Characteristics of the liquid state of matter. Characteristics of the liquid state of matter
The main property of a liquid, which distinguishes it from other states of aggregation, is the ability to change its shape indefinitely under the action of tangential mechanical stresses, even arbitrarily small, while practically maintaining volume. A substance in a liquid state exists in a certain temperature range, below which it passes into a solid state (crystallization occurs or transformation into a solid amorphous state - glass), above - into a gaseous state (evaporation occurs). The boundaries of this interval depend on the pressure.
3.1Physical properties of liquids:
ü Fluidity(The main property. Unlike plastic solids, a liquid has no yield strength: it is enough to apply an arbitrarily small external force to let the liquid flow.
ü Preservation of volume. One of the characteristic properties of a liquid is that it has a certain volume (with constant external conditions). A liquid is extremely difficult to compress mechanically because, unlike a gas, there is very little space between molecules. free space. Liquids typically increase in volume (expand) when heated and decrease in volume (contract) when cooled.
ü Viscosity. In addition, liquids (like gases) are characterized by viscosity. It is defined as the ability to resist the movement of one of the parts relative to the other - that is, as internal friction. When adjacent layers of a liquid move relative to each other, a collision of molecules inevitably occurs in addition to that due to thermal motion. The liquid in the vessel, set in motion and left to itself, will gradually stop, but its temperature will rise.
ü Free Surface Formation and Surface Tension.Due to volume conservation, the liquid is able to form a free surface. Such a surface is the phase separation surface of a given substance: on one side there is a liquid phase, on the other - a gaseous (vapor) phase. If the liquid and gaseous phases of the same substance come into contact, forces arise that tend to reduce the interface area - forces surface tension. The interface behaves like an elastic membrane that tends to shrink.
ü Evaporation and condensation
ü Boiling
ü wetting - surface phenomenon, which occurs when a liquid contacts a solid surface in the presence of steam, that is, at the interfaces of three phases.
ü Miscibility- the ability of liquids to dissolve in each other. An example of miscible liquids: water and ethyl alcohol, an example of immiscible liquids: water and liquid oil.
ü Diffusion. When two miscible liquids are in a vessel, the molecules, as a result of thermal motion, begin to gradually pass through the interface, and thus the liquids gradually mix. This phenomenon is called diffusion (it also occurs in substances in other states of aggregation).
ü Overheating and hypothermia. A liquid can be heated above the boiling point in such a way that boiling does not occur. This requires uniform heating, without significant temperature differences within the volume and without mechanical influences such as vibration. If in superheated liquid throw something, it instantly boils. Superheated water is easy to obtain in a microwave oven. Subcooling is the cooling of a liquid below the freezing point without turning into a solid state of aggregation.
Unlike gases, rather large forces of mutual attraction act between liquid molecules, which determines the peculiar nature of molecular motion. The thermal motion of a liquid molecule includes vibrational and forward movement. Each molecule oscillates around a certain equilibrium point for some time, then moves and again occupies a new equilibrium position. This determines its fluidity. The forces of intermolecular attraction do not allow molecules to move far from each other during their movement. The total effect of the attraction of molecules can be represented as the internal pressure of liquids, which reaches very high values. This explains the constancy of volume and the practical incompressibility of liquids, although they easily take any form.
The properties of liquids also depend on the volume of molecules, their shape and polarity. If the liquid molecules are polar, then two or more molecules combine (associate) into a complex complex. Such liquids are called associated liquids. Associated liquids (water, acetone, alcohols) have higher boiling points, less volatility, higher permittivity. For example, ethyl alcohol and dimethyl ether have the same molecular formula (C 2 H 6 O). Alcohol is an associated liquid and boils at more high temperature than dimethyl ether, which is a non-associated liquid.
The liquid state is characterized by such physical properties as density, viscosity, surface tension.
Surface tension.
The state of the molecules in surface layer, differs significantly from the state of molecules deep in the liquid. Consider a simple case - liquid - vapor (Fig. 2).
Rice. 2. Action of intermolecular forces on the interface and inside the liquid
On fig. 2, the molecule (a) is inside the liquid, the molecule (b) is in the surface layer. The spheres around them are the distances over which the forces of intermolecular attraction of the surrounding molecules extend.
The molecule (a) is uniformly affected by intermolecular forces from the surrounding molecules, so the forces of intermolecular interaction are compensated, the resultant of these forces is zero (f=0).
The density of a vapor is much less than the density of a liquid, since the molecules are far apart from each other. Therefore, the molecules in the surface layer almost do not experience the force of attraction from these molecules. The resultant of all these forces will be directed inside the liquid perpendicular to its surface. Thus, the surface molecules of a liquid are always under the influence of a force that tends to draw them in and, thereby, reduce the surface of the liquid.
To increase the liquid interface, it is necessary to expend work A (J). The work required to increase the interface S by 1 m 2 is a measure of the surface energy or surface tension.
In this way, surface tension d (J / m 2 \u003d Nm / m 2 \u003d N / m) - the result of uncompensated intermolecular forces in the surface layer:
e = F/S (F is the surface energy) (2.3)
Exists big number methods for determining surface tension. The most common are the stalagmometric method (the method of counting drops) and the method of the highest pressure of gas bubbles.
Using the methods of X-ray diffraction analysis, it was found that in liquids there is some orderliness in the spatial arrangement of molecules in individual microvolumes. Near each molecule, the so-called short-range order is observed. At some distance from it, this regularity is violated. And in the entire volume of the liquid there is no order in the arrangement of particles.
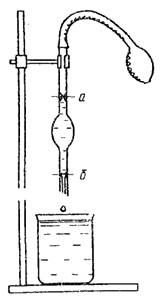
Rice. 3. Stalagmometer 4. Viscometer
Viscosity h (Pa s) - the property to resist the movement of one part of the liquid relative to the other. In practical life, a person is faced with a large variety of liquid systems, the viscosity of which is different - water, milk, vegetable oils, sour cream, honey, juices, molasses, etc.
