (at t=20 o C) | 2450 | 586 | Liquid oxygen | 214 | 51 |
| Water (at t=100 o C) | 2260 | 539 | Magnesium | 5440 | 1300 |
| Water (at t=370 o C) | 440 | 105 | Copper | 4800 | 1290 |
| Water (at t=374.15 o C) | 0 | 0 | Tin | 3010 | 720 |
| Mercury | 293 | 70 | |||
| Lead | 860 | 210 | |||
| Ethanol | 906 | 216 | |||
| Ethyl ether | 356 | 85 | |||
Specific heat of vaporization (vaporization) of some solids
Note. The direct transition of a substance from a solid to a gaseous state, bypassing the transformation into a liquid state, is called sublimation .
Critical parameters of some substances
thermal phenomena
OPTION #1
Level A
1. Heat exchange by convection can be carried out
in gases, liquids and solids
in gases and liquids
only in gases
only in liquids
1) 47 kJ 2) 68.4 kJ 3) 760 kJ 4) 5700 kJ
3. If at an atmospheric pressure of 100 kPa 200 g of vapor of a certain substance condenses at 100 ° C, then in environment the amount of heat equal to 460 kJ is transferred. The specific heat of vaporization of this substance is approximately equal to
1) 2.1 10 8 J/kg 2) 2.1 10 7 J/kg 3) 2.3 10 6 J/kg 4) 2.3 10 4 J/kg
4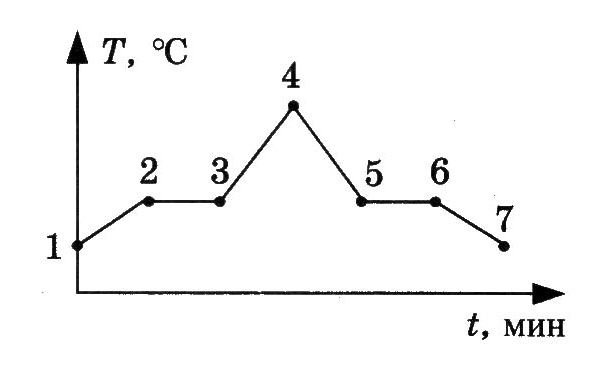 . The figure shows a graph of the dependence of the temperature of naphthalene on time during heating and cooling. At the initial moment, naphthalene was in a solid state. Which section of the graph corresponds to the process of naphthalene solidification?
. The figure shows a graph of the dependence of the temperature of naphthalene on time during heating and cooling. At the initial moment, naphthalene was in a solid state. Which section of the graph corresponds to the process of naphthalene solidification?
5
. Using the psychrometric table, determine the difference in dry and wet bulb readings if the room temperature is 20 ° C, and relative humidity air 44%.
1) 7 °С 2) 20 °С 3) 27 °С 4) 13 °С
6. heat engine receives 50 J from the heater per cycle and does useful work equal to 100 J. What is thermal efficiency cars?
200% 3) 50%
67% 4) Such a machine is impossible
Level B
To 
to the table
Level C
8. Pieces of melting ice are thrown into the calorimeter with water. At some point, the pieces of ice stop melting. The initial mass of water in the vessel is 330 g, and at the end of the process, the mass of water increases by 84 g. What was starting temperature water in the calorimeter? The specific heat capacity of water is 4200 J/(kg °C), the specific heat of ice melting is 330 kJ/kg.
thermal phenomena
^ OPTION #2
Level A
1. On Earth, the cycle of air masses is carried out on a huge scale. The movement of air masses is associated mainly with
1) thermal conductivity and radiation 3) radiation
2) thermal conductivity 4) convection
2. Before hot stamping, a brass ingot weighing 2 kg was heated from 150°C to 750°C. How much heat did the blank receive? The specific heat capacity of brass is 380 .
32 J 2) 456 kJ 3) 1050 kJ 4) 760 kJ
108 J 2) 108000 J 3) 6.75 kJ 4) 6750 J
1–2–3
5
. The wet bulb of the psychrometer shows a temperature of 16°C and the dry bulb of 20°C. Determine, using the psychrometric table, the relative humidity of the air.
100% 3) 66%
62% 4) 74%
Level B
7. Establish a correspondence between physical quantities and the formulas by which these quantities are determined.
To 
each position of the first column, select the corresponding position of the second and write down to the table selected numbers under the corresponding letters.
