A substance that is in a solid state. Thus, the total pressure is equal. Pressure under curved surface
Short-range order (fluidity, incompressibility, quasi-crystallinity, potential energy of molecules).
surface tension.
Pressure under a curved surface.
Wetting.
capillary phenomena.
Surface tension.
The potential energy of a molecule inside a liquid is less than outside the liquid. The surface layer is in different conditions. To transfer molecules to the surface, a certain potential barrier must be overcome.
It is impossible to make a clay cup without entering another category - it will only be a cup without a stick. Kosterlitz and Tauuss considered phenomena occurring in very thin, almost two-dimensional layers. Haldane also studied the properties of matter in the form of almost one-dimensional structures - chains of atoms.
For a long time, scientists believed that even at the temperature of absolute zero biscuit, thermal fluctuations destroy all order in a thin layer of matter. And if there are no ordered phases, there are no phase transitions either. Topological phase transition not like the usual passage of ice into water. The main role is played by small vortices in a flat material. At low temperatures, they form closely related pairs. When the temperature rises, a phase transition occurs - the vortices suddenly move away from each other and "sail" over the material.
r- radius of molecular action (sphere of molecular action).
The resulting force inside the liquid is 0. On the surface of the gas - its action can be neglected. The resulting force is reduced. The entire layer lying near the surface of the liquid is subjected to forces directed normally into the liquid. The surface layer exerts pressure on the liquid - molecular pressure.
It has found application, for example, in atomic physics and statistical mechanics. This has also been confirmed experimentally. David Thouless and Duncan Haldane presented an innovative theoretical work, which cast doubt on current theories about what materials can conduct electricity. It turned out that these changes can only take strictly defined integer values without intermediate states. Topology turned out to be the right key to explaining this puzzle: the electrons in the conducting layer between the semiconductor layers move freely, creating the so-called topological quantum fluid.
The mass of fluid, which is not acted upon by external forces, must take on a spherical shape. Of all geometric bodies A sphere has the smallest surface area for a given volume. The surface of a liquid is like a stretched film. To stretch a film, normally a force must be applied to its boundary  tangent to the surface of the liquid, called the surface tension force. These forces are the greater, the longer the length of the film boundary:
tangent to the surface of the liquid, called the surface tension force. These forces are the greater, the longer the length of the film boundary:
Changes in resistance are quantum - they happen gradually, not continuously. However, Haldane expected a more peculiar effect. It turned out that their properties are completely different depending on the nature of the atomic magnets, which are divided into two categories - even and odd. As Haldane showed, even magnets form topological circuits, while odd ones are illogical.
Further research in this area led to the discovery of topological states relating not only to chains or thin layers, but also to conventional three-dimensional materials. Topological insulators, superconductors or metals are among the "hot" research topics.
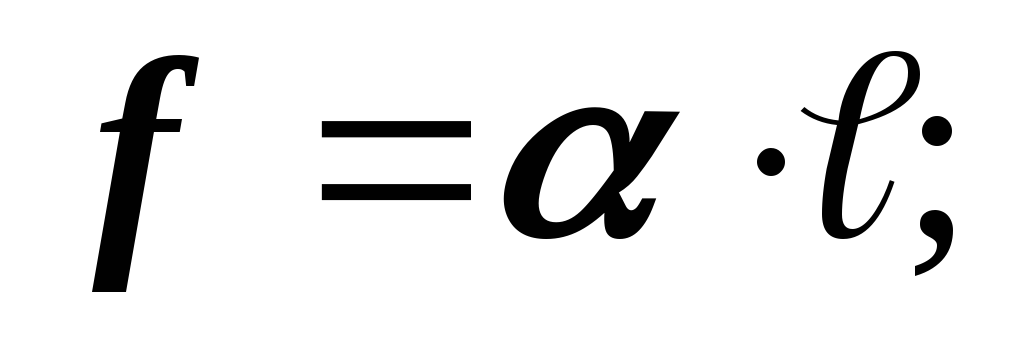
 - coefficient surface tension. FROMT
- coefficient surface tension. FROMT and
and  . AtT
. AtT  T Crete.
T Crete.
 0
. Let
0
. Let  - some platform.
- some platform.  - work to create her strengthF.
- work to create her strengthF.
Thanks to the discoveries of the laureates Nobel Prize This year, physicists have better understood the properties of matter. Theoretical progress can translate into the development of new materials and progress in building devices such as quantum computers. Part of a higher organization of matter. No dead volume, nor its shape, represents the greatest extensibility, but the most compressibility. Attractive forces between gas particles are weak, degassed cohesion between particles is very small, as well as density. The diffusion rate is inversely proportional to the particle mass and proportional to the gas pressure.

