How and when the internal energy of the body changes. Ways to change internal energy
Find out how you can calculate the change internal energy during heat transfer. In order to do this as accurately as possible, it is necessary to minimize the unaccounted for heat losses during heat exchange. Therefore, when scientific research heat transfer is carried out in a calorimeter (Fig. 6.1), the use of which allows you to accurately determine the heat given or received by the body in the process of heat transfer.
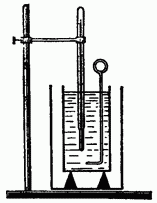
The calorimeter consists of two vessels: external and internal. The inner vessel is made of a good conductor of heat (brass, copper), since its temperature must be the same as that of the liquid poured into it.
The outer vessel protects the inner vessel from heat loss by convection and radiation. Therefore, it is usually painted with white paint or made of shiny tin.
To protect the inner vessel from heat loss by conduction, it is placed on wooden supports (wood has poor thermal conductivity). Place a stirrer (of the same material as the vessel) and a thermometer into the inner vessel.
Heat exchange is carried out as follows. With the help of balances, the mass of the inner vessel of the calorimeter and the stirrer is determined, and then the mass of the liquid poured into it, for example water. After that, the mass of the body is measured, heated to a known temperature, and, noticing starting temperature liquid, lower the heated body into the calorimeter. By measuring the final temperature of the liquid, it is possible to calculate how much heat the body gave off in the process of heat transfer.
With the help of such experiments, it is easy to establish that the change in the internal energy of a body is directly proportional to its mass and change in body temperature.
![]()
here c is the coefficient of proportionality. Since the change in internal energy during heat transfer is estimated by the amount of heat, we have
The initial temperature of the body is usually denoted as the final temperature. Then, in the case of heating the body, and in the case of cooling
Experiments show that it depends on the type of substance, on external conditions, from the aggregate state of matter. These dependencies are expressed by the coefficient c in formulas (6.1) and (6.2).
The value c characterizing the dependence of the change in the internal energy of the body during heating or cooling on the type of substance and on external conditions is called specific heat substances. The specific heat capacity of a substance is measured by the amount of heat required to heat a unit mass of a substance per unit temperature:
![]()
We derive the unit specific heat in SI:
In SI, the unit of specific heat is the specific heat of such a substance for which 1 J of energy is spent on heating a mass of 1 kg per 1 K. For small changes in temperature, the specific heat capacity can be considered constant. To solve problems, it is taken from tables.
It should be borne in mind that when determining the amount of heat necessary for heating or released during cooling of a body, the heat capacity of the body C is sometimes used - a value measured by the amount of heat necessary to heat the body per unit temperature. Consequently,
It is especially convenient to use the heat capacity of the whole body in calculations when individual parts of the body are made of different substances. In SI, the unit of heat capacity of a body is taken (Show this using formula (6.3).)
We also note that the specific heat capacity of a gas depends on the nature of the process in which it is heated. For example, the specific heat capacity of a gas at constant pressure greater than its specific heat capacity at constant volume since in the first case it is necessary not only to increase the internal energy of the gas, but also to expend energy to perform the work done by the gas on external bodies in the process of its expansion (§ 5.10). In the second case, the heat supplied to the gas goes only to increase its internal energy.
internal energy is the sum of the kinetic energies of all the particles that make up the body, and potential energies interactions between these particles. This includes the interaction energy of electrons with nuclei and the interaction energy constituent parts kernels.
The internal energy depends on its temperature. Temperature characterizes the average kinetic energy of the particles of a substance. When the temperature changes, the distance between the particles changes, therefore, the interaction energy between them also changes.
Internal energy also changes when a substance passes from one state of aggregation to another. Processes associated with a change in temperature or state of aggregation of a substance are called thermal. Thermal processes are accompanied by a change in the internal energy of the body.
chemical reactions, nuclear reactions are also accompanied by a change in the internal energy of the body, tk. the interaction energy of the particles involved in the reactions changes. Internal energy changes when atoms emit or absorb energy during the transition of electrons from one shell to another.
One of ways to change internal energy is Work. So, during the friction of two bodies, their temperature rises, i.e. their internal energy increases. For example, in the processing of metals - drilling, turning, milling.
When two bodies with different temperatures come into contact, energy is transferred from the body with high temperature to a body with a low temperature. The process of transferring energy from one body to another at a lower temperature is called heat transfer.
Thus, in nature there are two processes in which the internal energy of the body changes:
a) transformation mechanical energy to the internal and vice versa; while work is being done;
b) heat transfer; while no work is done.
If you mix hot and cold water, then by experience you can see that the amount of heat given off hot water, and the amount of heat received by cold water are equal to each other. Experience shows that if heat exchange occurs between bodies, then the internal energy of all heating bodies increases by as much as the internal energy of cooling bodies decreases. Thus, energy passes from one body to another, but the total energy of all bodies remains unchanged. it law of conservation and transformation of energy.
In all phenomena occurring in nature, energy does not arise and does not disappear. It only changes from one species to another, while its value is preserved.
