What is a process that occurs at constant volume called? What is thermodynamics
The set of changes in the state of a thermodynamic system during the transition from one state to another under the influence of the environment is called a thermodynamic process.
The state of a gas in which each of its parameters throughout the mass has the same value is called the equilibrium state.
Thermodynamic processes can be equilibrium and non-equilibrium.
An equilibrium process is a process that goes through equilibrium states, i.e. when the gas parameters (specific volume, pressure and temperature) are the same at all points in the volume. Equilibrium processes proceeding infinitely slowly and consist of an infinitely large number of equilibrium states, and at any time between the working body and environment an equilibrium is established, that is, between the environment and the working fluid, the difference in temperature and pressure is infinitesimal. Equilibrium processes for any moment of time have fixed values of the main parameters, so they can be depicted graphically using diagrams (PV, TS-diagrams).
Real processes are non-equilibrium. The non-uniformity of real processes is determined, first of all, by the fact that under the influence of external conditions they proceed at finite speeds and the equilibrium state does not have time to be established in the working body (the state parameters throughout the mass of the gas are not the same).
Thermodynamics primarily considers equilibrium states and equilibrium processes of changing the state of a gas. Equilibrium processes have the property of reversibility, which means that they can be reverse direction through all states of the direct process.
Reversible processes are processes that proceed both in the forward and in the opposite direction through the same equilibrium states themselves. At the same time, at the end of them, no changes remain in the system itself or in the environment.
The main conditions for the reversibility of processes are:
The condition for external reversibility is the presence of thermal and mechanical equilibria, i.e. equality of temperatures and pressures of the working fluid and the environment;
The condition of internal reversibility is the absence of friction, diffusion and other unilaterally directed processes.
In heat engines and apparatuses, processes proceed at a finite temperature difference between the source and the working fluid, so such processes are irreversible. All real processes in terrestrial conditions are irreversible.
All analytical dependences in thermodynamics, which characterize the processes of state change, refer to reverse processes. The transition from reversible processes to real, irreversible ones is carried out by introducing empirical coefficients that take into account the deviations of real processes from reversible ones.
Thermodynamics is the science that studies thermal phenomena occurring in bodies without linking them to the molecular structure of matter.
In thermodynamics, it is considered that all thermal processes in bodies are characterized only by macroscopic parameters- pressure, volume and temperature. And since they cannot be applied to individual molecules or atoms, then, unlike the molecular-kinetic theory, in thermodynamics the molecular structure of a substance in thermal processes is not taken into account.
All concepts of thermodynamics are formulated as a generalization of the facts observed in the course of experiments. Because of this, it is called the phenomenological (descriptive) theory of heat.
Thermodynamic systems

Thermodynamics describes thermal processes occurring in macroscopic systems. Such systems consist of a huge number of particles - molecules and atoms, and are called thermodynamic.
thermodynamic system can be considered any object that can be seen naked eye or with the help of microscopes, telescopes and other optical instruments. The main thing is that the dimensions of the system in space and the time of its existence make it possible to measure its parameters - temperature, pressure, mass, chemical composition elements, etc., using devices that do not respond to the effects of individual molecules (pressure gauges, thermometers, etc.).
For chemists, a thermodynamic system is a mixture of chemicals interacting with each other in the process chemical reaction. Astrophysicists will call such a system heavenly body. mixture of fuel and air in a car engine Earth, our body, a writing pen, a notebook, a machine tool, etc. are also thermodynamic systems.
Each thermodynamic system is separated from the environment by boundaries. They can be real - the glass walls of a test tube with chemical, cylinder body in the engine, etc. And they can be conditional, when, for example, they study the formation of a cloud in the atmosphere.
If such a system is not exchanged with external environment neither energy nor matter, it is called isolated or closed .
If the system exchanges energy with the external environment, but does not exchange matter, then it is called closed .
open system exchanges energy and matter with the environment.
Thermodynamic equilibrium

This concept is also introduced into thermodynamics as a generalization of experimental results.
Thermodynamic equilibrium called such a state of the system in which all its macroscopic quantities - temperature, pressure, volume and entropy - do not change in time if the system is isolated. Any closed thermodynamic system can spontaneously pass into such a state if all external parameters remain constant.
The simplest example of a system in thermodynamic equilibrium is a thermos with hot tea. The temperature in it is the same at any point in the liquid. Although a thermos can be called an isolated system only approximately.
Any closed thermodynamic system spontaneously tends to go into thermodynamic equilibrium if the external parameters do not change.
Thermodynamic process

If at least one of the macroscopic parameters changes, then they say that the system is thermodynamic process . Such a process can occur if external parameters change or the system begins to receive or transmit energy. As a result, it goes into another state.
Consider the example of tea in a thermos. If we dip a piece of ice into the tea and close the thermos, then immediately there will be a difference in temperatures in different parts of the liquid. The liquid in the thermos will tend to equalize temperatures. From areas with a higher temperature, heat will be transferred to where the temperature is lower. That is, a thermodynamic process will occur. In the end, the temperature of the tea in the thermos will again become the same. But it will already be different from the first initial temperature s. The state of the system has changed because its temperature has changed.
The thermodynamic process occurs when the sand heated on the beach on a hot day cools down at night. By morning, his temperature drops. But as soon as the sun rises, the heating process will start again.
Internal energy

One of the main concepts of thermodynamics is internal energy .
All macroscopic bodies have internal energy, which is the sum of the kinetic and potential energies of all particles (atoms and molecules) that make up the body. These particles interact only with each other and do not interact with the particles of the environment. The internal energy depends on the kinetic and potential energy particles and does not depend on the position of the body itself.
U = E k + E p
Internal energy changes with temperature. The molecular kinetic theory explains this by changing the speed of movement of particles of matter. If the temperature of the body rises, then the speed of movement of particles increases, the distance between them becomes greater. Consequently, their kinetic and potential energy increases. When the temperature drops, the reverse process occurs.
For thermodynamics, it is not the quantity that is more important internal energy, but its change. And you can change the internal energy using the heat transfer process or by making mechanical work.
Change in internal energy by mechanical work

Benjamin Rumford
The internal energy of a body can be changed by doing mechanical work on it. If work is done on the body, then mechanical energy is converted into internal energy. And if the work is done by the body, then its internal energy is converted into mechanical energy.
Almost to late XIX century it was believed that there is a weightless substance - caloric, which transfers heat from body to body. The more caloric flows into the body, the warmer it will be, and vice versa.
However, in 1798, the Anglo-American scientist Count Benjamin Rumford began to doubt the theory of caloric. The reason for this was the heating of the gun barrels during drilling. He suggested that the cause of heating is the mechanical work that is done during the friction of the drill on the barrel.
And Rumfoord did an experiment. To increase the friction force, they took a blunt drill, and the barrel itself was placed in a barrel of water. By the end of the third hour of drilling, the water in the barrel began to boil. This meant that the barrel received heat when mechanical work was done on it.
Heat transfer
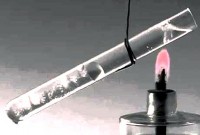
heat transfer called physical process transfer of thermal energy (heat) from one body to another either by direct contact or through a separating partition. As a rule, heat is transferred from a warmer body to a colder one. This process ends when the system comes to a state of thermodynamic equilibrium.
The energy that a body receives or gives off during heat transfer is called amount of heat .
