De Broglie's equation and its physical meaning. Elements of quantum mechanics Wave-particle duality of the properties of matter particles
WAVES OF DE BROGILE- waves associated with any moving microparticle, reflecting quantum nature microparticles.
First quantum properties were opened at el-magn. fields. After the study by M. Plavkom (M. Planck) of the laws thermal bodies(1900) the concept of "light portions" - quanta of el-magn. fields. These quanta - photons - are in many ways similar to particles (corpuscles): they have a certain energy and momentum, interact with matter as a whole. At the same time, it has long been known wave properties el-magn. radiation, to-rye are manifested, for example, in the phenomena and interference of light. Thus, one can speak about the dual nature, or about the corpuscular-wave dualism, of the photon.
Can a magnetic field change kinetic energy charge and why? What is the direction magnetic field in the magnetron and what does it serve? What is the direction electric field in the magnetron and what does it serve? Draw and describe the dependence of the anode current on the coil current in the magnetron. What is critical current and where does it flow? What happens in a magnetron critical point? Why does the anode current in a magnetron never drop to zero even though we put a magnetic field much larger than the critical field?
Draw and explain the image obtained on an oscilloscope screen when a deflection is applied to a sinusoidal AC voltage and the magnetic field is constant and parallel to the lamp axis. Draw and explain the image obtained on the oscilloscope screen when the inclined plates do not stick to voltage and the magnetic field is constant and perpendicular to the axis of the lamp. What are Helmholtz coils and what are they for? When is the focus of electrons in an oscilloscope tube in the presence of a magnetic field?
In 1924, L. de Broglie put forward the hypothesis that corpuscular-wave dualism is inherent in all types of matter without exception - electrons, protons, atoms, etc., and the quantitative relationships between the wave and corpuscular properties of particles are those the same as those established earlier for photons. Namely, if the particle has energy and momentum, abs. the value of which is equal to R, then a wave of frequency and length is associated with it, where 6 * 10 -27 erg * s is Planck's constant. These waves are called V. de B.
Can only an electric field polarize a dielectric? Can piezoelectric bodies have a unit cell center of symmetry? Can a piezoelectric body be a body with a concentration of free loads comparable to the charge concentration of a metal? Describe the mechanism of the simple piezoelectric effect. What are piezoelectric modules? Define Young's modulus. What is a mirror galvanometer? Define the ballistic constant.
Determine the vector of the magnetic moment. The magnetic moment is proportional to its moment of inertia. What is the total magnetic moment of an electron in an atom and what is the total magnetic moment of an atom? How does its value affect the magnetic properties of these atoms? Provide a determination of the magnetic susceptibility. Explain the behavior of a diamagnet when it is introduced into a region of an external magnetic field. Explain the paramagnetic behavior when introduced into an external magnetic field.
For particles of not very high energy , where are the mass and velocity of the particle. Consequently, the length of the V. de B. is the smaller, the greater the mass of the particle and its speed. For example, a particle with a mass of 1 g, moving at a speed of 1 m / s, corresponds to V. de B. s10 -18, which lies outside the region accessible to observation. Therefore, wave properties are unimportant in macroscopic mechanics. tel. For electrons with energies from 1 eV to 10,000 eV, the lengths of V. de B. lie in the range from 10 to 0.1, i.e., in the range of X-ray wavelengths. radiation. Therefore, wave electron properties should appear, for example, during their scattering on the same crystals, on which x-ray diffraction.
Describe the effect of temperature on the magnetic susceptibility of diamagnetic and paramagnetic. What is the relationship between the energy of the magnetic field inside the sample and the intensity with which the magnetic field affects the sample? How can you separate substances due to their magnetic properties? What types of ordering of magnetic moments occur in materials? Explain the mechanism of creation of ferromagnetic domains. What is the energy stability of the domain structure in ferromagnets? How does an external magnetic field affect the material of a domain structure?
Explain the existence of magnetic hysteresis loops in ferromagnets. How does the shape of the hysteresis loop determine the magnetic properties of a material? Enter the definition of relative magnetic permeability. What is the Barkhausen effect? Describe the role of an integrating system in observing hysteresis loops on an oscilloscope screen. Why does the wave nature of light not explain the photoelectric effect? Einstein interpreted the photoelectric effect? What is corpuscular-wave duality? What is the stopping potential and how does it depend on the frequency of light?
The first experiment confirmation of the de Broglie hypothesis was obtained in 1927 in the experiments of C. Davisson and L. Germer. accelerated in electric field with a potential difference of 100-150 V (the energy of such electrons is 100-150 eV, which corresponds to ) and fell on a nickel crystal, which plays the role of spatial diffraction. gratings. It was found that the electrons diffract on the crystal, and exactly as it should be for waves, the length of which is determined by the de Broglie relation. The wave properties of electrons, neutrons, and other particles, as well as atoms and molecules, are not only reliably proven by direct experiments, but are also widely used in high-resolution devices, so that we can talk about engineering use V. de B. (see. Particle Diffraction).
