International system of units of measurement in clinical and biochemical research
By decision of the XI General Conference on Weights and Measures in 1960, the International System of Units SI (Sisteme Internationale, SI) was adopted as a universal system of units for all branches of science and technology.
By the Decree of the State Committee of the Russian Federation for Standardization and Metrology dated February 4, 2003, GOST 8.417 - 81 was replaced by GOST 8.417 - 81 from September 1, 2003. GOST 8.417 - 2002 " State system ensuring the uniformity of measurements. Units of values". This GOST defines the basic (Table 11) and derived quantities and SI units (Tables 12, 13).
The unit of quantity of a substance is the mole - an important concept for expressing the results of laboratory tests. Molecular concentration reflects the relationship between substances at the functional level, since chemical reaction proceeds not in weight, but in molar ratios. For substances whose molecular weight is known, use molar unit measurements, not mass concentration.
Table 11
Basic SI units
Note. In addition to the thermodynamic temperature (designation T), it is also allowed to use the Celsius temperature (designation t) defined by the expression t\u003d T - T 0, where T 0 \u003d 273.15 K. The thermodynamic temperature is expressed in kelvins, and the Celsius temperature is expressed in degrees Celsius. In size, 1 degree Celsius is equal to a kelvin (degree Celsius is a special name used in this case instead of the name "kelvin").
Table 12
Examples of SI derived units whose name and symbol are formed using SI base units

Also, mass and weight should not be confused. The unit of mass is the kilogram (see Table 11), and the unit of weight (gravity) is the Newton (see Table 13).
Designations of derived units that do not have special names should contain a minimum number of designations for SI units with special names and basic units with the lowest possible exponents.
GOST 8.417 - 2002 allows for use without time limit, along with SI units, some non-systemic units of a traditional nature (Tables 14, 15). For example, a centner or ton is used as a unit of mass, a liter is used as a unit of volume. But it is not recommended to form derivative names from these units: you cannot write kiloton, but you should write 1000 tons, 1 gigagram (1 Gg; 1 Gg; 1 10 9 g).
Table 13
SI derived units having special
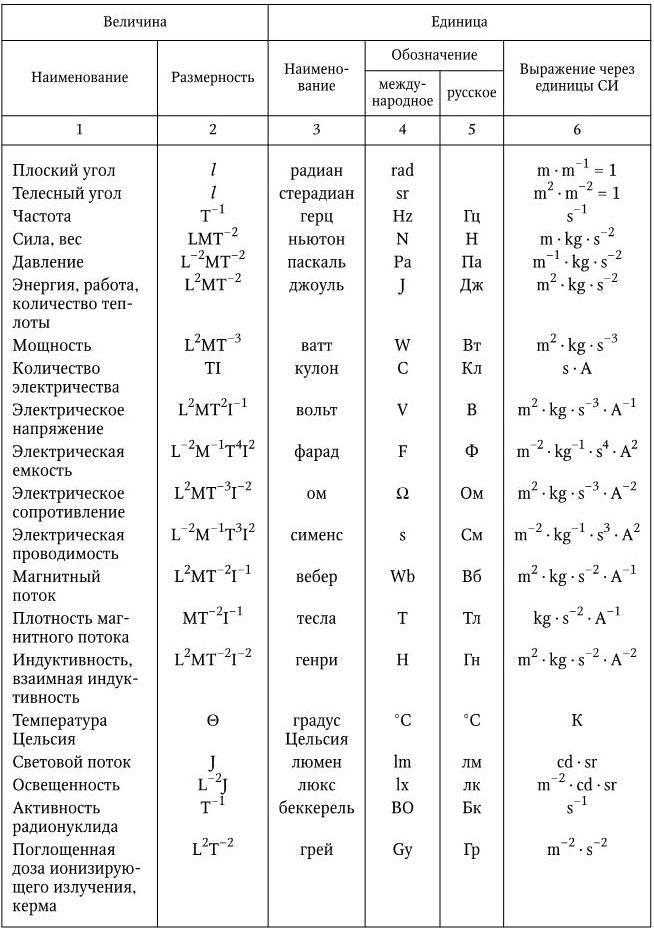

*The unit "katal" was introduced in accordance with Resolution 12 of the XXI General Conference on Weights and Measures (October 1999).