For example, a lead bullet flying at a certain speed hits an obstacle and heats up.
Or, an ice floe, falling from a snow cloud, melts near the ground.
Or, a kettle of water is heated on a gas stove, some of the water evaporates.
The law of conservation of energy is the scientific basis for calculations in all areas of science and technology. It should be borne in mind that completely internal energy cannot be converted into mechanical energy.
Internal energy can be changed in two ways.
If work is done on a body, its internal energy increases.
Internal energy of the body(denoted as E or U) is the sum of the energies of molecular interactions and thermal motions of a molecule. The internal energy is a single-valued function of the state of the system. This means that whenever a system finds itself in a given state, its internal energy assumes the value inherent in this state, regardless of the system's history. Consequently, the change in internal energy during the transition from one state to another will always be equal to the difference between its values in the final and initial states, regardless of the path along which the transition was made.
The internal energy of a body cannot be measured directly. Only the change in internal energy can be determined:
This formula is a mathematical expression of the first law of thermodynamics
For quasi-static processes, the following relationship holds:
Temperature measured in Kelvin
Entropy, measured in joules/kelvin
Pressure measured in pascals
Number of particles in systems
Heat of combustion of fuel. conditional fuel. The amount of air needed to burn the fuel.
The quality of a fuel is judged by its calorific value. The index is used to characterize solid and liquid fuels. specific heat combustion, which is the amount of heat released during the complete combustion of a unit mass (kJ / kg). For gaseous species fuel, an indicator of the volumetric heat of combustion is used, which is the amount of heat released during the combustion of a unit volume (kJ / m3). In addition, gaseous fuel in some cases is estimated by the amount of heat released during the complete combustion of one mole of gas (kJ / mol).
The heat of combustion is determined not only theoretically, but also empirically, by burning a certain amount of fuel in special devices called calorimeters. The heat of combustion is estimated by the increase in water temperature in the colorimeter. The results obtained by this method are close to the values calculated from the elemental composition of the fuel.
Question 14Change in internal energy during heating and cooling. The work of gas with a change in volume.
The internal energy of the body depends on the average kinetic energy of its molecules, and this energy, in turn, depends on temperature. Therefore, by changing the body temperature, we also change its internal energy. When a body is heated, its internal energy increases, and when it cools, it decreases.
The internal energy of the body can be changed without doing work. So, for example, it can be increased by heating a kettle of water on the stove or by lowering a spoon into a glass of hot tea. The fireplace in which the fire is kindled, the roof of the house illuminated by the sun, etc. are heated. An increase in the temperature of bodies in all these cases means an increase in their internal energy, but this increase occurs without doing work.
Change in internal energy body without doing work is called heat transfer. Heat transfer occurs between bodies (or parts of the same body) that have different temperatures.
How, for example, does heat transfer occur when a cold spoon comes into contact with hot water? First, the average speed and kinetic energy hot water molecules exceed the average speed and kinetic energy of the metal particles from which the spoon is made. But in those places where the spoon comes into contact with water, the hot water molecules begin to transfer part of their kinetic energy to the particles of the spoon, and they begin to move faster. In this case, the kinetic energy of water molecules decreases, and the kinetic energy of the particles of the spoon increases. Along with the energy, the temperature also changes: the water gradually cools down, and the spoon heats up. The change in their temperature occurs until it becomes the same for both the water and the spoon.
Part of the internal energy transferred from one body to another during heat exchange is denoted by a letter and is called the amount of heat.
Q is the amount of heat.
The amount of heat should not be confused with temperature. Temperature is measured in degrees, and the amount of heat (like any other energy) is measured in joules.
Upon contact of bodies with different temperatures a hotter body gives off some heat, and a colder body receives it.
Work at isobaric gas expansion. One of the main thermodynamic processes, taking place in most heat engines, is the process of gas expansion with the performance of work. It is easy to determine the work done during the isobaric expansion of a gas.
If during the isobaric expansion of gas from volume V1 to volume V2 the piston moves in the cylinder at a distance l (Fig. 106), then the work A "performed by the gas is equal to
Where p is the gas pressure, is the change in its volume.
Work with an arbitrary gas expansion process. An arbitrary process of gas expansion from volume V1 to volume V2 can be represented as a set of alternating isobaric and isochoric processes.
Work with isothermal gas expansion. Comparing the areas of the figures under the sections of the isotherm and isobar, we can conclude that the expansion of gas from volume V1 to volume V2 at the same initial value of gas pressure is accompanied in the case of isobaric expansion by more work.
Work with gas compression. When the gas expands, the direction of the gas pressure force vector coincides with the direction of the displacement vector, so the work A "performed by the gas is positive (A" > 0), and the work A of external forces is negative: A \u003d -A "< 0.
When compressing gas the direction of the external force vector coincides with the direction of movement, therefore the work A of the external forces is positive (A > 0), and the work A "performed by the gas is negative (A"< 0).
adiabatic process. In addition to isobaric, isochoric and isothermal processes, adiabatic processes are often considered in thermodynamics.
adiabatic process is a process occurring in a thermodynamic system in the absence of heat exchange with surrounding bodies, i.e., under the condition Q = 0.