The viscosity of liquids is due to intermolecular effects that limit the mobility of molecules. It depends on the nature of the liquid, temperature, pressure.
Viscosity is measured by devices called viscometers. The choice of viscometer and method for determining the viscosity depends on the state of the system under study and its concentration.
For liquids with a low viscosity or low concentration, capillary-type viscometers are widely used.
2.1 Bernoulli's law.
2.2 Pascal's law.
2.3 Laminar flow of liquids.
2.4 Poisel's law.
2.5 Turbulent flow of liquids.
3.1 Measuring the viscosity of a liquid.
3.2 Liquid volume and flow measurement
1. Liquid state of matter and its properties.
Liquids are intermediate between gaseous and solids. At temperatures close to boiling points, the properties of liquids approach those of gases; at temperatures close to melting points, the properties of liquids approach those of solids. If solid substances are characterized by a strict ordering of particles, extending over distances of up to hundreds of thousands of interatomic or intermolecular radii, then in a liquid substance there are usually no more than a few tens of ordered particles - this is explained by the fact that the ordering between particles in different places liquid substance arises just as quickly as it is again “smeared out” by the thermal oscillations of particles. At the same time, the total packing density of particles of a liquid substance differs little from that of a solid substance - therefore, their density is close to the density solids, and the compressibility is very low. For example, to reduce the volume occupied by liquid water by 1%, it is required to apply a pressure of ~ 200 atm, while the same decrease in the volume of gases requires a pressure of the order of 0.01 atm. Therefore, the compressibility of liquids is approximately 200: 0.01 = 20,000 times less than the compressibility of gases.
It was noted above that liquids have a certain volume of their own and take the shape of the vessel in which they are located; these properties are much closer to those of a solid than a gaseous substance. The close proximity of the liquid state to the solid state is also confirmed by the data on standard enthalpies of vaporization ∆Н° exp and standard enthalpies of melting ∆Н° pl. The standard enthalpy of vaporization is the amount of heat required to convert 1 mole of liquid to vapor at 1 atm (101.3 kPa). The same amount of heat is released when 1 mole of vapor condenses into a liquid at 1 atm. The amount of heat spent on the transformation of 1 mole of a solid into a liquid at 1 atm is called standard enthalpy melting (the same amount of heat is released when "freezing" ("solidifying") 1 mol of liquid at 1 atm). It is known that ∆Н° pl is much less than the corresponding values of ∆Н° exp, which is easy to understand, since the transition from solid state to liquid is accompanied by a lesser violation of intermolecular attraction than the transition from liquid to gaseous state.
A number of other important properties of liquids are more reminiscent of the properties of gases. So, like gases, liquids can flow - their property is called fluidity. The resistance to flow is determined by the viscosity. Fluidity and viscosity are affected by attractive forces between liquid molecules, their relative molecular weight, and a number of other factors. The viscosity of liquids is ~100 times greater than that of gases. Just like gases, liquids can diffuse, albeit much more slowly, because liquid particles are packed much more densely than gas particles.
One of the most important properties of a liquid is its surface tension (this property is not inherent in either gases or solids). A molecule in a liquid is subjected to uniform intermolecular forces from all sides. However, on the surface of the liquid, the balance of these forces is disturbed, and as a result, the “surface” molecules are under the action of a certain resultant force directed inside the liquid. For this reason, the surface of the liquid is in a state of tension. Surface tension is the minimum force that restrains the movement of liquid particles into the depth of the liquid and thereby keeps the surface of the liquid from contracting. It is surface tension that explains the "teardrop" shape of freely falling fluid particles.
Due to volume conservation, the liquid is able to form a free surface. Such a surface is the phase interface of a given substance: on one side there is a liquid phase, on the other - a gaseous (steam), and, possibly, other gases, such as air. If the liquid and gaseous phases of the same substance are in contact, forces arise that tend to reduce the interface area - surface tension forces. The interface behaves like an elastic membrane that tends to shrink.
Surface tension can be explained by the attraction between liquid molecules. Each molecule attracts other molecules, seeks to "surround" itself with them, and therefore, to leave the surface. Accordingly, the surface tends to decrease. Therefore, soap bubbles and bubbles during boiling tend to take on a spherical shape: for a given volume, a ball has a minimum surface. If only surface tension forces act on a liquid, it will necessarily take on a spherical shape - for example, water drops in weightlessness.
Small objects with a density greater than the density of a liquid are able to "float" on the surface of the liquid, since the force of gravity is less than the force that prevents the increase in surface area.
Wetting is a surface phenomenon that occurs when a liquid contacts a solid surface in the presence of steam, that is, at the interfaces of three phases. Wetting characterizes the “sticking” of a liquid to the surface and spreading over it (or, conversely, repulsion and non-spreading). There are three cases: non-wetting, limited wetting and complete wetting.
Miscibility is the ability of liquids to dissolve in each other. An example of miscible liquids: water and ethyl alcohol, an example of immiscible liquids: water and liquid oil.
When two miscible liquids are in a vessel, the molecules, as a result of thermal motion, begin to gradually pass through the interface, and thus the liquids gradually mix. This phenomenon is called diffusion (it also occurs in substances in other states of aggregation).
A liquid can be heated above the boiling point in such a way that boiling does not occur. This requires uniform heating, without significant temperature differences within the volume and without mechanical influences such as vibration. If something is thrown into a superheated liquid, it instantly boils. Superheated water is easy to get in the microwave.
Subcooling - cooling of a liquid below the freezing point without turning into a solid state of aggregation. As with superheating, subcooling requires the absence of vibration and significant temperature fluctuations.