then

This work goes to increase the energy of the film:


They are called saturated steam. It depends on the volume of gas in which the liquid is located. Surface tension shows up in liquids. Flow is a property specific to liquids. Crystal chemistry Factors affecting crystal structure- volume of particles - polarization - nature of interaction forces - electromagnetic. When evaporation occurs in the entire mass of the liquid, the process is called boiling. The pressure at which a liquid coexists at a certain temperature is called the vapor pressure.
These are compact structures. Layer near the wall of a stationary container. Crystals are limited by facets and edges meeting at the corners. Crystal packaging. The particles are arranged so that they can completely occupy the space. To build a network, consider compound atoms or ions as particles. The amount of heat required to transform one mole of liquid vapor is called the molar heat of vaporization. others flow with increasing speed until the maximum speed is reached. which means the maximum number of particles placed in the smallest possible space.
Surface tension energy.
Energy - is a part internal energy film, which is converted into work during an isothermal process.
Free energy
Surface tension explains: droplet formation:

The lever is oriented from the inside of the liquid to the inside and tends to reduce the surface of the liquid. The viscosity of a fluid is expressed by a viscosity coefficient representing the frictional force between two parallel layers of relative displacement fluid on a surface block. The process by which a liquid evaporates is called evaporation. Another fluid that shows up in fluids is internal resistance, which represents the deaeration force. gaseous molecules at a distance less than the radius of action of intermolecular forces.
For a drop:

Pressure under curved surface
Consider the surface of the liquid, based on a flat contour.

If the surface of the liquid is not flat, then its tendency to contract will lead to the addition of pressure in relation to the flat liquid.
Capillarity is the process of liquids rising in narrow pipes to form curved surfaces called meniscuri. Fluid displacement counteracts the intrinsic stability and viscosity of fluids. Depending on the adhesion of the liquid to the walls of the vessel, concave and convex markings are formed. Solid - attractive attraction between components - resistance to heat and thermal deformation. Definition in crystal - characterized by an ordered distribution of particles of amorphous particles - a distribution of particle regularity.

In the case of a convex surface, this pressure is positive; in the case of a concave one, it is negative.


Compute
 for a spherical liquid surface.
for a spherical liquid surface.

Due to surface tension, both hemispheres are attracted.


These forces press both hemispheres on the surface, and they cause additional pressure:

Surface curvature:
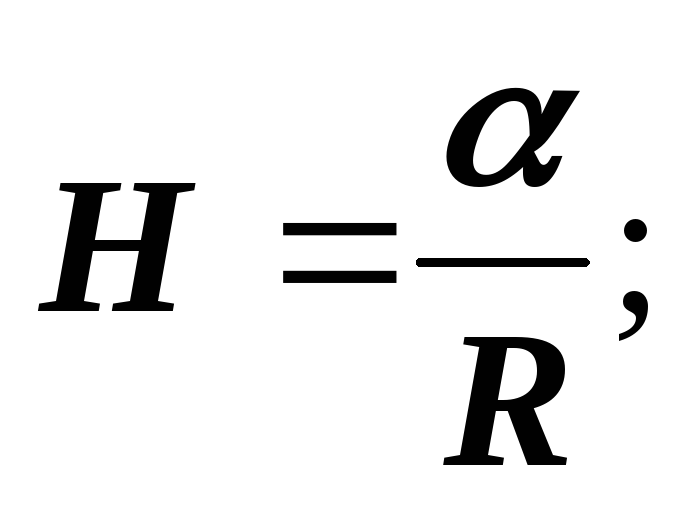
In geometry, it is proved that the half-sum of the reciprocal radii of curvature of any pair of mutually perpendicular sections has the same value H :
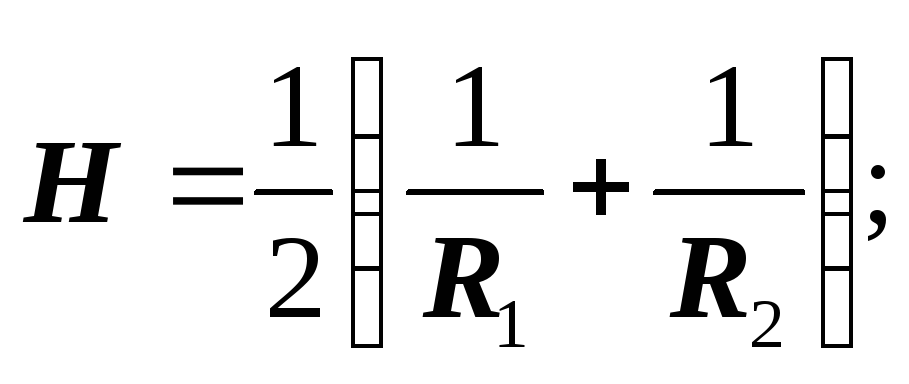
For sphere: R 1 = R 2 = R :

