The composition of the nuclei of atoms of various elements. The composition of the nucleus of an atom. Nuclear forces. Mass defect and binding energy of the atomic nucleus. Nuclear reactions. Nuclear energy
The nucleus of any atom, except for the light hydrogen atom, consists of particles - nucleons two types: Z protons and N neutrons. The neutron was discovered in 1932 by James Chadwick, at the same time by Karl Anderson - the positron. The nucleus of the light hydrogen atom consists of one proton.
Proton open in is a charged particle – qp = +e. The mass of a proton is m p= 1.67265 10 -27 kg. AT nuclear physics it is customary to express the energy of particles in units of energy (eV), for which the mass is multiplied by the square of the speed of light c 2, then the proton mass m p = 938.26 MeV. The proton has a spin equal to s = 1/2.
Neutron also has spin s= 1/2. Its mass is close to the mass of a proton and is m n\u003d 1.67495 10 -27 kg or in units of energy (eV) m p = 939.55 MeV. However, the neutron has no electric charge. In the free state, the neutron is radioactive, it spontaneously decays, turning into a proton. In this case, an antineutrino is released.
The neutron is stable in the nucleus.
An atom is characterized by a charge number Z(which is equal to the number of protons in the nucleus). Number Z determines the atomic number in the periodic table. Mass number A=N+Z shows the total number of nucleons in the nucleus. Mass of all nucleons A makes the main contribution to the mass of the entire atom. The nucleus is also called a nuclide. The adopted scheme of the nuclide has the following form: Except nucleons there are no other particles in the nucleus. However, nucleons are not elementary particles: each of them consists of three quarks, which will be discussed in another lecture.
Atoms whose nuclei have the same charge numbers Z and various mass numbers A, have the same Chemical properties and are called isotopes. Isotopes of the same chemical element differ from each other only by the number of neutrons in the nucleus. Most substances with atoms of the same Z are a mixture of different isotopes. So, hydrogen, carbon and oxygen have 3 isotopes each: - ordinary hydrogen, - deuterium, - tritium; ![]() ;
; ![]() ; Tin has 10 isotopes.
; Tin has 10 isotopes.
Atoms whose nuclei have the same mass numbers A, are called isobars. Isobars, i.e. kernels with different Z, correspond to the nuclei of atoms of various chemical elements.
In Rutherford's scattering experiments α -particles on the atoms of matter, it was found that the nuclei have a finite size. A lot of time has passed since that moment, but experiments on the scattering of particles on atomic nuclei are still the most preferable in determining the size of the nucleus. Since electrons experience only electrostatic interaction with nuclei, the charge distribution inside the nucleus is studied using electron scattering. The distribution of nuclear matter inside the nucleus is judged by the scattering of neutrons, since in this case the interaction between particles is reduced only to a specific nuclear one. In order for the nucleus to "feel" the incident particle, taking into account the masses, the energy of the electron must be at least 124 MeV, and the energy of the neutron must be at least 8 MeV. Experiments with electrons and neutrons of various (but satisfying the specified conditions) energies showed that the volume of a nucleus is proportional to the number of nucleons in its composition:
In nuclei with a spin greater than or equal to 1, a deviation from the spherical shape is indeed observed. Such nuclei can be compressed or prolate ellipsoids of revolution, and the difference between their major and minor axes never exceeds 20% and, as a rule, is much smaller. In the first approximation, the nucleus can be considered a ball, the radius of the nucleus is thus: (13.3)
Constant R0≈ 1.3·10 –15 m. Its approximate value is due to the fact that the value of the radius of the nucleus, obtained from the distribution of nuclear matter, differs from the value of the radius obtained from the charge distribution. This means that the charge and matter are distributed inside the nucleus in a different way.
In the framework of nuclear theory, the quantity is used 1 fermi = 1 f= 10 -15 m.
Then the core radius is ![]() .
.
The nuclear spin I is the total angular momentum of the nucleus. For a nucleus with a mass number A it is equal to: 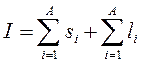 (13.4)
(13.4)
In this expression, the first term on the right is equal to the total spin moment of nucleons, and the second term is equal to the total orbital moment of nucleons in the nucleus. Values Si and l i are determined by the values of the corresponding quantum numbers: s p = s n= 1/2 and l = 0, 1, 2, ...
Magnetic moment of the nucleus μ i is the sum of the intrinsic magnetic moments of protons and neutrons and the orbital magnetic moments of protons (a neutron has a zero orbital magnetic moment for any l).