If the surface of the liquid is displaced from the equilibrium position, then under the action of restoring forces, the surface begins to move back to the equilibrium position. This movement, however, does not stop, but turns into oscillating motion near the equilibrium position and extends to other areas. This creates waves on the surface of a liquid.
If the restoring force is predominantly gravity, then such waves are called gravitational waves. Gravitational waves on water can be seen everywhere.
If the restoring force is predominantly a surface tension force, then such waves are called capillary. If these forces are comparable, such waves are called capillary-gravity waves. Waves on the surface of a liquid are attenuated by viscosity and other factors.
Formally speaking, for the equilibrium coexistence of a liquid phase with other phases of the same substance - gaseous or crystalline - strictly defined conditions are needed. So, at a given pressure, a strictly defined temperature is needed. Nevertheless, in nature and in technology everywhere liquid coexists with vapor, or also with solid state of aggregation- for example, water with water vapor and often with ice (if we consider steam as a separate phase present along with air). This is due to the following reasons.
Unbalanced state. It takes time for the liquid to evaporate, until the liquid has completely evaporated, it coexists with the vapor. In nature, water is constantly evaporating, as well as the reverse process - condensation.
closed volume. The liquid in a closed vessel begins to evaporate, but since the volume is limited, the vapor pressure rises, it becomes saturated even before the liquid has completely evaporated, if its amount was large enough. When the saturation state is reached, the amount of evaporated liquid is equal to the amount of condensed liquid, the system comes into equilibrium. Thus, in a limited volume, the conditions necessary for the equilibrium coexistence of liquid and vapor can be established.
The presence of the atmosphere in the conditions of terrestrial gravity. Affects the liquid Atmosphere pressure(air and steam), while for steam, practically only its partial pressure. Therefore, liquid and vapor above its surface correspond to different points on the phase diagram, in the area of existence of the liquid phase and in the area of existence of the gaseous, respectively. This does not cancel evaporation, but evaporation takes time during which both phases coexist. Without this condition, liquids would boil and evaporate very quickly.
2.1 Bernoulli's law - is a consequence of the law of conservation of energy for a stationary flow of an ideal (that is, without internal friction) incompressible fluid:
is the density of the fluid, is the velocity of the flow, is the height at which the considered element of the liquid is located, is the pressure at the point in space where the center of mass of the considered element of the liquid is located, is the acceleration of free fall.The constant on the right hand side is usually called pressure, or full pressure, and also Bernoulli integral. The dimension of all terms is a unit of energy per unit volume of liquid.
This ratio, derived by Daniel Bernoulli in 1738, was named after him. Bernoulli equation. For horizontal pipe h= 0 and the Bernoulli equation takes the form:
This form of the Bernoulli equation can be obtained by integrating the Euler equation for a stationary one-dimensional fluid flow, at a constant density ρ:
According to Bernoulli's law, the total pressure in a steady flow of fluid remains constant along this flow.
Full pressure consists of the weighted (ρ gh), static (p) and dynamic (ρν 2 /2) pressures.
It follows from Bernoulli's law that as the flow cross section decreases, due to an increase in velocity, that is, dynamic pressure, the static pressure decreases. This is the main reason for the Magnus effect. Bernoulli's law is also valid for laminar gas flows. The phenomenon of a decrease in pressure with an increase in the flow rate underlies the operation of various types of flow meters (for example, a Venturi tube), water and steam jet pumps. And the consistent application of Bernoulli's law led to the emergence of a technical hydromechanical discipline - hydraulics.
Bernoulli's law is valid in its pure form only for liquids whose viscosity is zero, that is, liquids that do not stick to the surface of the pipe. In fact, it has been experimentally established that the velocity of a liquid on the surface of a solid body is almost always exactly zero (except in cases of jet separation under certain rare conditions).
2.2 Pascal's law is formulated like this:
 The pressure exerted on a liquid (or gas) in any one place on its boundary, for example, by a piston, is transmitted without change to all points of the liquid (or gas).
The pressure exerted on a liquid (or gas) in any one place on its boundary, for example, by a piston, is transmitted without change to all points of the liquid (or gas). Basic property of liquids and gases- transfer pressure without change in all directions - is the basis for the design of hydraulic and pneumatic devices and machines.
How many times the area of one piston is greater than the area of the other, the same number of times the hydraulic machine gives a gain in strength.
2.3 Laminar flow(lat. lamina- plate, strip) - a flow in which a liquid or gas moves in layers without mixing and pulsations (that is, random rapid changes in speed and pressure).
Laminar flow is possible only up to a certain critical value of the Reynolds number, after which it becomes turbulent. The critical value of the Reynolds number depends on the specific type of flow (flow in a round pipe, flow around a ball, etc.). For example, for a flow in a round pipe
The Reynolds number is determined by the following relationship:
ρ is the density of the medium, kg/m 3 ;
v- characteristic speed, m/s;
L- characteristic size, m;
η - dynamic viscosity of the medium, N*s/m 2 ;
ν - kinematic viscosity of the medium, m 2 / s ();
Q- volumetric flow rate;
A- sectional area of the pipe.
The Reynolds number as a criterion for the transition from laminar to turbulent flow and vice versa works relatively well for pressure flows. When passing to free-flow flows, the transition zone between laminar and turbulent regimes increases, and the use of the Reynolds number as a criterion is not always justified. For example, in reservoirs, the formally calculated values of the Reynolds number are very high, although laminar flow is observed there.
2.4 Equation or Poiseuille's law- the law that determines the flow rate of a fluid in a steady flow of a viscous incompressible fluid in a thin cylindrical pipe of circular cross section.
According to the law, the second volumetric flow rate of a liquid is proportional to the pressure drop per unit length of the tube (pressure gradient in the pipe) and the fourth power of the radius (diameter) of the pipe:
- Q- fluid flow in the pipeline;
- D- pipeline diameter;
- v- fluid velocity along the pipeline;
- r- distance from the axis of the pipeline;
- R- pipeline radius;
- p 1 − p 2 - pressure difference at the inlet and outlet of the pipe;
- η is the viscosity of the liquid;
- L- pipe length.