Level C
8. Water weighing 500 g at a temperature of 95°C was poured into a heat-insulated vessel, where there was solid naphthalene at a temperature of 80°C. After the establishment of thermal equilibrium, the water temperature turned out to be 80°C, while all the naphthalene passed into a liquid state. Neglecting heat losses, estimate how many grams of naphthalene were in the vessel. The specific heat capacity of water is 4200 J/(kg °C), the specific heat of fusion of naphthalene is 150 kJ/kg, the melting point of naphthalene is 80°C.
thermal phenomena
^ OPTION #3
Level A
1. Due to what type of heat transfer (mainly) does water in reservoirs heat up on a summer day?
Convection 3) Radiation
Thermal conductivity 4) Convection and radiation
0.38 J/kg °C) 3) 380 J/kg °C)
760 J/kg °C) 4) 2000 J/kg °C)
1) 3.5 kJ 2) 5.6 kJ 3) 10 kJ 4) 18 kJ
4. The figure shows a graph of the temperature of naphthalene versus time during heating and cooling. At the initial moment of time, naphthalene was in a solid state. Which of the graph points corresponds to the beginning of naphthalene solidification?
5
. The relative humidity in the room is 60%. The difference in dry and wet thermometer readings is 4°C. Using the psychrometric table, determine the dry bulb reading.
18 °С 2) 14 °С 3) 10 °С 4) 6 °С
Level B
7. Establish a correspondence between physical quantities and the formulas by which these quantities are determined.
To 
each position of the first column, select the corresponding position of the second and write down to the table selected numbers under the corresponding letters.
Level C
1. A piece of ice, which had a temperature of 0 °C, was lowered into a beaker of a calorimeter containing 177 g of water. The initial temperature of the calorimeter with water is 45°C. After all the ice melted, the temperature of the water and the calorimeter became 5°C. Determine the mass of ice. Ignore the heat capacity of the calorimeter. The specific heat capacity of water is 4200 J/(kg °C), the specific heat of ice melting is 330 kJ/kg.
thermal phenomena
^ OPTION #4
Level A
1. In a metal rod, heat transfer is carried out mainly by
1) radiation 3) thermal conductivity
2) convection 4) radiation and convection
2. It took 1800 J of heat to heat 100 g of aluminum from 120°C to 140°C. Determine the specific heat capacity of aluminum from these data.
1) 0.9 J/kg °C 2) 9 J/kg °C 3) 360 J/kg °C 4) 900 J/kg °C
3. The mass of silver is 10 g. How much heat will be released during its crystallization if silver is at the melting point? The specific heat of fusion of silver is 88 kJ/kg.
880000 J 2) 8.8 kJ 3) 880 J 4) 88 kJ
5. Use the psychrometric chart to determine the wet bulb reading if the room temperature is 16°C and the relative humidity is 62%.
20 °C 3) 12 °C
22 °C 4) 16 °C
17,5%
>100%
Level B
7. Establish a correspondence between physical quantities and the formulas by which these quantities are determined.
To 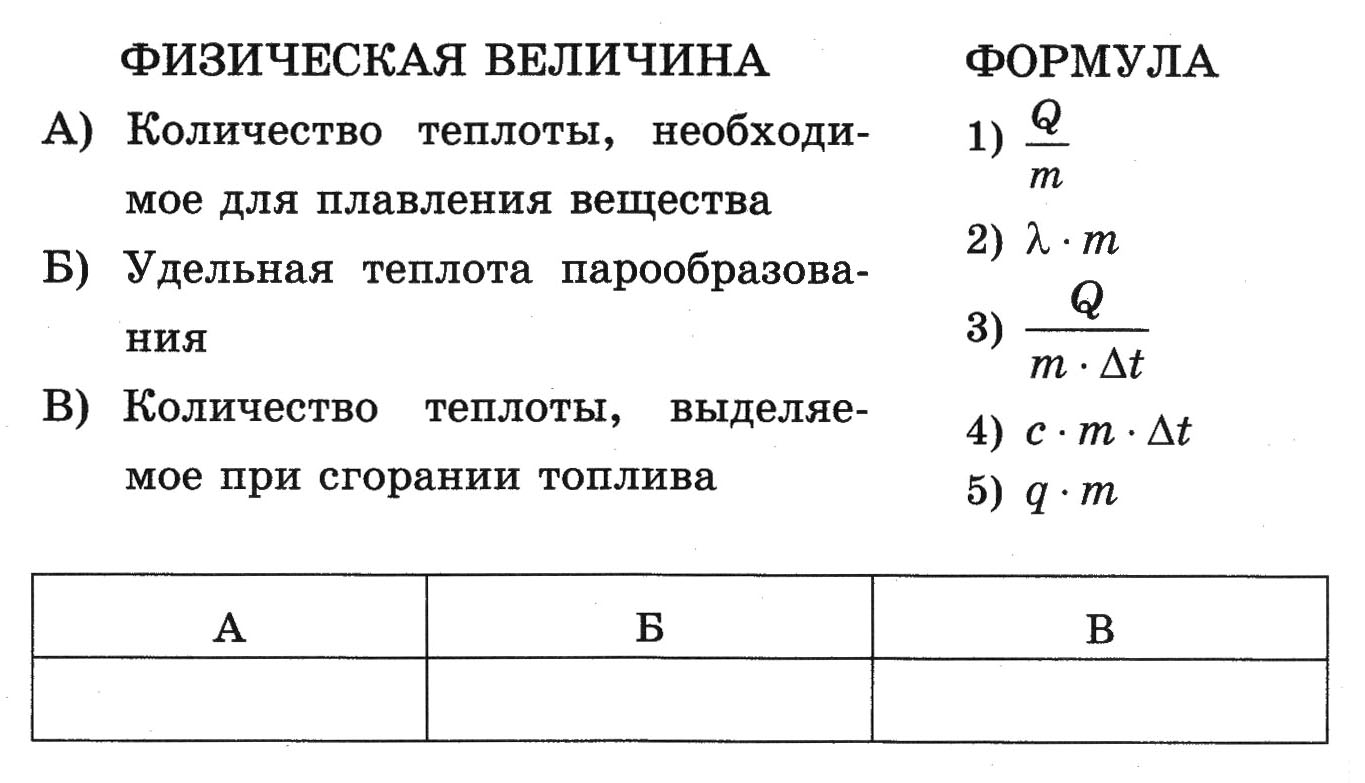
each position of the first column, select the corresponding position of the second and write down to the table selected numbers under the corresponding letters.