So, the core contains A nucleons. However, not all combinations of protons and neutrons form stable nuclei. This is due to the existence of nuclear energy levels. Since both protons and neutrons are fermions (their spin s = 1/2), then at each level there can be no more than two protons and two neutrons. Levels are filled according to the principle of minimizing the system of united particles. For example, consider two isotopes and . Their first two levels (Fig. 13.1) are filled in the same way.

Rice. 13.1 Stable isotope of carbon and unstable isotope of boron
At the last level, the 12th neutron is located in the nuclide, while at the same time, at the previous level, there is not enough proton until it is completely filled. The energy of a system of three neutrons and one proton will be greater than the energy of a system of two protons and two neutrons. Therefore, the isotope will not be stable and will decay rather quickly. At the same time, the isotope (containing 5 protons and 6 neutrons) is stable.
In light nuclei ( A< 20), как правило, число протонов и нейтронов одинаково (или отличается не единицу в случае ядер с нечетным числом нуклонов, причем число нейтронов обязательно more number protons). In heavy nuclei, the proportion of neutrons is getting larger. In such nuclei, in addition to the principle of energy minimization, the Coulomb repulsion of protons turns out to be significant. In nuclei with more than 10 protons, this repulsion is so strong that for the stability of the nucleus, this force must be compensated by something. Only attractive nuclear forces act between neutrons. Therefore, an increase in the number of neutrons in the composition of the nucleus leads to a balance of forces, i.e. to kernel stability.
The nucleus of an atom of any substance consists of protons and neutrons. ( Common name protons and neutrons - nucleons.) The number of protons is equal to the charge of the nucleus and coincides with the number of the element in the periodic table. The sum of the number of protons and neutrons is equal to the mass number. For example, the nucleus of an oxygen atom consists of 8 protons and 16 - 8 = 8 neutrons. The nucleus of an atom consists of 92 protons and 235 - 92 = 143 neutrons.
The forces that hold protons and neutrons in the nucleus are called nuclear forces. This is the strongest type of interaction.
If we compare the masses of nuclei with the masses of nucleons, it turns out that the mass of the nucleus of heavy elements is greater than the sum of the masses of protons and neutrons in the nucleus, and for light elements the mass of the nucleus is less than the sum of the masses of protons and neutrons in the nucleus. Therefore, there is a mass difference between the mass of the nucleus and the sum of the masses of protons and neutrons, called the mass defect. M = Mn - (Mp + Mn).
Since there is a connection between mass and energy, then during the fission of heavy nuclei and during the synthesis of light nuclei, energy must be released that exists due to the mass defect, and this energy is called the binding energy of the atomic nucleus.
This energy can be released during nuclear reactions. A nuclear reaction is a process of changing the charge of the nucleus and its mass, which occurs when the nucleus interacts with other nuclei or elementary particles. During the course of nuclear reactions, the conservation laws are fulfilled electric charges and mass numbers: the sum of charges (mass numbers) of nuclei and particles entering into a nuclear reaction is equal to the sum of charges (mass numbers) of the final products (nuclei and particles) of the reaction.
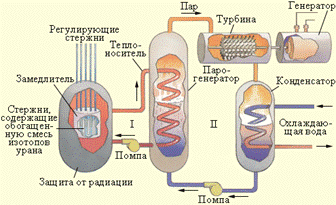
 A fission chain reaction is a nuclear reaction in which the particles causing the reaction are formed as products of that reaction. The uranium isotope 235 U has the ability to carry out a nuclear chain reaction. In the presence of certain critical parameters (critical mass - 50 kg, spherical shape with a radius of 9 cm), three neutrons released during the fission of the first nucleus fall into three neighboring nuclei, etc. The process goes in the form of a chain reaction that proceeds in a fraction of a second in the form nuclear explosion. Uncontrolled nuclear reaction is used in atomic bombs. For the first time, the physicist Enrico Fermi solved the problem of controlling the chain reaction of nuclear fission. They invented nuclear reactor in 1942. In our country, the reactor was launched in 1946 under the leadership of IV Kurchatov.
A fission chain reaction is a nuclear reaction in which the particles causing the reaction are formed as products of that reaction. The uranium isotope 235 U has the ability to carry out a nuclear chain reaction. In the presence of certain critical parameters (critical mass - 50 kg, spherical shape with a radius of 9 cm), three neutrons released during the fission of the first nucleus fall into three neighboring nuclei, etc. The process goes in the form of a chain reaction that proceeds in a fraction of a second in the form nuclear explosion. Uncontrolled nuclear reaction is used in atomic bombs. For the first time, the physicist Enrico Fermi solved the problem of controlling the chain reaction of nuclear fission. They invented nuclear reactor in 1942. In our country, the reactor was launched in 1946 under the leadership of IV Kurchatov.