Poiseuille's law works only for laminar flow and provided that the length of the tube exceeds the so-called length of the initial section, which is necessary for the development of laminar flow in the tube.
The Poiseuille flow is characterized by a parabolic velocity distribution along the tube radius. In each cross section of the tube average speed half the maximum speed in this section.
2.5 T urbulent t(from Latin turbulentus - turbulent, chaotic), the form of the flow of a liquid or gas, in which their elements make disorderly, unsteady movements along complex trajectories, which leads to intense mixing between layers of moving liquid or gas (see Turbulence). T. t. in pipes, channels, boundary layers near solids flowed around by liquid or gas, as well as so-called. free T. t. - jets, traces behind solids moving relative to a liquid or gas, and zones of mixing between flows of different speeds that are not separated by c.-l. solid walls. T. t. differ from the corresponding laminar flows both in their complex internal structure (Fig. 1), and in the distribution of the average velocity over the flow cross section and integral characteristics - the dependence of the average cross section or max. speed, flow, as well as the coefficient. resistance from the Reynolds number Re. The profile of the average velocity of a thermometer in pipes or channels differs from parabolic. profile of the corresponding laminar flow with a faster increase in velocity near the walls and less curvature towards the center. parts of the flow (Fig. 2). With the exception of a thin layer near the wall, the velocity profile is described by a logarithmic law (i.e., the velocity depends linearly on the logarithm of the distance to the wall). Drag coefficient:
- friction stress on the wall,is the density of the liquid,
- its speed, average over the flow section) is related to Re by the ratio
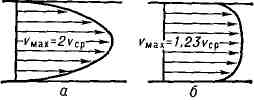
Average velocity profile: a - for laminar flow, 6 - for turbulent flow.
3.1 Fluid Viscosity Measurement .
Kinematic viscosity is a measure of the flow of a resistive fluid under the influence of gravity. When two liquids of equal volume are placed in identical capillary viscometers and move by gravity, the viscous liquid takes longer to flow through the capillary. If one fluid takes 200 seconds to flow out and another takes 400 seconds, the second fluid is twice as viscous as the first on the kinematic viscosity scale.
Absolute viscosity, sometimes called dynamic or simple viscosity, is the product of kinematic viscosity and fluid density:
Absolute Viscosity = Kinematic Viscosity * Density
The dimension of kinematic viscosity is L 2 /T, where L is length and T is time). SI UNIT kinematic viscosity - 1 cSt (centiStokes)=mm 2 /s. Absolute viscosity is expressed in centipoise (cPoise). SI UNIT of absolute viscosity - millipascal second 1 MPa * s = 1 cPas.
A device for measuring viscosity is called a viscometer. Viscometers can be classified into three main types:
BUT. Capillary viscometers measure the flow of a fixed volume of liquid through a small orifice at a controlled temperature. The shear rate can be measured from about zero to 106 s -1 by changing the capillary diameter and applied pressure. Types of capillary viscometers and their modes of operation:
Glass capillary viscometer (ASTM D 445) - Liquid passes through a hole of a set diameter under the influence of gravity. The shear rate is less than 10 s -1 . The kinematic viscosity of all automotive oils is measured by capillary viscometers.
High Pressure Capillary Viscometer (ASTM D 4624 and D 5481) - A fixed volume of liquid is extruded through a diameter glass capillary under the action of an applied gas pressure. The shear rate can be changed up to 106 s -1 . This technique is commonly used to model the viscosity of engine oils in working main bearings. This viscosity is called High Temperature High Shear Viscosity (HTHS) and is measured at 150°C and 106 s -1 . HTHS viscosity is also measured with a tapered bearing simulator, ASTM D 4683 (see below).
B. Rotational viscometers use torque on a rotating shaft to measure the resistance of a fluid to flow. Rotational viscometers include cold cranking simulator (CCS), mini rotational viscometer (MRV), Brookfield viscometer, and tapered bearing simulator (TBS). The shear rate can be changed by changing the dimensions of the rotor, the gap between the rotor and the stator wall, and the rotational speed.
Cold Scroll Simulator (ASTM D 5293) - CCS measures apparent viscosity in the range of 500 to 200,000 cPas. The shear rate is between 104 and 105 s -1 . normal range operating temperature- from 0 to -40°C. CCS showed excellent correlation with engine starting at low temperatures. The SAE J300 viscosity classification defines the low temperature viscosity performance of engine oils by CCS and MRV limits.
Mini Rotary Viscometer (ASTM D 4684) - The MRV test, which is related to the mechanism of oil pumpability, is a measurement at low shear rate. main feature method - slow sample cooling rate. The sample is prepared to have a specific thermal history that includes heating, slow cooling, and impregnation cycles. The MRV measures the apparent residual stress, which, if greater than a threshold value, indicates a potential pumping failure problem due to air intrusion. Above a certain viscosity (currently defined as 60,000 centipoise SAE J 300), the oil can cause pumpability failure through a mechanism called the "limited flow effect". An SAE 10W oil, for example, should have a maximum viscosity of 60,000 cPas at -30°C without residual stress. This method also measures the apparent viscosity at shear rates from 1 to 50 s -1 .
Brookfield viscometer - determines viscosity over a wide range (from 1 to 105 Poise) at low shear rates (up to 102 s -1).
ASTM D 2983 is primarily used to determine the low temperature viscosity of automotive gear oils, automatic transmission oils, hydraulic oils and tractor oils. Temperature - testing ranges from -5 to -40°C.