According to the method of heat transfer, heat transfer can be divided into 3 types: thermal conductivity, convention, thermal radiation.
Thermal conductivity

If there is a temperature difference between bodies or parts of bodies, then a heat transfer process will occur between them. thermal conductivity called the process of transfer of internal energy from a more heated body (or part of it) to a less heated body (or part of it).
For example, heating one end of a steel bar on fire, after a while we will feel that its other end also becomes warm.
We easily hold a glass rod, one end of which is hot, by the other end, without burning ourselves. But if we try to do the same experiment with an iron rod, we will fail.
Different substances conduct heat differently. Each of them has its own coefficient of thermal conductivity, or conductivity, numerically equal to the amount of heat that passes through a sample 1 m thick, with an area of 1 m 2 in 1 second. 1 K is taken as the unit of temperature.
Metals conduct heat best. This is their property we use in everyday life, cooking in metal pots or pans. But their handles should not get hot. Therefore, they are made from materials with poor thermal conductivity.
The thermal conductivity of liquids is less. And gases have poor thermal conductivity.
Animal fur is also a poor conductor of heat. Thanks to this, they do not overheat in hot weather and do not freeze in cold weather.
Convention
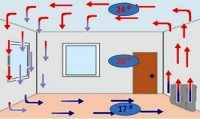
With convention, heat is transferred by jets and flows of gas or liquid. There is no convention in solids.
How does a convention arise in a liquid? When we put a kettle of water on the fire, the lower layer of the liquid heats up, its density decreases, it moves up. Its place is taken by a colder layer of water. After some time, it will also heat up and also change places with a colder layer. Etc.
A similar process occurs in gases. It is no coincidence that heating batteries are placed at the bottom of the room. After all, heated air always rises to the top of the room. And the lower, cold one, on the contrary, falls. Then it also heats up and rises again, while the upper layer cools down and sinks during this time.
Convention is natural and forced.
Natural convention is constantly taking place in the atmosphere. As a result of this, there are constant movements of warm air masses up, and cold ones - down. The result is wind, clouds and other natural phenomena.
When natural convention is not enough, I use forced convention. For example, warm air flows in a room with the help of fan blades.
thermal radiation

The sun heats the earth. There is no heat transfer or convention involved. So why do bodies get warm?
The fact is that the Sun is a source of thermal radiation.
thermal radiation - this is electromagnetic radiation generated by the internal energy of the body. All the bodies around us radiate thermal energy. This can be visible light from a table lamp, or sources of invisible ultraviolet, infrared, or gamma rays.
But bodies don't just radiate heat. They also consume it. Some to a greater extent, others to a lesser extent. Moreover, dark bodies both heat up and cool faster than light ones. In hot weather, we try to wear light-colored clothes, because they absorb less heat than dark-colored clothes. A dark-colored car heats up in the sun much faster than a light-colored car standing next to it.
This property of substances to absorb and radiate heat in different ways is used in the creation of night vision systems, missile homing systems, etc.
As a result of the impact on the working fluid (gas, steam) of the external environment, for example, compression, expansion, heating, etc., the parameters of its state change.
Any change in the parameters of the state of the working body is called thermodynamic process.
As noted above, there are equilibrium and non-equilibrium states of the working fluid. The thermodynamic process in which a system goes through a continuous series of equilibrium states is called equilibrium. The main condition for the equilibrium state of the working fluid is the equality of temperature and pressure throughout its mass. Equilibrium processes are reversible.
Non-equilibrium processes are called real processes proceeding at a finite rate, while the work expended on compression will be greater than in an equilibrium process (due to friction forces, inertia, etc.).
Non-equilibrium processes are irreversible.
When studying thermodynamic processes, they are used graphic images, in particular, in the coordinate system r and J, called the rJ-diagram.
Absolute pressures r are plotted along the ordinate axis, and specific volumes of gas are plotted along the abscissa axis. For known values of r and J, the equilibrium state is represented by a dot.
Figure 1 shows an arbitrary equilibrium process. When the gas passes from the initial state 1 to the final state 2, there is a decrease in pressure and an increase in specific volume. This is an expansion process that is considered a direct process. The compression process (from point 2 to point 1) is considered reverse.
All topics in this section:
THERMODYNAMICS AND ITS PROBLEMS
The processes of energy exchange take place in any phenomena of the surrounding world. Therefore, thermodynamics, as the science of the mutual transformation of heat and work, provides methods for studying energy fields.
STATES OF THE WORKING BODY
A thermodynamic system is a set of material bodies that are in mechanical and thermal interaction with each other and with surrounding bodies. Thermody
AND REAL GASES
Real gases at low pressures are close to ideal, since in this case the forces of intermolecular interaction and the volume of molecules can be neglected. This applies in particular to oxygen, air
GAS MIXTURES
In practice, as a rule, not any homogeneous gas is used as a working fluid, but a gas mixture: air, combustion products various kinds fuels, natural gases, etc.
TRUE AND AVERAGE HEAT CAPACITY
Heat capacities can be mass, volume, molar. The heat capacity of 1 kg of gas is called mass: it is denoted by the letter c and is measured in J / (kg. K). Those
HEAT CAPACITIES OF AN IDEAL GAS
As mentioned above, the temperature of the gas with the same amount of supplied heat q changes differently depending on the nature of the thermodynamic process. It means,
Values of molar heat capacities and coefficient k depending on atomicity
Gas mсJ mav mсJ mav k
HEAT CAPACITY OF GAS MIXTURES
The heat capacity of a gas mixture, as well as individual gases, can be referred to 1 kg, 1 m3 or 1 kmol. If the mixture is given by mass fractions, then its mass heat capacity
AND INTERNAL ENERGY
Work is done only when the volume of the gas changes. If the gas expands, then the work is done against external forces; when compressed, on the contrary, the gas takes work
HEAT
Heat is a form of motion of the smallest particles of a body. The transfer of heat from one body to another is carried out either by direct contact between them (thermal conductivity,
THE FIRST LAW OF THERMODYNAMICS
The first law of thermodynamics is special case the universal law of conservation and transformation of energy in relation to thermal processes. The formulation of the first law
THERMODYNAMIC PROCESSES IN GASES
Any change in the state of the working fluid (gas) is generally characterized by a change in its main parameters: r, J, T. The state of the gas changes in two ways: by telling it those
ENTROPY OF GAS
In the study, all processes are considered as equilibrium and reversible. Before considering the procedure for studying thermodynamic processes, we introduce the fifth parameter
ISOCHORIC PROCESS
A process that occurs at a constant specific volume is called isochoric. The isochoric process is used, in particular, in the calculations theoretical cycles carbureted engines
ISOBAR PROCESS
The process taking place at constant pressure, is called isobaric. Such a thermodynamic process can take place in a cylinder whose piston moves without friction, so
GAS ENTHALPY
In the processes associated with the calculation of boiler plants, steam turbines, as well as with drying and cooling of agricultural products, the parameter of the state of the working fluid (ha
ISOTHERMAL PROCESS
The process taking place at constant temperature working fluid is called isothermal. It is possible, for example, in the cylinder of a reciprocating machine, if, as heat q is supplied to the working
ADIABATIC PROCESS
An adiabatic process is a process that is carried out without heat exchange between the gas and the environment (q = 0). The practical use of this process is in the nozzles of steam t
POLYTROPIC PROCESS
In all real heat engines (engines internal combustion- internal combustion engines, compressors, gas turbine units, etc.) processes of compression of the working fluid (gas), combustion of fuel, expansion
Results of the analysis of polytropic processes
Group Limits of change in the polytropic index Change in internal energy Supply (removal) of heat Warm
CIRCULAR PROCESSES
A closed process, as a result of which the gas, after passing through a number of different states, returns to its original state, is called circular process(cycle). Let us explain this definition.