Table 14
Off-system units allowed for use
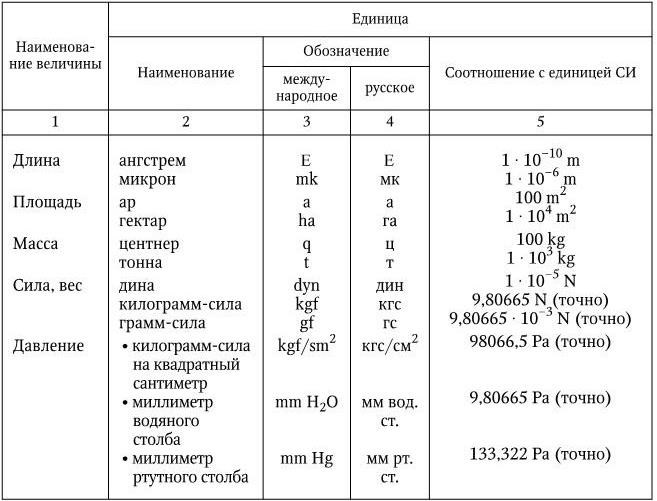

A detailed presentation of the ratios of some off-system units with SI units are presented in Appendix "B" GOST 7.417 - 2000 (Table 16).
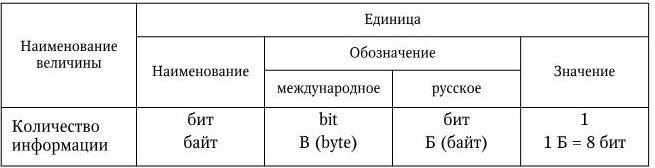
GOST 8.417 - 2002 provides for the use of units of the amount of information. The term "amount of information" is used in devices for digital processing and transmission of information, for example, in computer technology (computers) to record the amount of storage devices, the amount of memory used computer program(Table 17).
Historically, such a situation has developed that with the name “byte” it is incorrect (instead of 1000 = 10 3 1024 = 2 10 is accepted) SI prefixes are used: 1 KB = 1024 bytes, 1 GB = 1024 MB.
Table 15
Units allowed for use in clinical laboratory diagnostics along with SI units
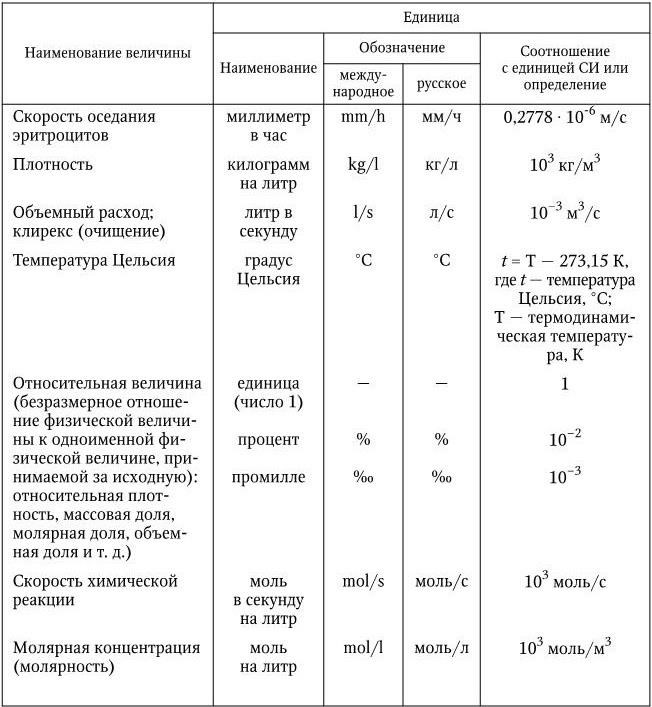
In this case, the Kbyte designation begins with an uppercase (large) letter, as opposed to the lowercase letter "k", denoting a factor of 10 3 (kilo).