ASTM D 5133, the Brookfield Scan method, measures the Brookfield viscosity of a sample when cooled at a constant rate of 1°C/hour. Like MRV, the ASTM D 5133 method is designed to determine the pumpability of an oil at low temperatures. This test determines the nucleation point, defined as the temperature at which the sample reaches a viscosity of 30,000 cPas. The nucleation index is also defined as the highest rate of increase in viscosity from -5°C to the lowest test temperature. This method finds application in motor oils and is required by ILSAC GF-2. Tapered Bearing Simulator (ASTM D 4683) - This technique also measures the viscosity of motor oils at high temperature and high shear (see High Pressure Capillary Viscometer). Very high shear rates are obtained due to the extremely small gap between the rotor and the stator wall.
Viscosity Index (VI) is an empirical number that indicates the degree of change in the viscosity of an oil within a given temperature range. A high VI means a relatively small change in viscosity with temperature, and a low VI means a large change in viscosity with temperature. Most mineral base oils have a VI between 0 and 110, but polymer oil (multigrage) VI often exceeds 110.
To determine the viscosity index, it is required to determine the kinematic viscosity at 40°C and 100°C. After that, the IV is determined from the tables according to ASTM D 2270 or ASTM D 39B. Since VI is determined from the viscosity at 40°C and 100°C, it is not related to low temperature or HTHS viscosity. These values are obtained using CCS, MRV, low temperature Brookfield viscometer and high shear viscometers.
The SAE has not used IV to classify motor oils since 1967 because the term is technically obsolete. However, the American Petroleum Institute's API 1509 method describes a base oil classification system using VI as one of several parameters to ensure the principles of oil interchangeability and the universality of the viscosity scale.
3.2. Measurement of the volume and flow of liquid.
To measure the flow of liquids, flowmeters based on various principles of operation are used: variable and constant pressure drop, variable level, electromagnetic, ultrasonic, vortex, thermal and turbine flowmeters.
To measure the amount of a substance, flow meters with integrators or counters are used. The integrator continuously sums up the readings of the device, and the amount of the substance is determined by the difference in its readings over the required period of time.
The measurement of flow and quantity is a complex task, since the physical properties of the measured flows affect the readings of the instruments: density, viscosity, phase ratio in the flow, etc. Physical properties the measured flows, in turn, depend on the operating conditions, mainly on temperature and pressure.
If the operating conditions of the flow meter differ from the conditions under which it was calibrated, then the error in the readings of the device may significantly exceed the allowable value. Therefore, for mass-produced devices, limitations have been established for the scope of their application: according to the properties of the measured flow, maximum temperature and pressure, the content of solid particles or gases in the liquid, etc.
Variable pressure flowmeters
The operation of these flowmeters is based on the occurrence of a pressure drop across the narrowing device in the pipeline when a liquid or gas flow passes through it. When the flow rate Q changes, the value of this pressure drop? p also changes.
 For some narrowing devices as flow converters into differential pressure, the transfer coefficient is determined experimentally and its values are summarized in special tables. Such narrowing devices are called standard.
For some narrowing devices as flow converters into differential pressure, the transfer coefficient is determined experimentally and its values are summarized in special tables. Such narrowing devices are called standard. The simplest and most common constriction device is the diaphragm. The standard diaphragm is a thin disk with a round hole in the center. The transmission coefficient of the diaphragm essentially depends on the resistance of the diaphragm, and especially the input edge of the hole. Therefore, diaphragms are made of materials that are chemically resistant to the measured medium and resistant to mechanical wear. In addition to the diaphragm, a Venturi nozzle and a Venturi pipe are also used as standard narrowing devices, which create less hydraulic resistance in the pipeline.
The orifice of a variable pressure differential flow meter is a primary converter in which the flow rate is converted into a differential pressure.
Differential pressure gauges serve as intermediate converters for variable pressure flowmeters. Differential pressure gauges are connected to the narrowing device by impulse tubes and are installed in close proximity to it. Therefore, variable pressure flowmeters usually use differential pressure gauges equipped with an intermediate converter for transmitting measurement results to the operator's shield (for example, diaphragm differential pressure gauges DM).
As well as when measuring pressure and level, separation vessels and membrane separators are used to protect differential pressure gauges from the aggressive effects of the medium being measured.
A feature of the primary converters of variable pressure drop meters is the quadratic dependence of the pressure drop on the flow rate. In order for the readings of the measuring device of the flow meter to linearly depend on the flow rate, a linearizing transducer is introduced into the measuring circuit of the variable pressure flow meters. Such a converter is, for example, a linearization block in the intermediate converter NP-PZ. With a direct connection of a differential pressure gauge with a measuring device (for example, KSD), linearization is performed in the device itself using a pattern with a quadratic characteristic.
Constant Differential Pressure Flowmeters
The flow rate of a liquid or gas can also be measured at a constant differential pressure. To maintain a constant pressure drop when the flow rate through the orifice changes, it is necessary to automatically change the area of its flow section. The easiest way is to automatically change the flow area in the rotameter.The rotameter is a vertical conical tube containing a float. The measured flow Q, passing through the rotameter from the bottom up, creates a pressure difference before and after the float. This pressure difference, in turn, creates a lift force that balances the weight of the float.
If the flow through the rotameter changes, then the pressure drop will also change. This will lead to a change in lift and, consequently, to an imbalance in the float. The float will begin to stir. And since the tube of the rotameter is conical, the area of the flow section in the gap between the float and the tube will change, as a result, the pressure drop will change, and hence the lifting force. When the pressure difference and lifting force will return to the previous values again, the float will balance and stop.
Thus, each value of the flow through the rotameter Q corresponds to a certain position of the float. Since for a conical tube the area of the annular gap between it and the float is proportional to the height of its rise, the rotameter scale is uniform.