DIRECT REVERSIBLE CARNO CYCLE
In 1824, S. Carnot proposed a cycle, which was given his name. A direct reversible (that is, consisting only of equilibrium, reversible processes) Carnot cycle is an ideal cycle
INVERSE REVERSIBLE CARNO CYCLE
This cycle is the perfect cycle refrigeration machines. The image of the reverse Carnot cycle is shown in Figure 10. The cycle consists of the same processes as the direct cycle, but the state p
THE SECOND LAW OF THERMODYNAMICS
The first law of thermodynamics establishes the equivalence of heat and work as two forms of energy transfer. However, this law says nothing about the conditions for the transformation of heat and
COMPRESSORS
Internal combustion engines (ICE) are called thermal reciprocating machines, in which the products of combustion of liquid or gaseous fuels are used as a working fluid, burning
INTERNAL COMBUSTION ENGINES
For analysis ICE operation and determining the main indicators (indicator power, mechanical efficiency) on a working cylinder, an indicator diagram is recorded using an indicator, etc.
CYCLES OF GAS TURBINE PLANT
Gas turbine units (GTUs) have a significant advantage over ICEs - the absence of reciprocating mechanisms in GTUs makes it possible to build them with high-speed
PISTON COMPRESSORS
Compressed air is widely used in technological processes, in particular for driving pneumatic mechanisms, hammers, vibrators, pneumatic hoists, for transporting bulk materials.
WATER VAPOR
Water vapor as a working fluid is widely used in steam turbines, which are the main heat engines in thermal and nuclear power plants. In quality
RV- AND Ts-WATER VAPOR DIAGRAM
Vaporization can be carried out by evaporation or boiling. Evaporation is called vaporization, occurring only from the surface of the liquid. This process is about
Hs-WATER VAPOR DIAGRAM
In engineering practice, thermodynamic processes with water vapor are calculated using the hs diagram. This diagram was built in the USSR up to a pressure of 100.0 MPa and a temperature of 1000
PARAMETERS OF WATER AND WATER STEAM
In studies, it is generally accepted that at 0 0С and any pressure, the enthalpy h0, internal energy u0, entropy s0 of water are equal to zero. In pic
CARNO CYCLE FOR WATER VAPOR
The most perfect ideal cycle of a steam power plant is the direct reversible Carnot cycle, the thermal efficiency of which, as noted above, is maximum in a given temperature range and does not exceed
RANKIN CYCLE
The main ideal cycle of steam power plants is the Rankine cycle. Figure 24 shows a schematic diagram of a steam power plant operating according to the Rankine cycle, and Figure 25 shows rJ- and Ts-di
RANKIN CYCLE
Study of the expression for thermal cycle efficiency Rankine at different initial (at the steam turbine inlet) and final (at the condenser inlet) steam parameters allows us to conclude that initially
WET AIR
As a working fluid, atmospheric air is used in drying, heating, cooling various materials, in air conditioning units, etc. atmospheric air co
HUMID AIR
According to Dalton's law, the pressure of a mixture of gases is equal to the sum of the partial pressures of its components: pnl.v. = pс.v. + pv.p., (128) &nbs
HD-CHART HUMID AIR
This chart allows you to most simply and quickly determine the parameters of humid air. In the Hd diagram (Fig. 27, a), the moisture content d (g / kg of dry air) is plotted along the abscissa axis,
BASIC CONCEPTS AND DEFINITIONS
One of the basic concepts of thermal conductivity is the temperature field. Temperature is one of the main parameters characterizing the thermal state of the working fluid or medium. Aggregate
FOURIER LAW
Fourier's law establishes a quantitative relationship between the temperature field and the intensity of heat propagation in it through heat conduction. According to the Fourier law, ve
IN STATIONARY MODE
Thermal conductivity of a single-layer flat wall. The scheme of heat propagation for this case is shown in Figure 30. Let heat propagate in a wall bounded by steam
CONVECTIVE HEAT TRANSFER
In practice, it is most often necessary to calculate the convective heat transfer between a liquid (gas) and a surface solid body or channel (pipe) through which it flows. If pr
WHEN LIQUID BOILS
Heat transfer during liquid boiling is accompanied by a change state of aggregation working body. This phenomenon has specific features and is of great practical importance for energy
AND THE LAWS OF RADIATION
Heat transfer by radiation is the process of heat transfer in the form of electromagnetic waves (photons). This type of heat transfer is carried out in three stages: the internal energy of the body at the beginning of
HEAT TRANSFER BY RADIATION
Knowing the laws of radiation makes it possible to obtain calculation formulas for radiant heat transfer between bodies. In particular, formula (155) is used in determining the emissivity
HEAT TRANSFER. BASICS FOR CALCULATION OF HEAT EXCHANGERS
The division of the process of heat transfer into thermal conductivity, convective heat transfer and heat transfer by radiation is convenient for its study. In reality, there is a complex heat exchange,
HEAT TRANSFER THROUGH A FLAT WALL
Let a single-layer flat wall (Fig. 37) of thickness d made of a material whose thermal conductivity coefficient l be washed on one side by a hot liquid with a temperature tl1
HEAT TRANSFER THROUGH A CYLINDRICAL WALL
In practice, the most common element of heat exchange devices is a pipe. A diagram of the heat transfer process through a cylindrical wall (pipe) is shown in Figure 38.
THERMAL INSULATION
When solving practical problems of heat transfer, it is required either to increase the intensity of heat transfer from the heating medium to the heated one, or, conversely, to slow down this process. Intensi
HEAT EXCHANGERS
Heat exchangers (heat exchangers) are devices designed to transfer heat from a heating coolant (with a higher temperature) to a heated heat
ENERGY FUEL
Energy fuels are such combustible substances that are economically feasible when burned in technical devices to produce heat. as a fuel
THE CONCEPT OF CONVENTIONAL FUEL
The calorific value of a fuel indicates how much heat (in kilojoules) is released during the complete combustion of a solid, liquid or gaseous fuel under normal conditions.