Level C
8. Solid naphthalene is in a heat-insulated vessel at a temperature of 80°C. Molten naphthalene weighing 600 g is poured into the vessel, the initial temperature of which is 100°C. From a certain point in time, the pieces of naphthalene in the vessel cease to melt, and the mass of liquid naphthalene reaches 700 g. Based on the results of this experiment, determine the specific heat of liquid naphthalene. The specific heat of fusion of naphthalene is 150 kJ/kg. The melting point of naphthalene is 80°C.
O  answers:
answers:
Control and independent work in physics. Grade 8: to the textbook by A.V. Peryshkin "Physics. Grade 8 / O.I. Gromtsev. -M.: Publishing house "Exam", 2010. - 111, p. (Series "Educational and methodical set")
total thermal capacity. What is the coefficient "C": (sp.) specific heat capacity of NAPHTHALENE (aromatic hydrocarbon). How do these types of thermophysical characteristics differ, why is it impossible to get by with one physical parameter that describes thermal properties, and why was it necessary to introduce the coefficient "to multiply entities, complicating the life of normal people"?Not specific, but total thermal capacity, in the generally accepted physical sense, is called the ability of a substance to heat up. At least that's what any textbook on thermal physics tells us - this is the classical definition of heat capacity(correct wording). In fact, this is an interesting physical feature. Little known to us in everyday life "side of the coin." It turns out that when heat is supplied from the outside (heating, warming up), not all substances react equally to heat ( thermal energy) and heat up differently. Ability NAFTHALINA receive, receive, retain and accumulate (accumulate) thermal energy is called the heat capacity of NAPHTHALEEN. And herself heat capacity, is a physical characteristic describing the thermophysical properties of an aromatic hydrocarbon. At the same time, in different applied aspects, depending on a specific practical case, one thing may turn out to be important for us. For example: the ability of a substance to take warm or the ability to accumulate thermal energy or "talent" to keep it. However, despite some difference, in the physical sense, the properties we need will be described heat capacity.
A small, but very "nasty snag" of a fundamental nature is that the ability to heat up - thermal capacity, is directly related not only to chemical composition, the molecular structure of a substance, but also with its quantity (weight, mass, volume). Due to such an "unpleasant" connection, the general heat capacity becomes too inconvenient physical characteristic of the substance. Since, one measured parameter simultaneously describes "two different things". Namely: really characterizes thermophysical properties of NAPHTHALEEN, however, "in passing" also takes into account its quantity. Forming a kind of integral characteristic, in which "high" thermal physics and a "banal" amount of substance (in our case: aromatic hydrocarbon) are automatically connected.