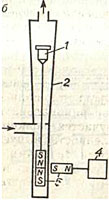 The industry produces rotameters with glass and metal tubes. For rotameters with a glass tube, the scale is printed directly on the surface of the tube. For remote measurement of the position of a float in a metal tube, intermediate linear displacement converters are used into a unified electrical or pneumatic signal.
The industry produces rotameters with glass and metal tubes. For rotameters with a glass tube, the scale is printed directly on the surface of the tube. For remote measurement of the position of a float in a metal tube, intermediate linear displacement converters are used into a unified electrical or pneumatic signal. In rotameters with an electrical output signal, the plunger of the differential transformer transducer moves with the float. Flowmeters with a pneumatic output signal use a magnetic coupling to transmit the float position to the transmitter. It consists of two permanent magnets. One - double - moves along with the float, the other, mounted on the lever of the displacement to compressed air pressure converter, moves along with the lever after the first magnet.
Rotameters are also available for measuring the flow of highly aggressive media. Rotameters are supplied with a jacket for steam heating. They are designed to measure the flow of crystallizing media.
Variable Level Flowmeters
From hydraulics it is known that if the liquid flows freely through the hole in the bottom of the tank, then its flow rate Q and the level in the tank H are interconnected. Therefore, by the level in the tank, one can judge the flow from it.
This principle is the basis for the operation of variable level flowmeters. It is obvious that the role of the primary converter here is played by the tank itself with a hole in the bottom. The output signal of such a converter is the level in the tank. Therefore, any of the considered level gauges can serve as an intermediate converter of the measuring circuit of the variable level flowmeter.
Variable level meters are commonly used to measure the flow of aggressive and contaminated liquids when they are discharged into tanks at atmospheric pressure.
Electromagnetic flowmeters
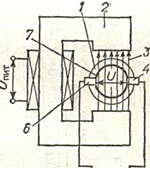 The operation of electromagnetic flowmeters is based on the law electromagnetic induction, according to which e will be induced in a conductor moving in a magnetic field. d.s., proportional to the speed of the conductor. In electromagnetic flowmeters, the role of a conductor is performed by an electrically conductive liquid flowing through pipeline 1 and crossing the magnetic field 3 of an electromagnet 2. In this case, an e will be induced in the liquid. d.s. U, proportional to the speed of its movement, i.e., the flow rate of the liquid.
The operation of electromagnetic flowmeters is based on the law electromagnetic induction, according to which e will be induced in a conductor moving in a magnetic field. d.s., proportional to the speed of the conductor. In electromagnetic flowmeters, the role of a conductor is performed by an electrically conductive liquid flowing through pipeline 1 and crossing the magnetic field 3 of an electromagnet 2. In this case, an e will be induced in the liquid. d.s. U, proportional to the speed of its movement, i.e., the flow rate of the liquid. The output signal of such a primary converter is taken by two insulated electrodes 4 and 6 installed in the pipeline wall. The section of the pipeline on both sides of the electrodes is covered with electrical insulation 7 to prevent shunting of the induced e. d.s. through the liquid and the pipeline wall.
The degree of aggressiveness of the measured media for electromagnetic flowmeters is determined by the insulation material of the pipe and electrodes of the primary converter. In flowmeters, rubber, acid-resistant enamel and fluoroplastic are used for this purpose. The most resistant to aggressive media is a flowmeter with a fluoroplastic insulating coating and graphitized fluoroplast electrodes.
During the operation of the flow meters, the zero and calibration of the device should be checked periodically, at least once a week. To check the primary converter is filled with the measured liquid. After that, the operation mode switch on the front panel of the measuring unit is moved to the “Measurement” position and the pointer of the measuring device is set to zero with the “Zero” potentiometer. When the switch is moved to the "Calibration" position, the arrow of the device should stop at 100%. Otherwise, the arrow is brought to this mark by the "Calibration" potentiometer.
A distinctive feature of electromagnetic flowmeters is the absence of additional pressure losses in the area. measurements. This is due to the absence of parts protruding into the pipe. A particularly valuable property of such flowmeters, in contrast to other types of flowmeters, is the ability to measure the flow rate of aggressive, abrasive and viscous liquids and slurries.
Ultrasonic flow meters
The operation of these flowmeters is based on the addition of the speed of propagation of ultrasound in the liquid and the speed of the liquid flow itself. The emitter and receiver of the ultrasonic pulses of the flow meter are located at the ends of the measuring section of the pipeline. The electronic unit contains a pulse generator and a time meter for the pulse to travel the distance between the emitter and the receiver.
Before starting operation, the flow meter is filled with the liquid, the flow rate of which will be measured, and the time it takes the pulse to travel this distance in a stagnant medium is determined. When the flow moves, its speed will add up with the speed of ultrasound, which will lead to a decrease in the pulse travel time. This time, converted in the block into a unified current signal, will be the smaller, the greater the flow rate, i.e., the greater its consumption Q.
Ultrasonic flowmeters have the same advantages as electromagnetic flowmeters, and, in addition, they can measure the flow of non-conductive liquids.
Vortex meters
The operation of such flowmeters is based on the occurrence of vortices when a flow meets a non-streamlined body. During operation of the flow meter, the vortices are detached alternately from opposite sides of the body located across the flow. The vortex separation frequency is directly proportional to the flow velocity, i.e., its volumetric flow rate Q. At the site of the vortex, the flow velocity increases, and the pressure decreases. Therefore, the frequency of the formation of vortices can be measured, for example, with a pressure gauge, the electrical output of which is fed to a frequency meter.
Thermal flow meters
 The thermal flow meter consists of a heater 1 and two temperature sensors 2 and 3, which are installed outside the tube 4 with the measured flow. At constant power heater, the amount of heat taken from it by the flow will also be constant. Therefore, with an increase in flow rate Q, the heating of the flow will decrease, which is determined by the temperature difference measured by temperature sensors 3 and 2. To measure high flow rates, not the entire flow Q is measured, but only its part Q1, which is passed through tube 4. This tube shunts pipeline section 5 , equipped with a choke 6. The flow area of the choke determines the upper limit of the range of measured flow rates: the larger this section, the greater the flow rate can be measured (at the same heater power).