MAIN FUEL COMPONENTS
The main component of the combustible part of the fuel is carbon. The heat of combustion of carbon is 33,650 kJ/kg. The carbon content in the combustible mass of fuel is: in anthracite - 87 ... 93%
FUEL TYPES
Wood. The use of wood as fuel is limited. The calorific value of firewood is largely determined by humidity. The more moisture in the wood, the less
THE ESSENCE OF THE FUEL COMBUSTION PROCESS
Depending on the rate of combustion, normal combustion and explosive combustion are distinguished. The burning rate is the rate of flame propagation. During normal combustion, the rate of propagation
FOR COMPLETE FUEL COMBUSTION
If the composition of the fuel is known, then the amount of air required for the complete combustion of any of its components can be determined from the expression C + O2 = CO2. E
VOLUME AND COMPOSITION OF COMBUSTION PRODUCTS
For the correct calculation and selection of heat engineering units, it is necessary to know the amount of combustion products formed. As a rule, the amount of combustion products is referred to 1 kg of TV
Numerical values of the enthalpies of the constituent products of combustion and air at different temperatures
Temperature, K НСО2, kJ/m3 НN2, kJ/m3 НО2, kJ/m3
REFERENCE INDEX
Alekseev G. N. General heat engineering. - M .: graduate School, 1980. Andryushchenko AI Fundamentals of thermodynamics of cycles of thermal power plants. - M., Higher school. Ar
INSTITUTE OF ENERGY AND AUTOMATION
Department of Thermal Engineering
And energy systems

TECHNICAL THERMODYNAMICS
Magnitogorsk
| INTRODUCTION .................................................. ................................................. ......................... | |
| Topic1. The subject and method of thermodynamics .............................................. ....................... | |
| Thermodynamic system .................................................................. ............................... | |
| Thermodynamic parameters of the state ............................................................... ...... | |
| Equation of state .................................................................. ......................................... | |
| Thermodynamic process .................................................................. ......................... | |
| Heat capacity of gases .................................................................. ............................................ | |
| Topic 2. Mixes ideal gases.................................................................................... | |
| Analytical expression of the first law of thermodynamics .............................................. | |
| Topic 3. Internal energy ............................................... ............................................... | |
| Extension operation .................................................................. ......................................... | |
| Heat................................................. ................................................. ............. | |
| Enthalpy................................................. ................................................. ........... | |
| Entropy................................................. ................................................. ........... | |
| Topic 4. General wording of the second law.................................................... ................... | |
| Direct Carnot Cycle .............................................................. ............................................... | |
| Reverse Carnot cycle .................................................................. ......................................... | |
| Change of entropy in non-equilibrium processes .............................................. | |
| Topic 5. Thermodynamic processes of ideal gases in closed systems Oh......... | |
| Exergy ............................................................ ................................................. ............. | |
| Topic 6. Thermodynamic processes of real gases .............................................. ..... | |
| Equation of state of real gases............................................................... ................. | |
| Topic 7. Equation of the first law of thermodynamics for flow................................................. | |
| Converging nozzle outflow .................................................................. .................... | |
| Main regularities of gas flow in nozzles and diffusers.................................................. | |
| Calculation of the expiration process with h-s diagrams .................................. | |
| Throttling of gases and vapors .............................................. ............................... | |
| Topic 8. Thermodynamic Efficiency of cycles of thermal power plants .......... | |
| Cycles piston engines internal combustion ............................................... | |
| Cycles of gas turbine plants .......................................................... ......................... | |
| Cycles of steam turbine plants .......................................................... ................... | |
| Carnot and Rankine cycles saturated steam. Heat recovery ............................... | |
| Rankine cycle on superheated steam .............................................. ......................... | |
| Thermal efficiency of the cycle ............................................................... ................................................ | |
| Heat supply .................................................................. ................................................. .... |
Topic 9. Theoretical process multistage compressor
. Control questions for conducting intermediate certification(exam) based on the results of mastering the discipline:
- Essence and formulations of the first law of thermodynamics.
- Analytical expression of the first law of thermodynamics.
- Show on the P - V diagram the useful work and the work of expansion (compression) for an arbitrary thermodynamic process.
- Internal energy and enthalpy as functions of state, their connection with heat capacity.
- What is called total heat capacity.
- Specific heat capacity - mass, volume and molar, their designation and dimension.
- Which heat capacity is greater - isobaric or isochoric and why.
- Basic thermodynamic processes, their representation on P–V and T–S diagrams.
- Relationship of parameters for basic thermodynamic processes.
- Calculation of the adiabatic process using the functions 0 and 0 .
- Reversible and irreversible processes, the main causes of irreversibility.
- Draw on the T - S diagram a reversible and irreversible adiabatic process of expansion and contraction.
- Essence and formulations of the second law of thermodynamics.
- Analytical expression of the second law of thermodynamics for reversible and irreversible processes.
- Entropy as a function of state, physical meaning entropy.
- What is called the thermodynamic cycle.
- Direct and reverse thermodynamic cycles.
- How to evaluate the efficiency of the direct and reverse cycle.
- Schematic diagram of a heat engine and a refrigeration unit.
- Direct Carnot cycle, its thermal efficiency, image on state diagrams.
- Real gases, Van-der-Waals equation of state.
- Phase P-T diagram for normal and anomalous matter.
- Show on the T-S diagram the heat spent on vaporization.
- Water vapor - saturated (dry and wet) and superheated - definitions.
- Water vapor state diagrams P - V, T - S, h - S.
- Critical and triple point.
- Thermodynamic processes of water vapor on state diagrams.
28. The equation of the first law of thermodynamics for the flow of the working fluid.
30.How to define a channel profile.
31. What nozzle is needed to obtain supersonic speed.
32. What process is called throttling.
33. Depict the throttling process on the h - s diagram.
34. How the parameters of the working fluid change in the process of throttling.
35. Which of the three compression processes in a compressor (isothermal, adiabatic, polytropic) is the most beneficial and why.
36. Multi-stage compression, its advantages compared to a single-stage compressor.
37. Cycles of internal combustion engines, their comparison.
38. Cycles of gas turbine plants, their comparison.
39. Methods for improving the efficiency of gas turbine plants.
40. Carnot cycle for water vapor.
41. Schematic diagram of a steam turbine plant.
42. Rankine cycle with saturated steam, its thermal efficiency.
43. Rankine cycle with superheated steam, its thermal efficiency.
44. Real Rankine cycle, definition of absolute internal efficiency.
45. Influence of initial and final steam parameters on thermal efficiency.
46. Scheme and cycle of a steam turbine plant with intermediate steam reheating.
47. Write the formula for the thermal efficiency of a cycle with reheat.
48. What cycle is called regenerative.
49. Scheme and cycle of a steam turbine plant with regenerative extractions.
50. Write the formula for the thermal efficiency of the regenerative cycle.
51. Thermodynamic fundamentals of district heating.
52. Heat utilization factor (KIT).
53. Cycles of nuclear power plants, prospects for the use of atomic energy.
54. Binary cycles (steam - steam and steam - gas).
55. Calculation of thermal efficiency of binary installations.
56. Machineless (direct) conversion of thermal energy into electrical energy.
57. Scheme, cycle and thermal efficiency of an installation with MHD generators.
58. Refrigeration units - gas and vapor compression.
59. What is called a heat pump, how to evaluate its effectiveness.
60. First and second laws of thermodynamics for chemical systems
THEME 1
Subject and method of thermodynamics
Thermodynamicsstudies the laws of energy conversion in various processes occurring in macroscopic systems and accompanied by thermal effects. A macroscopic system is any material object consisting of a large number particles. The sizes of macroscopic systems are incommensurably larger than the sizes of molecules and atoms.
Depending on the objectives of the study, they consider technical or chemical thermodynamics, thermodynamics of biological systems, etc. Technical thermodynamics studies the patterns of mutual transformation of thermal and mechanical energy and the properties of the bodies involved in these transformations.. On its basis, the calculation and design of all heat engines, as well as all kinds of technological equipment, are carried out.
Considering only macroscopic systems, thermodynamics studies the patterns of the thermal form of the motion of matter, due to the presence of a huge number of continuously moving and interacting microstructural particles (molecules, atoms, ions).
Physical Properties macroscopic systems are studied by statistical thermodynamic methods. The statistical method is based on the use of probability theory and certain models of the structure of these systems and is the content of statistical physics. The thermodynamic method does not require the involvement of model ideas about the structure of matter and is phenomenological(i.e., considers "phenomena" - phenomena as a whole).