Well, why do we need such thermophysical characteristics, in which "inadequate psyche" is clearly traced? From a physics point of view, the total heat capacity(in the most clumsy way), tries not only to describe the amount of thermal energy capable of accumulating in an organic compound, but also "in passing to inform us" about the amount NAFTHALINA. It turns out absurdity, and not a clear, understandable, stable, correct thermophysical characteristic. Instead of a useful constant suitable for practical thermophysical calculations, we are given a floating parameter, which is the sum (integral) of the amount of heat received NAPHTHALINE and its mass or volume.
Thank you, of course, for such "enthusiasm", but the quantity NAFTHALINA I can measure myself. Having received results in much more convenient, "human" form. Quantity NAFTHALINA I would like not to "extract" mathematical methods and calculations using a complex formula from the general heat capacity, at different temperatures, and find out the weight (mass) in grams (g, g), kilograms (kg), tons (tons), cubes (cubic meters, cubic meters, m3), liters (l) or milliliters (ml). Moreover, smart people have long come up with measuring instruments that are quite suitable for these purposes. For example: scales or other devices.
Especially the "annoying floating nature" of the parameter: general heat capacity of NAPHTHALENE. His unstable, changeable "mood". When changing the "serving size or dose", heat capacity of NAPHTHALENE at different temperatures changes immediately. More quantity, physical quantity, absolute value heat capacity- increases. Less quantity, value thermal capacity decreases. "Disgrace" some turns out! In other words, what we "have" cannot in any way be considered a constant describing thermophysical characteristics of NAPHTHALENE at different temperatures. And it is desirable for us to "have" an understandable, constant factor, a reference parameter characterizing thermal properties organic compound, without "references" to the amount of aromatic hydrocarbon (weight, mass, volume). What to do?
This is where a very simple but "very scientific" method comes to our rescue. It comes down to not only bailiff "ud. - specific", before physical quantity, but to an elegant solution involving the exclusion of the amount of matter from consideration. Naturally, "uncomfortable, superfluous" parameters: mass or volume NAFTHALINA absolutely impossible to rule out. At least for the reason that if there is no quantity, then there will be no “subject of discussion” itself. And the substance should be. Therefore, we choose some conditional standard of mass or volume, which can be considered a unit suitable for determining the value of the coefficient "C" we need. For weight of NAPHTHALENE, such a unit of mass, convenient in practical use, turned out to be 1 kilogram (kg).
Now we we heat one kilogram of NAPHTHALENE by 1 degree, and the amount of heat (thermal energy), we need in order to heat the organic compound by one degree - this is our correct physical parameter, coefficient "C", well, quite fully and clearly describing one of thermophysical properties of NAPHTHALENE at different temperatures. Please note that now we are dealing with a characteristic describing physical property substance, but not trying to "additionally inform us" about its quantity. Comfortable? There are no words. It's a completely different matter. By the way, now we are not talking about the general thermal capacity. Everything has changed. THIS IS THE SPECIFIC HEAT CAPACITY OF NAPHTHALENE, which is sometimes called by another name. How? Just MASSIVE HEAT CAPACITY OF NAPHTHALENE. Specific (ud.) And mass (m.) - in this case: synonyms, they mean here the one we need coefficient "C".
Table 1. Coefficient: specific heat capacity of NAFTHALEN (sp.). Mass thermal capacity of NAPHTHALEEN. Reference data.
In order to melt any substance in the solid state, it is necessary to heat it.
Experiments show that different substances of the same mass require different amounts of heat to completely melt it.
That is, there is a certain value on which depends how much heat a substance needs to absorb in order to melt. And this value is different for different substances. This value in physics is called the specific heat of fusion of a substance. The specific heat of fusion shows how much heat is needed to completely convert 1 kg of a substance from a solid state to a liquid, taken at the melting point. The specific heat of fusion is denoted by the Greek letter λ (lambda), and the unit is 1 J / kg.
Specific heat of fusion formula
The specific heat of fusion is found by the formula:
λ = Q/m,
where Q is the amount of heat required to melt a body of mass m.
The amount of heat required to melt a substance is equal to the product of the specific heat of fusion times the mass of the substance.
Q = λ*m,
Again, it is known from experiments that, during solidification, substances emit the same amount of heat that was required to be spent on their melting. Molecules, losing energy, form crystals, being unable to resist the attraction of other molecules. And again, the temperature of the body will not decrease until the moment when the whole body solidifies, and until all the energy that was spent on melting it is released. That is, the specific heat of fusion shows how much energy is needed to melt a body of mass m, and how much energy is released during the solidification of this body.
The amount of heat required for the crystallization of a substance is equal to the product of the specific heat of fusion times the mass of the substance.
Q = λ*m
When the body crystallizes Q it is written with a "-" sign, as heat is released.