The thermal flow meter consists of a heater 1 and two temperature sensors 2 and 3, which are installed outside the tube 4 with the measured flow. At constant power heater, the amount of heat taken from it by the flow will also be constant. Therefore, with an increase in flow rate Q, the heating of the flow will decrease, which is determined by the temperature difference measured by temperature sensors 3 and 2. To measure high flow rates, not the entire flow Q is measured, but only its part Q1, which is passed through tube 4. This tube shunts pipeline section 5 , equipped with a choke 6. The flow area of the choke determines the upper limit of the range of measured flow rates: the larger this section, the greater the flow rate can be measured (at the same heater power). Turbine meters
In such flowmeters, the measured flow drives an impeller that rotates in bearings. The speed of rotation of the impeller is proportional to the flow rate, i.e., the flow rate Q. To measure the speed of rotation of the impeller, its housing is made of a non-magnetic material. A differential transformer converter is installed outside the housing, and an edge is made of a ferromagnetic material at one of the blades of the turbine. When this blade passes by the converter, its inductive reactance changes and, with a frequency proportional to the flow rate Q, the voltage on the secondary windings U out changes. Measuring device Such a flow meter is a frequency meter that measures the frequency of voltage changes.
Speed counters
These meters are similar in design to turbine flow meters. The difference between them lies in the fact that the speed of rotation of the turbine is measured in flow meters, and the number of its revolutions is measured in meters, which is then converted to the amount of liquid that has passed through the meter for the time interval of interest to us, for example, per month.
Liquids occupy an intermediate position between gaseous and solid substances. At temperatures close to boiling points, the properties of liquids approach those of gases; at temperatures close to melting points, the properties of liquids approach those of solids.

Physical properties of liquids Fluidity Conservation of volume Evaporation (gradual transition of a substance from liquid to gaseous phase) and condensation (transition of a substance gaseous state into a liquid) Boiling (At a sufficiently high temperature, the vapor pressure becomes higher than the pressure inside the liquid, and vapor bubbles begin to form there) Miscibility (the ability of liquids to dissolve in each other) Diffusion (the mutual penetration of contacting substances into each other due to the thermal movement of the particles of the substance.)



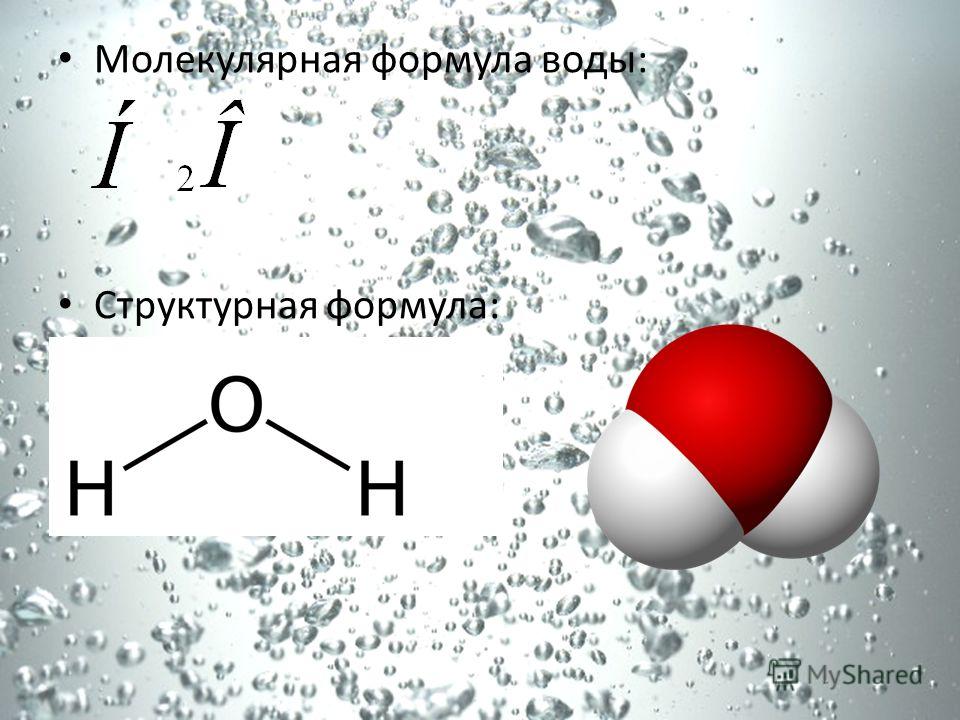


Chemical properties water Water reacts with many non-metal oxides. Unlike the previous ones, these reactions are not redox, but compound reactions: (sulphurous acid) Some metal oxides can also react with water: CaO + H2O \u003d Ca (OH) 2 (calcium hydroxide (slaked lime))

Chemical properties of water Water forms numerous compounds in which its molecule is completely preserved (hydrates, crystalline hydrates): (sulfuric acid hydrate) Synthesis of starch and other similar compounds (carbohydrates) by plants, occurring with the release of oxygen: (under the action of light)



Importance of water for humans Water is the most important component of our habitat. The water content in various organs is %. With age, the amount of water in the body changes. A three-month-old fetus contains 90% water, a newborn 80%, an adult - 70%. Water carries our body's waste, delivers lubricant to our joints, stabilizes our temperature, and is the lifeblood of the cell. Water is a heat carrier and thermostat. The amount of water required to maintain water balance depends on age, physical activity, ambient temperature and humidity. The daily requirement of an adult is about 2.5 liters.


Liquid, occupying an intermediate position between gases and crystals, combines the properties of both types of these bodies..