In this case, all the main conclusions of thermodynamics can be obtained by deduction, using only two basic empirical laws of thermodynamics.
In the future, based on the thermodynamic method, we will use molecular-kinetic ideas about the structure of matter for clarity.
Thermodynamic system
Thermodynamic system is a set of material bodies that are in mechanical and thermal interactions with each other and with external bodies surrounding the system("external environment").
The choice of the system is arbitrary and dictated by the conditions of the problem being solved. Bodies not included in the system are called environment. The system is separated from the environment control surface(shell). So, for example, for the simplest system - a gas enclosed in a cylinder under a piston, the external environment is the surrounding air, and the cylinder walls and the piston serve as control surfaces.
Mechanical and thermal interactions of a thermodynamic system are carried out through control surfaces. During mechanical interaction by the system itself or on the system, work is done. (In the general case, the system can also be affected by electric, magnetic and other forces, under the influence of which the system will perform work. These types of work can also be taken into account in the framework of thermodynamics, but we will not consider them further). In our example, mechanical work is performed by moving the piston and is accompanied by a change in volume. Thermal interaction consists in the transfer of heat between the individual bodies of the system and between the system and the environment. In the example under consideration, heat can be supplied to the gas through the walls of the cylinder.
In the most general case, the system can exchange with the environment and matter (mass transfer interaction). Such a system is called open. Gas or steam flows in turbines and pipelines - examples open systems. If the substance does not pass through the boundaries of the system, then it is called closed. In what follows, unless otherwise stated, we will consider closed systems.
A thermodynamic system that cannot exchange heat with its environment is called thermally insulated or adiabatic. An example of an adiabatic system is a gas in a vessel, the walls of which are covered with ideal thermal insulation, which excludes heat exchange between the gas enclosed in the vessel and the surrounding bodies. Such an insulating shell is called adiabatic. A system that does not exchange energy or matter with its environment is called isolated(or closed).
The simplest thermodynamic system is a working fluid that performs the mutual transformation of heat and work. In an internal combustion engine, for example, the working fluid is a combustible mixture prepared in a carburetor, consisting of air and gasoline vapors.
Thermodynamic state parameters
The properties of each system are characterized by a number of quantities, which are usually called thermodynamic parameters. Let us consider some of them, using the molecular-kinetic concepts known from the course of physics about an ideal gas as a collection of molecules that have vanishingly small dimensions, are in random thermal motion and interact with each other only during collisions.
Pressure due to the interaction of the molecules of the working fluid with the surface and is numerically equal to the force acting per unit area of the surface of the body along the normal to the latter. In accordance with the molecular kinetic theory, the gas pressure is determined by the relation
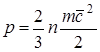 , (1.1)
, (1.1)
where F is the force; f-surface; n- number of molecules per unit volume;
t is the mass of the molecule; since 2- RMS speed forward movement molecules.
AT international system units (SI) pressure is expressed in pascals (1 Pa \u003d 1 N / m 2). Since this unit is small, it is more convenient to use 1 kPa = 1000 Pa and 1 MPa = 10 6 Pa.
Pressure is measured using pressure gauges, barometers and vacuum gauges.
Liquid and spring pressure gauges measure gauge pressure, which is the difference between total or absolute pressure R measured medium and atmospheric pressure p atm, i.e. ![]()
Devices for measuring pressures below atmospheric are called vacuum gauges; their readings give the value of vacuum (or vacuum):
![]() ,
i.e. excess atmospheric pressure over the absolute.
,
i.e. excess atmospheric pressure over the absolute.
Note that the state parameter is absolute pressure. This is what enters into the thermodynamic equations.
temperature called a physical quantity that characterizes the degree of heating of the body. The concept of temperature follows from the following statement: if two systems are in thermal contact, then if their temperatures are not equal, they will exchange heat with each other, but if their temperatures are equal, then there will be no heat exchange.
From the point of view of molecular kinetic concepts, temperature is a measure intensity of thermal motion of molecules. Its numerical value is related to the value of the average kinetic energy of the molecules of the substance:
In the SI system, the unit of temperature is the kelvin (K); in practice, the degree Celsius (°C) is widely used. The ratio between the absolute T and centigrade I temperatures has the form
![]() .
.
In industrial and laboratory conditions, temperature is measured using liquid thermometers, pyrometers, thermocouples and other instruments.
Specific volume v - is the volume per unit mass of a substance. If a homogeneous body of mass M occupies volume v, then by definition
v= V/M.
In the SI system, the unit of specific volume is 1 m 3 /kg. There is an obvious relationship between the specific volume of a substance and its density:
To compare the quantities characterizing systems in the same states, the concept of “normal physical conditions” is introduced:
p\u003d 760 mm Hg \u003d 101.325 kPa; T=273,15 K.
In various branches of technology and different countries introduce their own “normal conditions” somewhat different from those given, for example, “technical” ( p\u003d 735.6 mm Hg \u003d 98 kPa, t=15˚C) or normal conditions for assessing compressor performance ( p=101.325 kPa, t=20˚С), etc.
If all thermodynamic parameters are constant in time and the same at all points of the system, then this state of the system is calledequilibrium.
If there are differences in temperature, pressure and other parameters between different points in the system, then it isnonequilibrium. In such a system, under the influence of gradients of parameters, flows of heat, substances, and others arise, tending to return it to a state of equilibrium. Experience shows that isolated system over time, it always comes to a state of equilibrium and can never spontaneously get out of it. In classical thermodynamics, only equilibrium systems are considered.
State equation
For an equilibrium thermodynamic system, there is a functional relationship between the state parameters, which is called equation of state. Experience shows that the specific volume, temperature and pressure of the simplest systems, which are gases, vapors or liquids, are related thermal equation view states ![]() .
.
The equation of state can be given another form: ![]()
These equations show that of the three main parameters that determine the state of the system, any two are independent.
To solve problems by thermodynamic methods, it is absolutely necessary to know the equation of state. However, it cannot be obtained within the framework of thermodynamics and must be found either experimentally or by methods of statistical physics. The specific form of the equation of state depends on the individual properties of the substance.
Equation of state for ideal gases
Equations (1.1) and (1.2) imply that .
Consider 1 kg of gas. Considering that it contains N molecules and, therefore, we get: ![]() .
.
Constant value nk, referred to 1 kg of gas, denoted by the letter R and call gas constant. That's why
Or . (1.3)
The resulting relation is the Clapeyron equation.
Multiplying (1.3) by M, we obtain the equation of state for an arbitrary mass of gas M:
The Clapeyron equation can be given a universal form if we refer the gas constant to 1 kmole of gas, that is, to the amount of gas whose mass in kilograms is numerically equal to the molecular mass μ. Putting in (1.4) M=μ and V=V μ, we obtain for one mole the Clapeyron - Mendeleev equation:
Here, is the volume of a kilomole of gas, and is the universal gas constant.
In accordance with Avogadro's law (1811), the volume of 1 kmole, which is the same under the same conditions for all ideal gases, under normal physical conditions is 22.4136 m 3, therefore
The gas constant of 1 kg of gas is .
Equation of state of real gases
In real gases in the difference from the ideal is significant forces of intermolecular interactions (attractive forces when the molecules are at a considerable distance, and repulsive forces when they are sufficiently close to each other) and the intrinsic volume of the molecules cannot be neglected.