GOST 8.417-2002 recommends that the name of decimal, multiple and submultiple SI units be denoted using multipliers and prefixes indicated in Table. 18, for example, milligram, kilometer, decalitre, etc. Attaching two or more prefixes in a row to the name and designation of the unit is not allowed.
Table 16
Units of measurement temporarily allowed for use
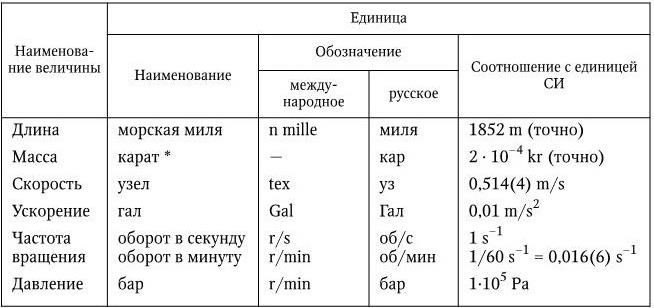
*To denote the mass of precious stones and pearls.
Table 17
Units of quantity of information
Due to the fact that the name of the basic unit of mass - kilogram - already contains the prefix "kilo", to form multiple and submultiple units of mass, one should use grams (0.001 kg) and attach prefixes to this unit, for example, milligram (mg, mg) instead of microkilograms ( mkg, mkg).
It is not allowed to use units of time (minute, hour, day), flat angle (degree, minute, second) and optical power (dioptre) with prefixes.
The prefix or its designation is written together with the name of the unit or, accordingly, with the designation of the latter. In the case when the unit is formed as a product or ratio of units, the prefix or its designation is attached to the name or designation of the first unit included in the product or ratio.
METROLOGY(Greek, metron measure + logos doctrine) - a field of knowledge and practice devoted to measurement methods, ensuring the uniformity of measurements and the required accuracy. It is customary to divide measurement into theoretical (scientific), applied, and legislative measurement. Theoretical measurement includes the general theory of measurements, including methods for assessing accuracy, and the development of units. physical quantities and their systems, the study of the relationship between them. The main tasks of applied measurement are to develop methods and means for reproducing units of physical quantities (standards), methods and means for exemplary and working measurements, and methods for transferring unit sizes from standards to exemplary means and from them to working measuring instruments. Legislative M. includes a set of interrelated rules, requirements, and norms aimed at ensuring the uniformity of measurements and the uniformity of measuring instruments and subject to control (supervision) by state and departmental metrological services (see the Metrological Health Service).
Historically, measurement arose from the description of various kinds of measures according to their names, subdivisions, and mutual correlation. The measures that were in use (linear, volumetric, weight, time), as well as monetary units, were extremely diverse and variegated (in different times in selected countries and even in different cities and localities, measures of various names and sizes were used).
A qualitative leap in measurement took place in connection with the development and introduction into practice of the metric system of measures. This led to the elimination in a number of countries of the isolation and complexity of national and local measures and the need for appropriate research to create a metric system. In 1875, with the participation of Russian scientists B. S. Jacobi, G. I. Wild and G. V. Struve, the Metric Convention was developed, to-ruyu was signed by representatives of 17 countries (including Russia). At the same time, the International Bureau of Weights and Measures was established with the general funds of the states that signed the Convention, which created new standards metric measures for all countries - parties to the Convention. The adoption by most countries of a unified system of weights and measures has led to the transformation of descriptive mathematics into a branch of modern physics based on high-precision physical experiments.