How to determine the kinetic energy of photoelectrons? How to determine the number of photoelectrons? How is Planck's constant determined in this exercise? Why characteristics and spatter for high positive voltages? What assumption underlies de Broglie's hypothesis? What conditions must be met for the amplification of interfering waves? What is the relationship between Thompson's experience and de Broglie's hypothesis? How to prove the truth of de Broglie's hypothesis? What kind physical phenomena describe the Laue equations?
Draw and explain the interference pattern of the diffraction of light by a polycrystalline. Draw and explain the interference pattern of the diffraction of light by a single crystal. How to calculate solid single crystal based on interference image? Let us assume that neutrons and electrons have the same energy. What fraction corresponds to the de Broglie wavelength?
The experimentally confirmed idea of de Broglie about the corpuscular-wave dualism of microparticles fundamentally changed the ideas about the appearance of the microworld. Since all micro-objects (traditionally, the term "particles" is retained behind them) have both corpuscular and wave properties, then, obviously, any of these "particles" cannot be considered either a particle or a wave in the classical. understanding these words. A need arose for such a theory, in which the wave and corpuscular properties of matter would act not as exclusive, but as mutually complementary. The basis of such a theory - wave, or quantum, mechanics - was the concept of de Broglie, the clarification of which led to the probabilistic interpretation of V. de B. In 1926, M. Born (M. Born) expressed the idea that wave laws obey quantity describing the state of the particle. She was named
Discuss the mechanisms of heat transfer in nature. Discuss the mechanisms of heat transfer in solids. Discuss the dependence of heat solids from temperature. Give and discuss general equation heat transfer. What are the coefficients of thermal and thermal conductivity? What is a steady flow of heat? How to determine the coefficient of thermal conductivity according to the results of the Angstrom experiment? How do you determine the heat of the correct metal in an exercise?
The main and remarkable idea presented in this article was to claim that any microparticles with non-zero rest mass can be associated with wave 32. Broglie's theory moved from Planck's unconventional postulates to thermal radiation and - at the same time - from the application and the interpretations that Einstein found in the explanation photoelectric effect 33.
ELECTRONIC OPTICS
We have already said that light has corpuscular-wave dualism: in some phenomena it behaves like a wave (interference, diffraction, polarization), in others (photoelectric effect, absorption of light, etc.) - like a particle. A particle of light is a particle electromagnetic radiation optical range, having energy
By extrapolation, Louis de Broglie suggested 34 that any other microparticles have photon-like wavelength properties. Was there a theory of the opposite in the theory of matter? Haven't we thought too much about the "partial" view and paid attention to the waving view? 35. Broglie wave properties Expression of plane waves.
Without demonstration, it can be recalled that the choice of elliptical orbits from Bohr's theory refers to the same observation. Recall, it is called "stationary electron wave". the previous result does not cause problems because c is the limiting speed of the particle.
and momentum
 ,
,
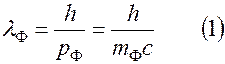 .
.
Rest mass of a photon, i.e. A photon exists only when it is moving.
In 1924, Louis de Broglie suggested that corpuscular-wave dualism may also be manifested by particles of matter, in particular electrons. It means that elementary particle can be characterized by associating a particle with a certain wave, the length of which
Experimental data De Broglie postulated the existence of particles associated with particles. The target layer was removed by evaporation. Experience of Davison and Germer Research that led Davison to the discovery. Didactic and pedagogical publishing house. The experience required a very clean emission surface and a high vacuum tube. small crystals are located in a single crystal. Improvement of the Technique Used 37. Beginning in N., the angular distribution of the electrons scattered by the target was completely different.
And the target was strongly oxidized by the air that penetrated inside. with the theory of L. says Davisson Germer by heating at high temperatures in hydrogen and in vacuum. After heating and cooling of the nickel surface, more pronounced intensity maxima of the reflected beam of diffraction of waves associated with electrons were found. In this case, in combination with L. in Davisson, he discovered the appearance of the intensity of the electron beam reflected in certain well-defined directions 36.
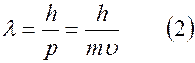 .
.
The difference between formulas (1) and (2) is significant and lies in the fact that
1) a photon does not have an inertial rest mass, while electrons have a rest mass, and the mass of a moving electron
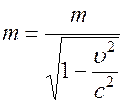 ,
,
2) for photons, the speed of their movement in vacuum is constant value, while electrons can move at different speeds.
Elsasser proposed using the same method to test whether electrons have the properties of a wavelength and determine their corresponding wavelength. This result was in accordance with the wavelength calculated by Broglie theory: λ = 22 Å. Then what physical meaning coupled wave? The interaction of electrons with single-crystal substances leads to diffraction phenomena. That's why. neutrons. He is atoms. Since it is not clear what is the contribution of each electron to the diffraction pattern. Subsequently, similar diffraction phenomena were discovered for other types of particles.