The presence of intermolecular repulsive forces leads to the fact that molecules can approach each other only up to a certain minimum distance. Therefore, we can assume that the volume free for the movement of molecules will be equal to , where b is the smallest volume to which a gas can be compressed. In accordance with this, the mean free path of molecules decreases and the number of impacts on the wall per unit time, and, consequently, the pressure increases compared to an ideal gas in relation to , i.e.
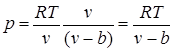 .
.
Attractive forces act in the same direction as the external pressure and give rise to molecular (or internal) pressure. The force of molecular attraction of any two small parts of a gas is proportional to the product of the number of molecules in each of these parts, that is, to the square of the density, so the molecular pressure is inversely proportional to the square of the specific volume of the gas: rmol= a/ v 2 , where a - coefficient of proportionality, depending on the nature of the gas.
From this we obtain the van der Waals equation (1873):
![]() ,
,
At large specific volumes and relatively low pressures of a real gas, the van der Waals equation practically degenerates into the Clapeyron equation of state for an ideal gas, since the quantity a/v 2
(compared with p) and b(compared with v) become negligible.
Qualitatively, the van der Waals equation describes the properties of a real gas quite well, but the results of numerical calculations do not always agree with experimental data. In a number of cases, these deviations are explained by the tendency of real gas molecules to associate into separate groups consisting of two, three, or more molecules. The association occurs due to the asymmetry of the external electric field of the molecules. The resulting complexes behave like independent unstable particles. During collisions, they break up, then recombine with other molecules, etc. As the temperature rises, the concentration of complexes with a large number molecules decreases rapidly, while the proportion of single molecules increases. Polar water vapor molecules exhibit a greater tendency to association.
Thermodynamic process
The change in the state of a thermodynamic system with time is called thermodynamic process. So, when the piston moves in the cylinder, the volume, and with it the pressure and temperature of the gas inside, will change, the process of expansion or compression of the gas will take place.
As already noted, the system, taken out of the state of equilibrium, and left to itself at constant parameters of the environment, after some time will again come to an equilibrium state corresponding to these parameters. Such a spontaneous (without external influence) return of the system to a state of equilibrium
called relaxation, and the time interval during which the system returns to equilibrium is called relaxation time. For different processes it is different: if it is always required to establish an equilibrium pressure in a gas, then ten are needed to equalize the temperature in the volume of the same gas; minutes, and in the volume of the heated solid - sometimes several hours.
The thermodynamic process is calledequilibrium, if all parameters of the system during its course change rather slowly compared to the corresponding relaxation process. In this case, the system is actually in a state of equilibrium with the environment all the time, which determines the name of the process.
For the process to be equilibrium, the rate of change of the system parameters must satisfy the relation
where BUT- the parameter that changes most rapidly in the process under consideration; With rel - the rate of change of this parameter in the relaxation process; τ rel - relaxation time.
Consider, for example, the process of compressing a gas in a cylinder. If the time of displacement of the piston from one position to another significantly exceeds the relaxation time, then in the process of moving the piston, pressure and temperature will have time to equalize throughout the entire volume of the cylinder.
This alignment is ensured by the continuous collision of molecules, as a result of which the energy supplied from the piston to the gas is fairly quickly and evenly distributed between them. If subsequent displacements of the piston will occur in a similar way, then the state of the system at each moment of time will be practically equilibrium. In this way, equilibrium process consists of a continuous series of successive equilibrium states, therefore, at each of its points, the state of the thermodynamic system can be described by the equation of state of the given working fluid. That is why classical thermodynamics in its research operates only with equilibrium processes. They are a convenient idealization of real processes, allowing in many cases to significantly simplify the solution of the problem. This idealization is quite justified, since condition (1.8) is satisfied quite often in practice. Since mechanical disturbances propagate in gases at the speed of sound, the process of compressing the gas and the cylinder will be in equilibrium if the speed of the piston is much less than the speed of sound.
Processes that do not satisfy the condition, proceed with imbalance, i.e. arenonequilibrium. If, for example, it rapidly increases the ambient temperature, then the gas in the cylinder will gradually warm up through its walls, relaxing to an equilibrium state corresponding to the new environmental parameters. In the process of relaxation, the gas is not in equilibrium with the environment and it cannot be characterized by the equation of state, if only because in different points volume of gas temperature has different values.
Heat capacity of gases
The ratio of the amount of heat received by a body with an infinitesimal change in its state to the change in body temperature associated with this is called heat capacity bodies in this process:
Usually, heat capacity is referred to a unit of the amount of a substance and, depending on the chosen unit, they distinguish:
specific mass heat capacityc , referred to 1 kg of gas,
J/(kg K);
specific volumetric heat capacityc´, referred to the amount of gas contained in 1 m 3 volume under normal physical conditions, J / (m 3 ·K);
specific molar heat capacity , referred to one kilomole, J / (kmol K).
Relationship between specific heat capacities is established by obvious relations: ;
Here is the density of the gas under normal conditions.
The change in body temperature with the same amount of heat supplied depends on the nature of the process taking place, therefore heat capacity is a function of the process. This means that the same working fluid, depending on the process, requires a different amount of heat for its heating by 1 K. Numerically, the value of c varies from +∞ to -∞.
In thermodynamic calculations, the following are of great importance:
heat capacity at constant pressure
equal to the ratio of the amount of heat imparted to the body in the process at constant pressure to the change in body temperature dT
heat capacity at constant volume
, (1.5)
equal to the ratio of the amount of heat , brought to the body in the process at constant volume, to a change in body temperature .
In accordance with the first law of thermodynamics for closed systems in which equilibrium processes take place ![]() , and
, and
For an isochoric process ( v=const) this equation takes the form ![]() , and, taking into account (1.5), we obtain that
, and, taking into account (1.5), we obtain that
![]() ,
,
i.e., the heat capacity of a body at constant volume is equal to the partial derivative of its internal energy with respect to temperature and characterizes the growth rate of internal energy in an isochoric process with increasing temperature.
For an ideal gas
For an isobaric process, from Eqs. (2.16) and (2.14) we obtain
This equation shows the relationship between heat capacities with p and cv. For an ideal gas, it is greatly simplified. Indeed, the internal energy of an ideal gas is determined only by its temperature and does not depend on volume, therefore and, in addition, it follows from the equation of state ![]() , where
, where
This ratio is called Mayer equation and is one of the main ideal gases in technical thermodynamics.
In the process v\u003d const the heat imparted to the gas goes only to change its internal energy, while in the process R= const heat is spent both on increasing internal energy and on doing work against external forces. That's why with p more cv the size of this work.
For real gases, since when they expand (at p= const) work is done not only against external forces, but also against the forces of attraction acting between molecules, which causes additional heat consumption.
Usually, heat capacities are determined experimentally, but for many substances they can be calculated by methods statistical physics.
The results of the classical theory of heat capacity are in good agreement with experimental data in the region of room temperatures (Table 2.1), but the experiment does not confirm the main conclusion about independence from temperature. The discrepancies, especially significant in the region of low and sufficient high temperatures, are related to the quantum behavior of molecules and find explanations in terms of quantum theory heat capacity.