Question 15 Conditions for the equilibrium of the body. Moment of power. Types of balance.
Equilibrium, or balance, of a number of related phenomena in the natural and human sciences.
A system is considered to be in a state of equilibrium if all influences on this system are compensated by others or are absent altogether. A similar concept is stability. Equilibrium can be stable, unstable or indifferent.
Typical examples of balance:
1. Mechanical balance, also known as static balance, - the state of a body at rest, or moving uniformly, in which the sum of the forces and moments acting on it is equal to zero.
2. Chemical equilibrium - the position in which chemical reaction proceeds to the same extent as the reverse reaction, and as a result there is no change in the amount of each component.
3. The physical balance of people and animals, which is maintained by understanding its necessity and, in some cases, by artificially maintaining this balance [source not specified 948 days].
4. Thermodynamic equilibrium - the state of the system in which its internal processes do not lead to changes in macroscopic parameters (such as temperature and pressure).
R equality to zero of the algebraic sum moments of forces also does not mean that the body is necessarily at rest. For several billion years, the rotation of the Earth around its axis continues with a constant period precisely because the algebraic sum of the moments of forces acting on the Earth from other bodies is very small. For the same reason, the spinning bicycle wheel continues to rotate at a constant frequency, and only external forces stop this rotation.
Types of balance. In practice, an important role is played not only by the fulfillment of the equilibrium condition for bodies, but also by the qualitative characteristic of equilibrium, called stability. There are three types of balance of bodies: stable, unstable and indifferent. The equilibrium is called stable if, after small external influences, the body returns to its original state of equilibrium. This happens if, with a slight displacement of the body in any direction from the initial position, the resultant of the forces acting on the body becomes non-zero and is directed towards the equilibrium position. In stable equilibrium is, for example, a ball at the bottom of the recess.
The general condition for the equilibrium of a body. Combining the two conclusions, we can formulate a general condition for the equilibrium of a body: a body is in equilibrium if the geometric sum of the vectors of all forces applied to it and the algebraic sum of the moments of these forces about the axis of rotation are equal to zero.
Question 16Vaporization and condensation. Evaporation. Boiling liquid. Dependence of liquid boiling on pressure.
Vaporization - property of dropping liquids to change their state of aggregation and turn into steam. Vaporization that occurs only on the surface of a dropping liquid is called evaporation. Vaporization over the entire volume of a liquid is called boiling; it occurs at a certain temperature, depending on the pressure. The pressure at which a liquid boils at a given temperature is called pressure. saturated vapors pnp, its value depends on the type of liquid and its temperature.
Evaporation is the process by which a substance moves from liquid state into gaseous (steam). The evaporation process is the reverse of the condensation process (transition from a vapor to a liquid state. Evaporation (vaporization), the transition of a substance from a condensed (solid or liquid) phase to a gaseous (steam); phase transition first kind.
Condensation - it is the reverse process of evaporation. During condensation, the vapor molecules return to the liquid. In a closed vessel, a liquid and its vapor can be in a state of dynamic equilibrium when the number of molecules leaving the liquid is equal to the number of molecules returning to the liquid from the vapor, that is, when the rates of evaporation and condensation are the same. Such a system is called a two-phase system. A vapor that is in equilibrium with its liquid is called saturated. The number of molecules emitted from a unit surface area of a liquid in one second depends on the temperature of the liquid. The number of molecules returning from vapor to liquid depends on the concentration of vapor molecules and on average speed their thermal motion, which is determined by the temperature of the steam.
Boiling- the process of vaporization in a liquid (the transition of a substance from liquid to gaseous state), with the appearance of phase separation boundaries. Boiling point at atmospheric pressure is usually given as one of the main physicochemical characteristics of a chemically pure substance.
Boiling is distinguished by type:
1. boiling with free convection in a large volume;
2. boiling under forced convection;
3. as well as in relation average temperature liquid to saturation temperature:
4. boiling of a liquid subcooled to saturation temperature (surface boiling);
5. boiling of a liquid heated to saturation temperature
Bubble
Boiling , in which steam is formed in the form of periodically emerging and growing bubbles, is called nucleate boiling. With slow nucleate boiling in a liquid (more precisely, as a rule, on the walls or at the bottom of the vessel), bubbles filled with vapor appear. Due to the intense evaporation of the liquid inside the bubbles, they grow, float, and the vapor is released into the vapor phase above the liquid. In this case, in the near-wall layer, the liquid is in a slightly overheated state, i.e. its temperature exceeds the nominal boiling point. Under normal conditions, this difference is small (on the order of one degree).
Film
When the heat flux increases to a certain critical value, the individual bubbles merge, forming a continuous vapor layer near the vessel wall, which periodically breaks through into the liquid volume. This mode is called film mode.
©2015-2017 site
All rights belong to their authors. This site does not claim authorship, but provides free use.