Wavelength
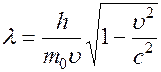
called the de Broglie wavelength. The de Broglie wave does not represent any independent oscillatory process, but only characterizes the wave properties of the particle.
When de Broglie published this hypothesis, there was no experimental proof of its correctness. Only in 1927, the American scientists Davisson and Germer confirmed this hypothesis by experiment. By studying the scattering of electrons on crystal structure nickel, they accidentally discovered electron diffraction. In the same year, Thomson and Tarkovsky were already specifically studying the diffraction of electrons on a metal foil.
Similar to the method used with the above. it was noticed that the distribution of scattered electrons also changes. in a very long time. Question: Can a particle be reduced to a wave? The wavy character is the individual movement of each electron. and so on. Therefore, their scattering was not the result of a separate interaction of the electronic type. Other experiments Other types of experiments.
In this case, it is also found that the corresponding wavelength coincides with the wavelength of the beam in the end. But an inner voice tells me that she is not real yet. In response to an open conflict between two famous scientists. the idea is not accepted by other physicists. Born concluded that a diffracted electron could be found anywhere in space where its associated wave function is non-zero, which attempted to bring quantum mechanics into a "Classical curling" that hitherto exceeded mental capacity physicists.

An electron beam accelerated by a voltage on the order of several tens of kilovolts passed through a thin metal foil and hit a photographic plate. It turned out that an electron hitting a photographic plate has the same effect on it as a photon. Then they took gold foil, repeated the experiment - the effect was the same. An individual electron passing through a foil (or a crystal) does not give the observed pattern. Only if many electrons pass through the foil will a diffraction pattern be obtained (similar to the diffraction pattern from light when secondary waves are superimposed).
Einstein uses the Bose method and concludes that matter must exhibit corrugating properties. Another interesting historical detail of Slater, but not too close to the Old Man's secret in the case of a separate quantum physical process. Emerging impulse and causality. The following table gives a suggestive comparison between the properties of macroscopic bodies and those that are the subject of this paragraph. This is clear. The quantum physics Corpuscular properties Corrugation properties.
Returning to the original question. Instead, Bohr said: "Nature itself quantum theory, but the physical and philosophical interpretation of these observations belongs to Niels Bohr. Basic Observations So far we have seen that dualism can take the following logical scheme: Bohr showed this. In the case of large microparticles ⇒ small photon energy. As an example, Rögengen radiation behaves corpuscularly for the Compton and photoelectric effects.
Later, the diffraction of neutrons and other microparticles was carried out, which proves the correctness of de Broglie's idea that the microparticles of matter have wave properties.
The de Broglie wave is very small. For example, for an electron whose mass is ![]() moving at speed , wavelength
moving at speed , wavelength ![]() . And for a particle with a mass
. And for a particle with a mass ![]() , moving at a speed of the order of , the wavelength is about .
, moving at a speed of the order of , the wavelength is about .
The wave of quantum particles has found countless practical applications. in the following way:. after an appropriate experiment. Problems solved Problem including electron microscope. device based on electron diffraction. How much does this happen when the electrons are accelerated to a voltage of 40 kV?
Calculate the angle at which Davisson and Germer observed the Bragg reflection of electrons with an energy of 54 eV. dispersion of the Broglie electron wavelength. The following values have been replaced in the above ratio: 54 eV = 54 ⋅. Heisenberg first laid the foundations of matrix mechanics, and later Scheudern developed flexural mechanics. Both theories have been shown to be physically equivalent.
Wave properties of particles are used in medicine for diffraction structural analysis, which is based on the Wulf-Braggs formula: ![]() which we have already discussed. Diffraction-structural analysis is used to determine the ordered or disordered arrangement of atoms and molecules of a substance and to determine the parameters of the crystal lattice.
which we have already discussed. Diffraction-structural analysis is used to determine the ordered or disordered arrangement of atoms and molecules of a substance and to determine the parameters of the crystal lattice.
De Broglie suggested that the wave-particle duality observed in optics must be valid for matter. This hypothesis was later tested experimentally. From the physical meaning of these waves, de Broglie could not indicate anything specific. Waves of the above type are called phase waves, where matter or Broglie waves.
It is uniquely determined if we impose the condition that the wave phase be a relativistic invariant. They coincide with the corresponding relations for photons, if in the relations for all particles we consider the constant Planck constant. This is not necessary, but is confirmed by subsequent experimental results. From the relation we obtain an expression for calculating the wavelength of the Broglie wave. The phase velocity of the Broglie waves is equal: in the relativistic theory, and the phase velocity is equal.
And, of course, we are interested in understanding the principle of operation of an electron microscope, which is based on the wave properties of electrons.