Laplace proved that the formulas are valid for a surface of any shape, if by H is meant the average curvature of the surface at the point at which the additional pressure is determined.
Average curvature

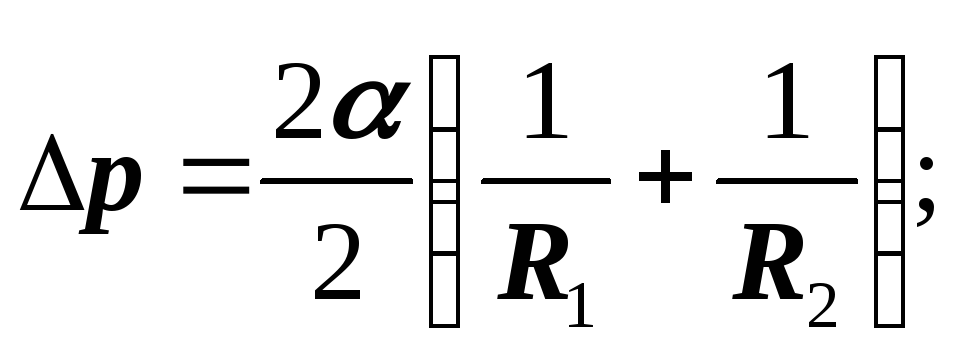
Laplace formula
Additional pressure changes the liquid level in narrow tubes (capillaries), which is sometimes called capillary pressure.
The floating of small bodies on the surface is explained by the Laplace pressure.
wetting
When considering phenomena at the liquid-solid boundary, it is necessary to consider the total surface energy of two substances.
If three substances border: liquid, solid and gas. Then the whole configuration corresponds minimum total energy (surface, in the liquid field).
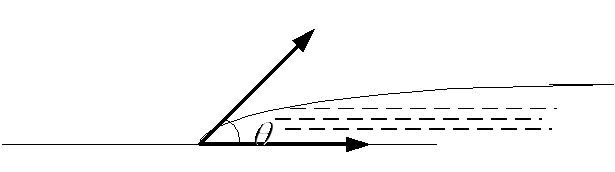
Angle between the surface of a solid and the tangent to the liquid
 - edge angle.
- edge angle.
If a
 less than π/2 the liquid wets the body.
less than π/2 the liquid wets the body.
If a
 more than π/2 the liquid does not wet the body.
more than π/2 the liquid does not wet the body.
At
 zero total wetting.
zero total wetting.
At
 complete nonwetting.
complete nonwetting.
Non-wetting can lead to curious phenomena: a needle does not sink in fat. Similarly, you can carry water in a sieve if the sieve is not wetted by water (cover the sieve threads with paraffin), if there is not much water.
Capillary phenomena
The existence of the contact angle leads to the curvature of the liquid surface near the walls of the vessel. In a narrow capillary tube, the surface turns out to be curved.
The liquid wets the surface:
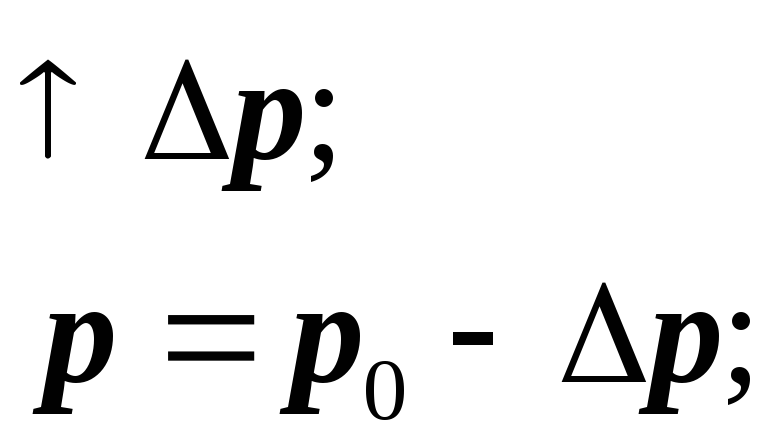
If the liquid does not wet:

If the surface of the liquid is curved, then the surface tension forces create additional pressure on the liquid:
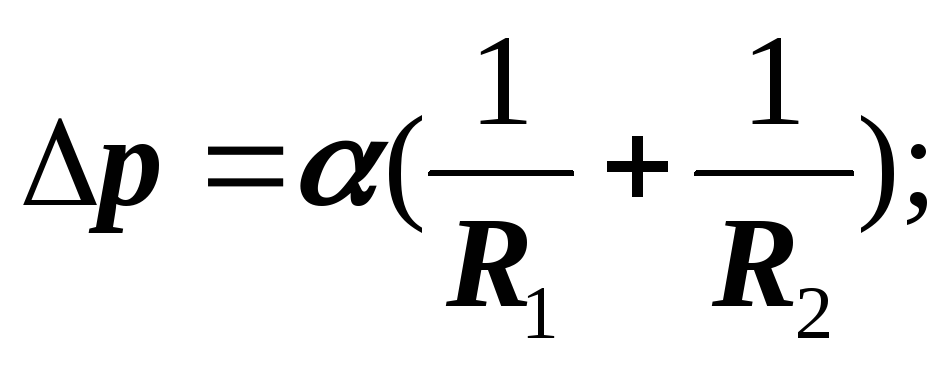
So the total pressure is:

 capillary, Laplacian pressure.
capillary, Laplacian pressure.
If the capillary is immersed with one end in a liquid, then when the capillary is wetted, the liquid level will be higher than the level in the vessel, and when not wetted, it will be lower.

Level height change in narrow tubes - capillarity.

If the capillaries are round section, then:
 and
and


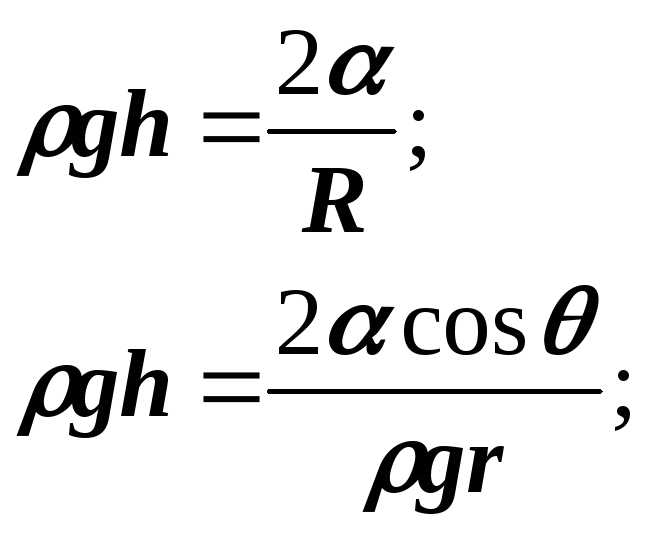

If the capillary is small, then with complete wetting
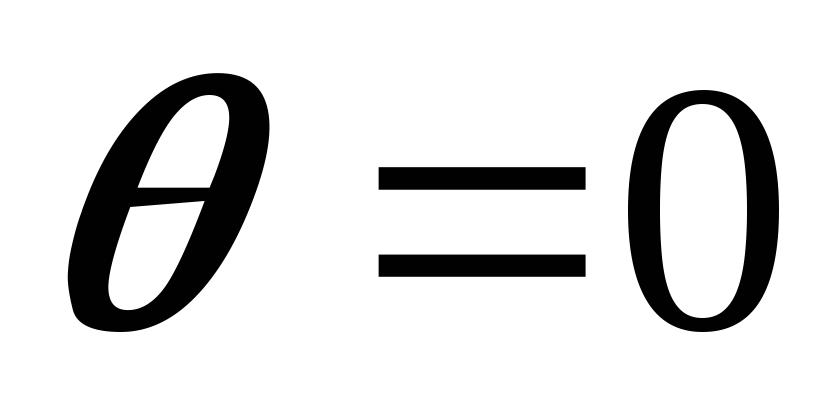 :
:
R = r