Heat capacity of some gases at t= 0°C in the ideal gas state
This theory establishes, first of all, the injustice of the theorem on the uniform distribution of energy over the degree of freedom in the region of low and high temperatures. As the gas temperature decreases, the number of degrees of freedom of the molecule freezes out. So, for a diatomic molecule, the rotational degrees of freedom “freeze out” and instead of five, it has three degrees of freedom, and, consequently, lower internal energy and heat capacity. With an increase in temperature, polyatomic molecules excite internal degrees of freedom due to the occurrence of vibrational motion of the atoms of the molecule (the molecule becomes an oscillator). This leads to an increase in internal energy and, consequently, in heat capacity with increasing temperature.
The heat capacity of a real gas depends on pressure, however, very weakly.
Since the heat capacity of a real gas depends on temperature, thermodynamics distinguishes between true and average heat capacities.
Average heat capacity With Wed this process in the temperature range from t1 before t2 is the ratio of the amount of heat supplied to the gas to the difference between the final and initial temperatures:
![]()
Expression
determines the heat capacity at a given temperature or the so-called true heat capacity. From * it follows that
 .
.
For practical calculations, the heat capacities of all substances are tabulated, and in order to reduce the volume of the tables, the average heat capacities are given in them for the temperature range from 0 to t.
LECTURE 2
Mixtures of ideal gases
All dependences obtained above for ideal gases are also valid for their mixtures, if the gas constant, molecular weight, and heat capacity of the mixture are substituted into them.
Dalton's Law. In engineering practice, one often has to deal with gaseous substances that are similar in properties to ideal gases and are a mechanical mixture of individual components of various gases that do not chemically react with each other. These are the so-called gas mixtures. Examples include products of fuel combustion in internal combustion engines, furnaces and steam boilers, moist air in drying plants, etc.
The main law that determines the behavior of a gas mixture is Dalton's law: the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of all its components:
Partial pressure pi- the pressure that a gas would have if it alone occupied the entire volume of the mixture at the same temperature.
Methods for setting a mixture. The composition of the gas mixture can be specified by mass, volume or mole fractions.
Mass fraction is the ratio of the mass of a single component Mi, to the mass of the mixture M:
It is obvious that and .
Mass fractions are often given as a percentage. For example, for dry air; .
Volumetric fraction is the ratio of the reduced volume of gas V, to the total volume of the mixture V: .
Given is the volume that a component of a gas would occupy if its pressure and temperature were equal to the pressure and temperature of the mixture.
To calculate the reduced volume, we write two equations of state i-th component:
![]() ; (2.1)
; (2.1)
![]() .
.
The first equation refers to the state of the gas component in the mixture when it has partial pressure pi and occupies the full volume of the mixture, and the second equation - to the reduced state, when the pressure and temperature of the component are equal, as for the mixture, R and T. It follows from the equations that
Summing relation (2.2) for all components of the mixture, we obtain, taking into account Dalton's law, whence . Volume fractions are also often given as a percentage. For air, .
Sometimes it is more convenient to specify the composition of the mixture in mole fractions. Mole fraction called the ratio of the number of moles Ni of the component under consideration to the total number of moles of the mixture N.
Let the gas mixture consist of N1 moles of the first component, N2 moles of the second component, etc. The number of moles of the mixture, and the mole fraction of the component will be equal to .
In accordance with Avogadro's law, the volumes of a mole of any gas at the same R and T, in particular, at the temperature and pressure of the mixture, in the ideal gas state they are the same. Therefore, the reduced volume of any component can be calculated as the product of the volume of a mole by the number of moles of this component, i.e., and the volume of the mixture - by the formula. Then ![]() , and, consequently, the assignment of mixing gases by mole fractions is equal to the assignment by its volume fractions.
, and, consequently, the assignment of mixing gases by mole fractions is equal to the assignment by its volume fractions.
Gas constant of a mixture of gases. Summing up equations (2.1) for all components of the mixture, we obtain 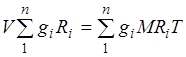 . Considering , we can write
. Considering , we can write
![]() , (2.3)
, (2.3)
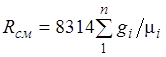 . (2.4)
. (2.4)
Equation (2.3) implies that a mixture of ideal gases also obeys the Clapeyron equation. Because the ![]() then from (2.4) it follows that the gas constant of the mixture [J/(kg-K)] has the form
then from (2.4) it follows that the gas constant of the mixture [J/(kg-K)] has the form
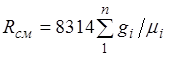 (2.5)
(2.5)
The apparent molecular weight of the mixture. Let us formally express the gas constant of the mixture R, by introducing the apparent ocular mass of the mixture: ![]() (2.6)
(2.6)
Comparing the right-hand sides of relations (2.5) and (2.6), we find
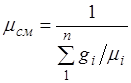 .
.
From the definition of mass fractions it follows that
Summing up this ratio for all components and taking into account that , we obtain an expression for the apparent molecular weight and mass of the mixture, given by volume fractions:
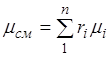 . (2.7)
. (2.7)
The ratio between volume and mass fractions. Taking into account (2.7), we obtain 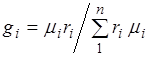 .
.
Because the  , then
, then
Dividing the numerator and denominator of this formula by the mass of the mixture M, we get
 .
.
Analytical expression of the first law of thermodynamics
The first law of thermodynamics is a special case of the universal law of conservation and transformation of energy as applied to thermal phenomena. According to Einstein's equation it is necessary to consider a single law of conservation and transformation of mass and energy. However, in technical thermodynamics we are dealing with such low object velocities that the mass defect is equal to zero, and therefore the law of conservation of energy can be considered independently.
The law of conservation and transformation of energy is a fundamental law of nature, which is obtained on the basis of generalization of a huge amount of experimental data and is applicable to all natural phenomena. He argues that energy does not disappear and does not arise again, it only passes from one form to another, and the decrease in energy of one type gives an equivalent amount of energy of another type.
Our compatriot M. V. Lomonosov (1711 - 1765) was among the first scientists who affirmed the principle of conservation of matter and energy.
Let some working body with volume V and weight M, having a temperature T and pressure R, an infinitesimal amount of heat is communicated from the outside. As a result of the supply of heat, the body is heated by dT and increases in volume dV.
An increase in body temperature indicates an increase in the kinetic energy of its particles. An increase in the body volume leads to a change in the potential energy of the particles. As a result, the internal energy of the body increases by dU. Since the working fluid is surrounded by a medium that exerts pressure on it, when it expands, it performs mechanical work against the forces of external pressure. Since no other changes occur in the system, then, according to the law of conservation of energy
![]() (2.8)
(2.8)
i.e., the heat imparted to the system goes to increase its internal energy and to perform external work.
The resulting equation is a mathematical expression of the first law of thermodynamics. Each of the three terms of this ratio can be positive, negative, or zero. Let's consider some particular cases.
1. - there is no heat exchange between the system and the environment, i.e., heat is not supplied to the system and is not removed from it. The process without heat exchange is called adiabatic. For it, equation (2.8) takes the form:
Therefore, the expansion work done by the system in adiabatic process, is equal to the decrease in the internal energy of the given system. With adiabatic compression of the working fluid, the work expended from the outside goes entirely to increase the internal energy of the system.
2. - while the volume of the body does not change, dV=0 . Such a process is called isochoric, for him
i.e., the amount of heat supplied to the system at a constant volume is equal to the increase in the internal energy of this system.
3. dU=0– the internal energy of the system does not change and
those. The heat imparted to the system is converted into external work equivalent to it.