The basic concepts of M. are the concepts of physical quantity and measurement. A physical quantity is a property that is qualitatively common to many physical objects, their states or processes occurring in them, but quantitatively individual for each object. An example of physical quantities are mass, pressure, length, temperature, etc. To express and compare the values of physical quantities, their units are used, that is, such quantities of physical quantities, to which numerical values \u200b\u200bare conventionally assigned equal to one. An example of units of measurement would be kilogram (kg), pascal (Pa), meter (m), degree, etc.
Basic and derived units of physical quantities are combined into systems of units, in which they are optimally related. Most countries, including the USSR, have adopted and are implementing the International System of Units of Physical Quantities - SI (see Units of Measurement).
Measurement is the experimental determination of the value of a physical quantity using special technical means. Distinguish between direct and indirect measurements, depending on whether the desired value is found directly from experimental data or on the basis of a known relationship between the sought value and quantities obtained directly from experience. An example of direct measurements is the measurement of mass on a balance scale. An example of indirect measurements is the measurement of the concentration of a substance in a solution by the optical density of a sample of this solution. As a rule, stable statistical relationships between physical quantities or dependencies due to physical laws are used in indirect measurements.
Any measurements are characterized by accuracy, i.e., the degree of approximation of the measurement results to the true value of the measured quantity. The deviation of the measurement results from the true value of the measured quantity is called the measurement error and is expressed quantitatively in absolute or relative units. In this case, two components of the measurement error are distinguished: the error of the measurement method and the instrumental error. So, when measuring body temperature, an inaccurate assessment of it may occur due to the incorrect location of the thermometer (measurement method error) and inaccurate thermometer readings (instrumental error).
In honey. science and practice use measurements of almost all known physical quantities characterizing the properties or states of biol, an object, samples taken from it, samples environment, measurement parameters various kinds radiations (light, thermal, X-ray, ultrasonic) used in the physiotherapeutic and surgical purposes, and also measurements at the dosed introduction of medicines and biol, preparations.
Along with constant or slowly changing quantities (anthropometric parameters, galvanic current, etc.), dynamic quantities are measured in medicine (pressure and flow rate of biol, gases and liquids, electrical biopotentials), and in the field of electromagnetic nature, measurements cover the entire spectrum of electromagnetic oscillations - from low-frequency, radio-frequency and optical ranges to hard ionizing radiation.
Modern methods of honey. research requires the application of the achievements of all natural sciences, general physical laws and principles, to-rye are implemented in the applied means of measuring physical quantities. Based on this, honey. science and practice make extensive use of all the basic provisions of theoretical, applied, and legislative measurement, and ensuring the uniformity of measurements in medicine is achieved by using the country's standard base, the national park of exemplary measuring instruments, verification schemes, and cadres of verifiers for known types of measurements, as well as the use of common for all spheres National economy rules of metrological supervision.
R. I. Utyamyshev, A. N. Grishin.
[International system of units (SI) in medicine. G. Lippert. Per. with him. M., Medicine, 1980; SI units in medicine. Geneva, WHO, 1979]
The handbook contains tables for substances and some physical quantities of interest to space biology and medicine. The data of these tables include the numerical values of the norm, the disputed (doubtful) area and the area of pathology.
The numbers of the norm are printed in bold, the disputed area in italics, the areas of pathology in normal font.
As an addendum, a table of unit conversion factors for substances used in laboratory practice is provided.