 Thermonuclear reactions are reactions of fusion of light nuclei that occur when high temperature(approximately 107 K and above). The necessary conditions for the synthesis of helium nuclei from protons are found in the interiors of stars. On Earth, a thermonuclear reaction has been carried out only in experimental explosions, although international research is underway to control this reaction.
Thermonuclear reactions are reactions of fusion of light nuclei that occur when high temperature(approximately 107 K and above). The necessary conditions for the synthesis of helium nuclei from protons are found in the interiors of stars. On Earth, a thermonuclear reaction has been carried out only in experimental explosions, although international research is underway to control this reaction.
it promising directions nuclear energy. Since this energy can be used for peaceful purposes. Nuclear power plants are an example of this. Naval ships, icebreakers powered by nuclear installations.
24/2. Experimental task on the topic "Kinematics": checking the dependence of the time of movement of the ball along the inclined chute on the angle of the chute (2-3 experiments).
You have a chute, a ruler, a ball, a stopwatch and a metal cylinder at your disposal.
Install one end of the chute at a small height H (1-2 cm) above the table surface, and place a cylinder at the end of the chute. Measure the time it takes the ball, launched from rest from the top of the chute, to reach the cylinder. Make the height of the top of the trough equal to 2H and again measure the time of the ball's movement.
Do the results of the experiments confirm the assumption that the time of the ball's movement decreased by 2 times when the height of the upper point of the trough was doubled?
25/1. Radioactivity. Types of radioactive emissions and methods for their registration. Effect of ionizing radiation on living organisms.
In 1896, Becquerel discovered that uranium salts spontaneously, without any external influences, create some kind of radiation. Like X-rays, this radiation ionized the air and discharged the electroscope. Further studies carried out by Maria Sklodowska-Curie and Pierre Curie showed that the radiation of THORium and the new elements they discovered - RADIUM and POLONIA - have the same properties. The phenomenon of spontaneous radiation is called RADIOACTIVITY.
 Classical composition determination experiment
Classical composition determination experiment
radioactive radiation was supplied by Rutherford. He placed a radioactive preparation at the bottom of a narrow lead channel, and passed a thin beam of rays emerging from the hole through a magnetic field. During the development of a photographic plate located in the path of the rays, three bright spots were found - the places where the rays hit.
Thus, it was found that radioactive radiation consists of three parts that behave differently in a magnetic field. The negative component of the radiation (beta rays) deviated most strongly, the positive component experienced less deviation (alpha rays), and a third of the rays (gamma rays) did not deviate at all.
Research has made it possible to elucidate the nature of these radiations.
ALPHA RAYS are the nuclei of helium atoms flying at a speed of about 15,000-30,000 km/s. They have positive charge and rejected magnetic field to the left (according to the figure). Due to the large mass of particles, the deviation is small. Alpha particles have low penetrating power. A sheet of paper delays them.
BETA RAYS are electrons flying at a speed close to the speed of light. They are deflected by the magnetic field to the right (according to the figure). Due to the small mass, the deflection of beta rays is many times greater than that of alpha particles. Beta rays have a higher penetrating power. To stop them, you need to place an aluminum plate on the way.
GAMMA RAYS are electromagnetic waves of very small wavelength (smaller than x-rays). Magnetic and electric fields they are not rejected. Gamma rays have similar properties to X-rays. They have great penetrating power. Even a sheet of lead 1 cm thick does not completely stop them. The speed of propagation of gamma rays is the same as that of other electromagnetic waves - 300,000 km / s.
Ionizing radiation is recorded using a Geiger counter, cloud chamber, bubble chamber and photoemulsion method. The Geiger counter allows you to register electrons and gamma rays of high energy. Alpha particles do not get inside the counter because of the low penetrating power. In 1912, a cloud chamber was invented, which made it possible not only to register particles, but also to observe their trajectories (tracks). By placing the camera in a magnetic field, it was possible to measure the charge-to-mass ratio of particles and recognize them.
Radioactive radiation has a detrimental effect on living organisms. Even at low radiation power, radiation sickness and death can occur. The effect of radiation is characterized by the ABSORBED RADIATION DOSE D, which is equal to the ratio of the absorbed energy E of ionizing radiation to the mass M of the irradiated substance:
In SI, the absorbed dose of radiation is expressed in GREYAH (1 Gy) 1 Gy is equal to the absorbed dose of radiation, at which ionizing radiation energy of 1 J is transferred to the irradiated substance with a mass of 1 kg. A radiation dose of 3 - 10 Gy received in a short time is lethal. In practice, another unit is often used - RENTGEN (1 R). 1R is approximately equal to 0.01 Gy.