For a system containing 1 kg of working fluid
![]() . (2.9)
. (2.9)
Integrating equations (2.8) and (2.9) for some process, we obtain the expression for the first law of thermodynamics in integral form:
![]() ; .
; .
Internal energy
The internal energy of the system includes:
kinetic energy of translational, rotational and vibrational motion of particles;
potential energy of particle interaction;
energy electron shells atoms;
intranuclear energy.
In most heat and power processes, the last two components remain unchanged. Therefore, in the future underinternal energy we will understand the energy of the chaotic motion of molecules and atoms, including the energy of translational, rotational and oscillatory movements both molecular and intramolecular, as well as the potential energy of the forces of interaction between molecules.
Kinetic energy molecules is a function of temperature, the value of potential energy depends on the average distance between molecules and, therefore, on the volume occupied by the gas V, i.e. is a function v. Therefore internal energy U is a function of the state of the body.
For complex system it is determined by the sum of the energies of the individual parts, i.e., it has the property of additivity. Value u=U/M, called specific internal energy (J / kg), is the internal energy of a unit mass of a substance.
In what follows, for brevity, we will refer to the quantity and just inner energy. Since the internal energy is a function of the state of the body, it can be represented as a function of any two independent parameters that determine this state:
; ; .
Its change in the thermodynamic process does not depend on the nature of the process and is determined only by the initial and final states of the body:
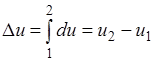 ;
;
The value of internal energy in the initial state, and - in the final. Mathematically, this means that an infinitesimal change in internal energy du there is total differential and; if we express the internal energy as a function of specific volume and temperature, then
The internal energy of an ideal gas, in which there are no interaction forces between molecules, does not depend on the gas volume or pressure, a is determined only by its temperature, so the derivative of the internal energy of an ideal gas with respect to temperature is the total derivative:
For the problems of technical thermodynamics, it is not the absolute value of the internal energy that is important, but its change in various thermodynamic processes. Therefore, the origin of the internal energy can be chosen arbitrarily. For example, in accordance with the international agreement for water, the value of internal energy at a temperature of 0.01 ° C and a pressure of 610.8 Pa is taken as zero, and for ideal gases - at 0 ° C, regardless of pressure.
Expansion work
Work in thermodynamics, as well as in mechanics, is determined by the product of the force acting on the working body and the path of its action.
Consider a gas with mass M and volume V, enclosed in an elastic shell with surface F.
 |
If a certain amount of heat is imparted to the gas, then it will expand, while doing work against external pressure R, exerted on him by the environment. The gas acts on each element of the shell dF with a force equal to pdf and moving it along the normal to the surface at a distance dn, commits elementary work pdfdn. The total work done during an infinitesimal process can be obtained by integrating this expression over the entire surface F shells: ![]() .
.
It can be seen from the figure that the volume change dV expressed as an integral over the surface: ![]() ,
Consequently
,
Consequently
With a finite change in volume, the work against the forces of external pressure, called expansion work, is equal to
From (3.1) it follows that and dV always have identical signs:
if dV>0, then and > 0, i.e., during expansion, the work of the body is positive, while the body itself does the work;
if dV<0, then and<0, т. е. при сжатии работа тела отрицательна: это означает, что не тело совершает работу, а на его сжатие затрачивается работа извне. Единицей измерения работы в СИ является джоуль (Дж).
Attributing the work of expansion to 1 kg of the mass of the working body, we obtain
l = L/M; .
The value representing the specific work performed by a system containing 1 kg of gas is equal to
Since in general R is a variable, then integration is possible only when the law of pressure change is known p = p(v).
Formulas (3.1) - (3.2) are valid only for equilibrium processes in which the pressure of the working fluid is equal to the pressure of the environment.
In thermodynamics, equilibrium processes are widely used p, v- a diagram in which the abscissa axis is the specific volume, and the ordinate axis is the pressure. Since the state of a thermodynamic system is determined by two parameters, then on p, v It is represented by a dot in the diagram. Point in the figure 1 corresponds to the initial state of the system, point 2 - final, and the line 12 - the process of expanding the working fluid from v1 before v2. With an infinitesimal change in volume, the area of the shaded vertical strip is ; hence the work of the process 12 is depicted by the area bounded by the process curve, the abscissa axis and the extreme ordinates.
In this way, the work of changing the volume is equivalent to the area under the process curve in the p, v diagram (Figure 3.1).
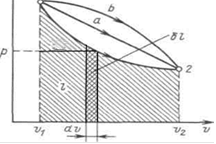
Figure 3.1 - Graphical representation of work in p, v- coordinates
Each path of the system transition from the state / to the state 2 (for example, 12, 1a2 or 1b2) corresponds to its expansion work. Therefore, work depends on the nature of the thermodynamic process, and is not a function of only the initial and final states of the system. On the other hand, depends on the path of integration and, therefore, the elementary work is not a total differential.
Work is always associated with the movement of macroscopic bodies in space, for example, the movement of a piston, the deformation of a shell, therefore it characterizes an ordered (macrophysical) form of energy transfer from one body to another and is a measure of the transferred energy. Since the value is proportional to the increase in volume, it is advisable to choose those that have the ability to significantly increase their volume as working bodies designed to convert thermal energy into mechanical energy. This quality is possessed by gases and vapors of liquids. Therefore, for example, at thermal power plants, water vapor serves as a working medium, and in internal combustion engines, gaseous products of combustion of a particular fuel.
Heat
In addition to the macrophysical form of energy transfer - work, there is also a microphysical, i.e., a form of energy exchange carried out at the molecular level between the system and the environment. In this case, energy can be transferred to the system without doing work. The measure of the amount of energy transferred by microphysical means is heat.
Heat can be transferred either through direct contact between bodies (thermal conduction, convection), or at a distance (by radiation), and in all cases this process is possible only if there is a temperature difference between the bodies.
As will be shown below, the elementary amount of heat, as well as L is not a total differential, unlike the internal energy differential dU. Behind this mathematical symbolism is hidden the deep physical meaning of the difference between the concepts of internal energy, heat and work.
Internal energy- it is a property of the system itself, it characterizes the state of the system. Warmth and work- these are the energy characteristics of the processes of mechanical and thermalinteractions systems with the environment. They characterize the amounts of energy that are transferred to the system or given away by it through its boundaries in a certain process.
Enthalpy
In thermodynamics, an important role is played by the sum of the internal energy of the system U and products of system pressure R to its volume V, called enthalpy and denoted H:
Since the quantities included in it are functions of the state, then the enthalpy is a state function. Just like internal energy, work and heat, it is measured in joules (J).
![]() ,
,
called specific enthalpy(h = H/M), represents the enthalpy of a system containing 1 kg of a substance and is measured in J/kg.
Since enthalpy is a state function, it can be represented as a function of any two state parameters:
![]() ;
; ![]() ;
; ![]() ,
,
and the value dh is a total differential.
The change in enthalpy in any process is determined only by the initial and final states of the body and does not depend on the nature of the process.
Let us find out the physical meaning of enthalpy using the following example. Let us consider an extended system including gas in a cylinder and a piston with a load with a total weight G.
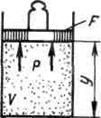 |
The energy of this system is the sum of the internal energy of the gas and the potential energy of the piston with the load in the field of external forces: . In equilibrium (G=pF) this function can be expressed in terms of gas parameters: . We get that , i.e. enthalpy can be interpreted as the energy of an expanded system.