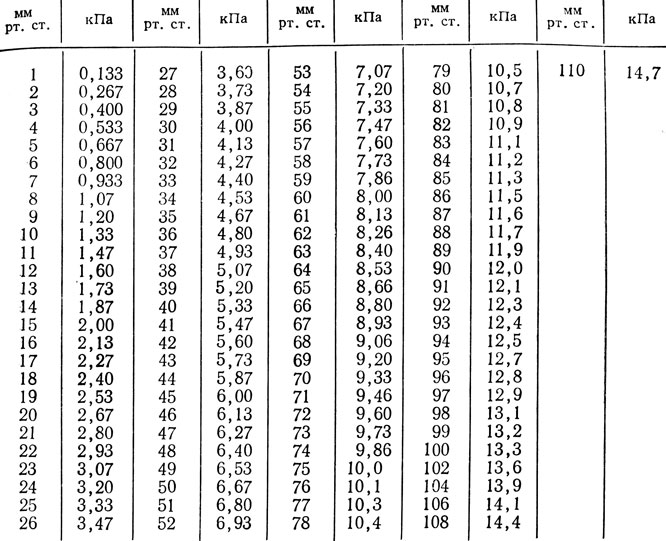

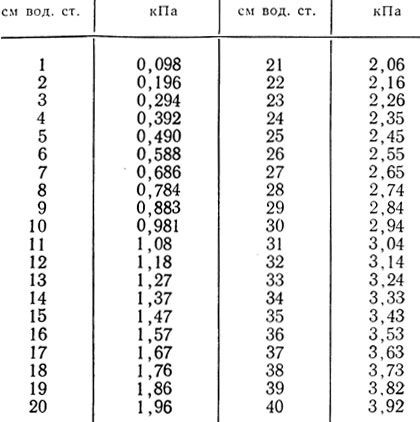

Table 27 st. → kPa for pCO 2, pO 2
Recalculation:

Recalculation: mmHg st.*0.1333 = kPa; kPa*7.501 = mmHg Art.

Recalculation: 1/min * 0.01667 = Hz; Hz*60 = 1/min.
Example: 72/min = 1.2 Hz.
Respiratory rate at rest 8-20 per 1 min.
Heart rate at rest 60-80 in 1 min.
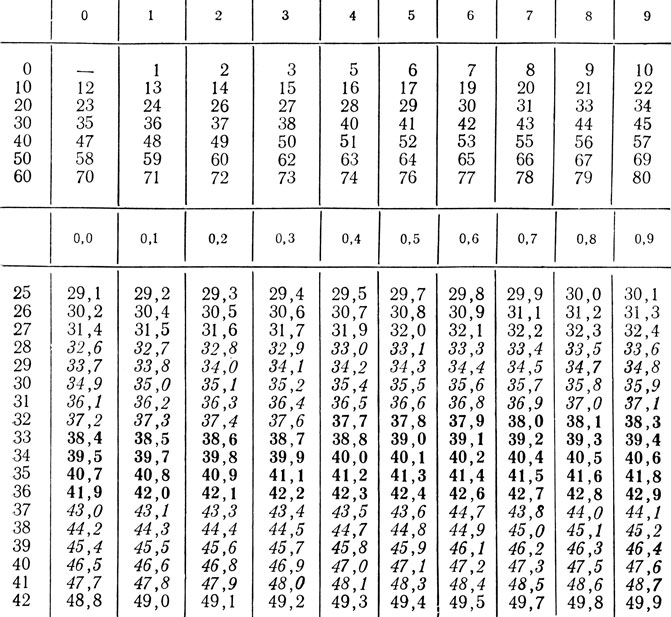
Table 30. Basic exchange kcal / m 2 h → J / m 2 s (W / m 2)
Recalculation: kcal / m 2 h * 1.163 \u003d J / m 2 s * 0.8598 \u003d kcal / m 2 h.
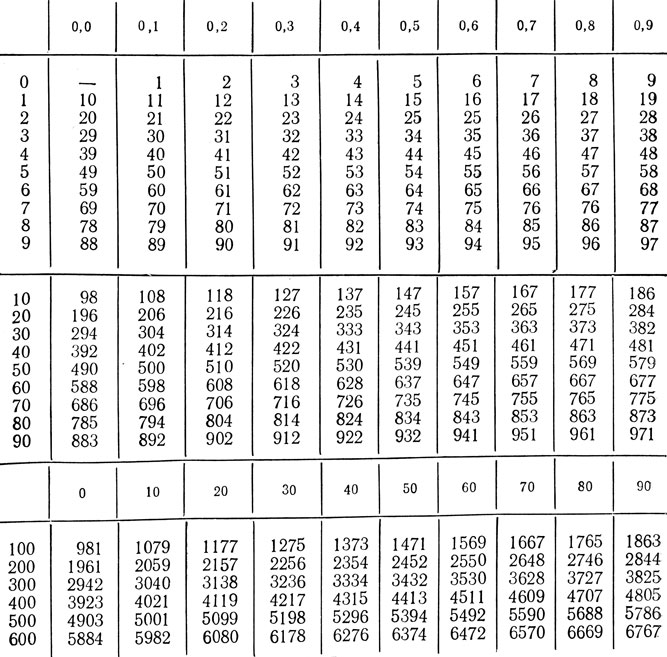
Recalculation: kgcm * 9.807 \u003d J; J * 0.102 \u003d 1 kgcm.