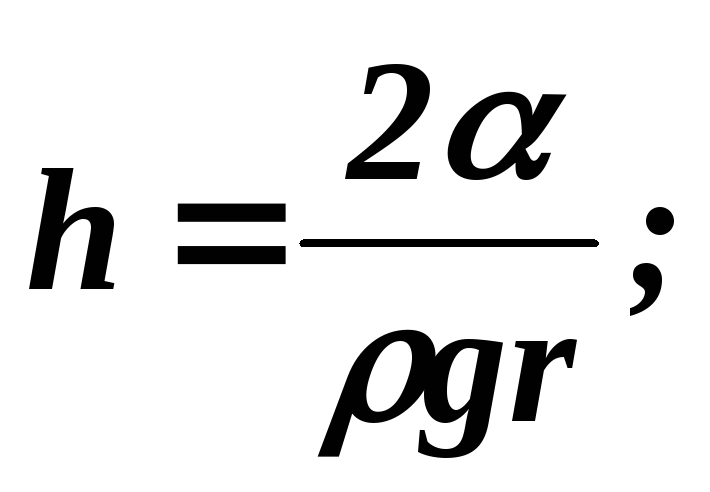
Liquid - state of aggregation of matter, intermediate between solid and gaseous. Liquids have the inherent property of solids - to retain their volume, form a surface, transparency, tensile strength. Gases: take the form of a vessel, continuously turns into a gas without a jump.
A number of features peculiar only to her: Feature - fluidity. Liquids are almost incompressible. Fluid testing with x-rays showed that internal structure they have much in common with the structure of solids.
In the arrangement of liquid particles, there is short range order .
The main property of a liquid that distinguishes it from others aggregate states, is the ability to unlimitedly change shape under the action of tangential mechanical stresses, even arbitrarily small, while practically maintaining volume. Substance in liquid state exists in a certain temperature range, below which it passes into a solid state (crystallization or transformation into a solid amorphous state - glass), above - into a gaseous state (evaporation occurs). The boundaries of this interval depend on the pressure.
3.1Physical properties of liquids:
ü Fluidity(Basic property.Unlike plastic solids, the liquid has no yield point: it is enough to apply an arbitrarily small external force to let the liquid flow.
ü Preservation of volume. One of the characteristic properties of a liquid is that it has a certain volume (with constant external conditions). A liquid is extremely difficult to compress mechanically because, unlike a gas, there is very little space between molecules. free space. Liquids typically increase in volume (expand) when heated and decrease in volume (contract) when cooled.
ü Viscosity. In addition, liquids (like gases) are characterized by viscosity. It is defined as the ability to resist the movement of one of the parts relative to the other - that is, as internal friction. When adjacent layers of a liquid move relative to each other, a collision of molecules inevitably occurs in addition to that due to thermal motion. The liquid in the vessel, set in motion and left to itself, will gradually stop, but its temperature will rise.
ü Free Surface Formation and Surface Tension.Due to volume conservation, the liquid is able to form a free surface. Such a surface is the phase separation surface of a given substance: on one side there is a liquid phase, on the other - a gaseous (vapor) phase. If the liquid and gaseous phases of the same substance are in contact, forces arise that tend to reduce the interface area - surface tension forces . The interface behaves like an elastic membrane that tends to shrink.
ü Evaporation and condensation
ü Boiling
ü wetting - surface phenomenon, which occurs when a liquid contacts a solid surface in the presence of steam, that is, at the interfaces of three phases.
ü Miscibility- the ability of liquids to dissolve in each other. An example of miscible liquids: water and ethyl alcohol, an example of immiscible liquids: water and liquid oil.
ü Diffusion. When two miscible liquids are in a vessel, the molecules, as a result of thermal motion, begin to gradually pass through the interface, and thus the liquids gradually mix. This phenomenon is called diffusion (it also occurs in substances in other states of aggregation).
ü Overheating and hypothermia. A liquid can be heated above the boiling point in such a way that boiling does not occur. This requires uniform heating, without significant temperature differences within the volume and without mechanical influences such as vibration. If in superheated liquid throw something, it instantly boils. superheated water easy to obtain in a microwave oven. Subcooling - cooling of a liquid below the freezing point without turning into a solid state of aggregation.