1. Like a solid, a liquid slightly compressible due to the dense arrangement of molecules. (However, if water could be completely released from compression, then the water level in the world ocean would rise by 35 m and water would flood 5,000,000 km 2 of land.)
2. Like a solid, a liquid saves volume but like a gas takes the form of a vessel .
3. For crystals typical long range order in the arrangement of atoms (crystal lattice), for gases- full chaos. For liquid there is an intermediate state short range order , i.e. the arrangement of only the nearest molecules is ordered. When moving away from this molecule at a distance of 3–4 effective molecular diameters, the order is blurred. Therefore, liquids are close to polycrystalline bodies, consisting of very small crystals (about 10 9 m), arbitrarily oriented relative to each other. Due to this, the properties of most liquids are the same in all directions (and there is no anisotropy, as in crystals).
4. Most liquids, like solids, with increasing temperature increase their volume , while reducing its density (at a critical temperature, the density of a liquid is equal to the density of its vapor). Water is different famous anomaly , consisting in the fact that at +4 С water has a maximum density. This anomaly is explained by the fact that water molecules are partially assembled into groups of several molecules (clusters), forming peculiar large molecules. H 2 O, (H 2 O) 2 , (H 2 O) 3 … with different density. At different temperatures, the ratio of the concentrations of these groups of molecules is different.
Exist amorphous bodies (glass, amber, resins, bitumen...), which are usually considered as supercooled liquids with a very high viscosity. They have the same properties in all directions (isotropic), short-range order in the arrangement of particles, they do not have a melting point (when heated, the substance gradually softens and passes into a liquid state).
Used in technology magnetic fluids - these are ordinary liquids (water, kerosene, various oils), into which (up to 50%) are introduced the smallest particles (several microns in size) of a solid ferromagnetic material (for example, Fe 2 O 3). The movement of the magnetic fluid and its viscosity can be controlled by a magnetic field. In the strong magnetic fields magnetic fluid hardens instantly.
Some organic substances, the molecules of which have a filamentous shape or the shape of flat plates, can be in a special state, possessing both the properties of anisotropy and fluidity. They're called liquid crystals . To change the orientation of the molecules of a liquid crystal (in this case, its transparency changes), a voltage of about 1 V and a power of the order of microwatts are required, which can be provided by direct supply of signals from integrated circuits without additional amplification. Therefore, liquid crystals are widely used in electronic clock indicators, calculators, and displays.
When freezing, water increases in volume by 11%, and if water freezes in a closed space, a pressure of 2500 atmospheres can be reached (water pipes, rocks are destroyed ...).
withdrawals one of the biggest: 1) the dielectric constant(therefore, water is a good solvent, especially salts with ionic bonds - the entire periodic table is contained in the World Ocean); 2) heat of fusion(slow melting of snow in spring); 3) heat vaporization; 4) surface tension; 5) heat capacity(mild coastal climate).
Exists light (1 g / cm 3) and heavy (1.106 g/cm3) water . Light water ("living") - biologically active - it is protium oxide H 2 O. Heavy water ("dead") - suppresses the vital activity of organisms - it is deuterium oxide D 2 O. Protium (1 amu), deuterium (2 amu) and tritium (3 amu) are isotopes of hydrogen. There are also 6 isotopes of oxygen: from 14 O up to 19 O that can be found in a water molecule.
In water treatment magnetic field its properties change: the wettability of solids changes, their dissolution accelerates, the concentration of dissolved gases changes, the formation of scale in steam boilers is prevented, the hardening of concrete is accelerated by 4 times and its strength increases by 45%, there is a biological effect on humans (magnetic bracelets and earrings, magnetophores, etc.) and plants (germination and crop yields increase).
silver water can be stored for a long time (about six months), since water is neutralized from microbes and bacteria by silver ions (it is used in astronautics, for canning food, disinfecting water in pools, for medicinal purposes to prevent and combat gastrointestinal diseases and inflammatory processes).
Drinking water disinfection in city water pipes carried out by chlorination and ozonation of water. There are also physical methods of disinfection using ultraviolet radiation and ultrasound.
Solubility of gases in water depends on temperature, pressure, salinity, presence of other gases in the aqueous solution. In 1 liter of water at 0 С, the following can be dissolved: helium - 10 ml, carbon dioxide - 1713 ml, hydrogen sulfide - 4630 ml, ammonia - 1300000 ml (ammonia). When diving to great depths, scuba divers use special breathing mixtures so that when they ascend, they do not get "carbonated blood" due to the dissolution of nitrogen in it.
All living organisms 60-80% water. The blood of humans and animals is similar in salt composition to ocean water. Man and animals can synthesize water in their bodies, form it during the combustion of food products and the tissues themselves. In a camel, for example, the fat contained in the hump can, as a result of oxidation, give 40 liters of water.
At electrolysis two types of water can be obtained: 1) acidic water (“dead”), which acts as an antiseptic (similar to how many pathogenic microbes die in acidic gastric juice); 2) alkaline water (“live”), which activates biological processes (increases productivity, heals wounds faster, etc.).
You can learn about other features of water (structured, energy-informational, etc.) from the Internet.
TRIZ task 27. Water worker
Most often, various mechanisms have "solid-state" working bodies. Give examples of technical devices in which the working body is water (liquid). What laws of development of technical systems does such a working body correspond to?
TRIZ task 28. Water in a sieve
In the famous problem How to carry water in a sieve? there is an explicit physical contradiction: there should be holes in the sieve so that bulk solids can be sieved through it, and there should be no holes so that water does not pour out. One of the possible solutions to this problem can be found in Ya.I. Perelman in "Entertaining Physics", where it is proposed to lower the sieve into molten paraffin so that the sieve mesh is not wetted with water. Based techniques for eliminating technical and physical contradictions suggest 10-20 other ways to solve this problem.