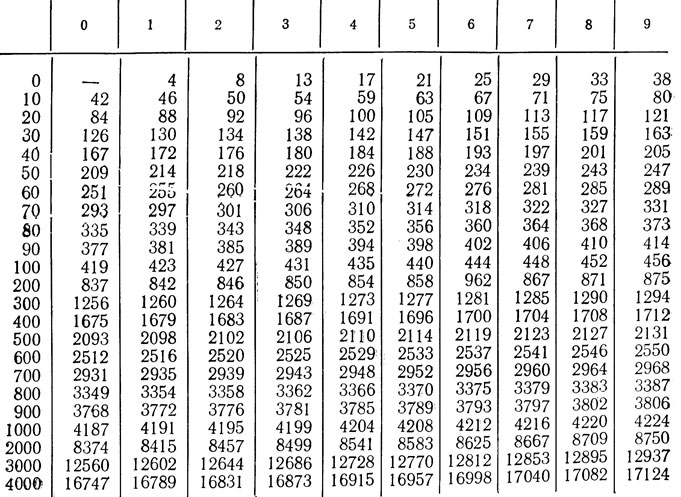
Recalculation: kcal * 4.1868 \u003d kJ; kJ*0.2388 = kcal.
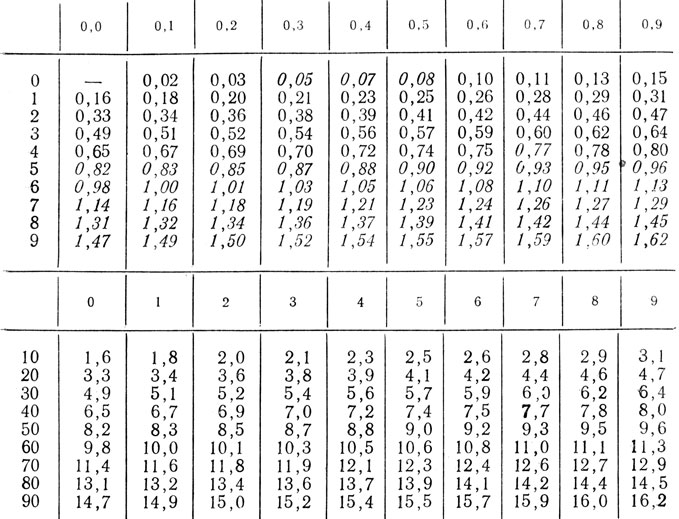
Recalculation: kgcm/min*0.1634 = W; W*6.118 = kgcm/min.

Recalculation: g% * 0.6206 = mmol / l; mmol / l * 1.611 \u003d g%.
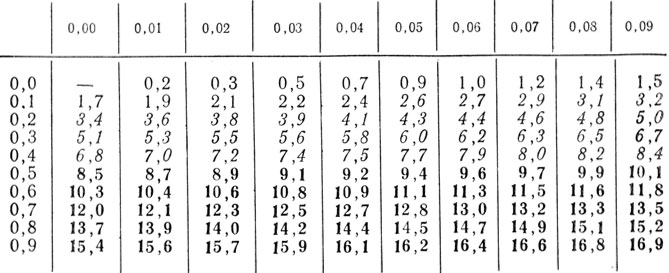
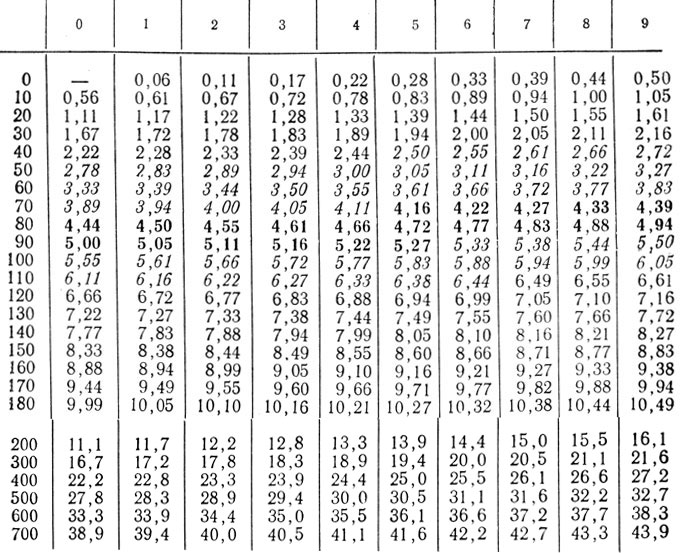
Recalculation: mg% * 0.05551 = mmol / l; mmol/l*18.02 = mg%.
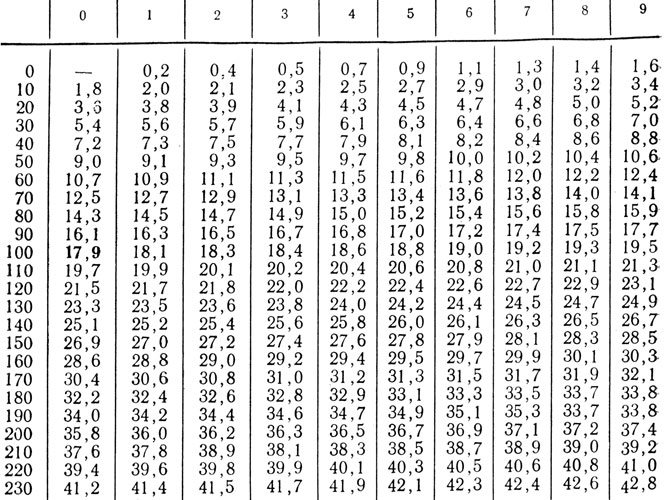

Recalculation: mg% * 0.2557 \u003d mmol / l \u003d mg * eq / l; mmol / l * 3.91 \u003d mg%.

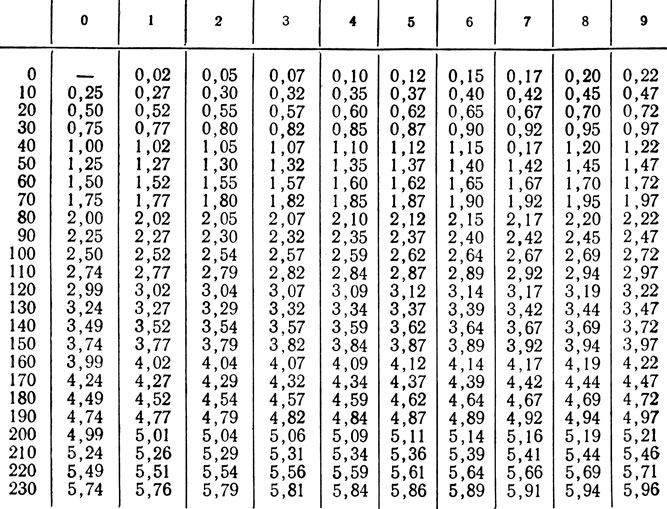
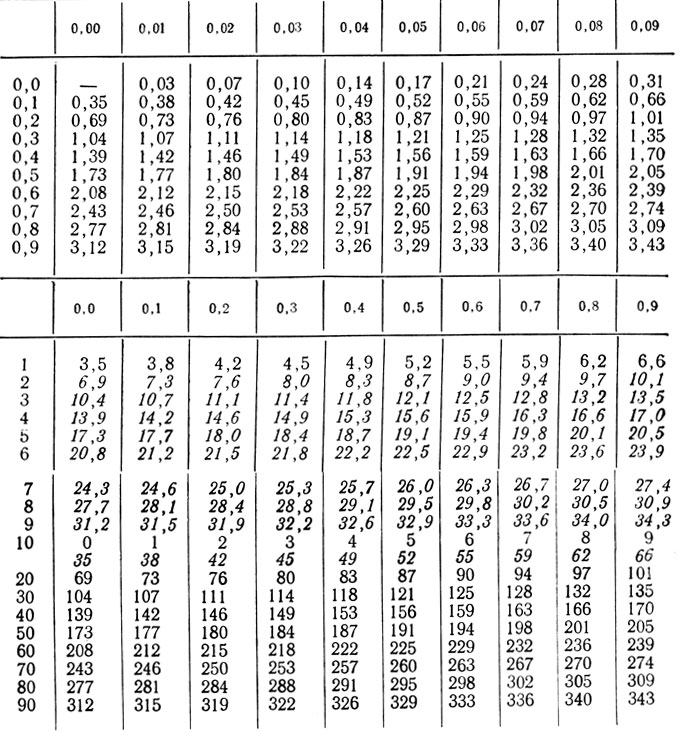
Recalculation: mg*3.467 = µmol*0.2884 = mg.
Note. 17-ketosteroids are calculated as dehydroepiandrosterone (DNEP), the calculation for etiocholanolone and androsterone differs only by 0.3%. The conversion table can also be used for "ketogenic" steroids (the rate is slightly lower). The norm is very dependent on age: the maximum rates at the age of 20-30 years are higher for men than for women.
The International System of Units (SI) as a single universal system for all branches of science, technology and production was adopted in 1960.
The XI General Conference on Weights and Measures, the XXX session of the World Health Assembly, held in 1974, recommended the adoption of the SI in all areas of medicine, including practical public health.
The SI is based metric system. The international system includes the basic physical quantities.
Along with the basic units, the SI also includes their derivatives. Derived units are formed from basic units in accordance with the rules of the International System of Units.
The results of biochemical studies should be expressed only in basic units or their derivatives:
1) the concentration of a substance with a known molecular weight in biological fluids (except urine) should be expressed in moles or its fractions per liter (mol / l, mmol / l, μmol / l, nmol / l, etc.);
2) in cases where the molecular weight of a substance is unknown or cannot be determined (in mixtures), the result of the determination must be expressed in units of mass per liter (g/l, mg/l, etc.);
3) withdrawal various substances with urine expressed in fractions of a mole per day (if the relative molecular weight is known) or in units of mass per day (if the relative molecular weight is unknown);
5) the density of substances is indicated in g / l, clearance in ml / s.
Some prefixes and SI multipliers for the formation of decimal multiples and submultiples
The SI unit of enzyme activity is "katal" (kat) and its derivatives (mkat, etc.). Catal - the amount of enzyme that catalyzes the conversion of 1 mol of substrate in 1 second (mol / s). Therefore, the activity of enzymes in clinical and biochemical studies should be expressed in katals and its fractions per liter, while it must be remembered that cat/l=mol/(s*l), mkat/l=mmol/(s*l), mkat /l=µmol/(s*l), nkat/l-nmol/(s*l).
Factors for converting various previously used enzymatic activity units IU/L to ncat/L:
1) mmol/min/l
*16667 x 0.00006
2) mmol/h/l
x 277.78 x 0.0036
3) µmol/h/l
x277.78 x 0.0036
4) µmol/h/l
xO,2778 x3,6
5) mmol/s/l
* 1000000 x l0-6
6) µmol/min/l (IU/l).
X 16.67 xO.06
Bibliography
1. Brevolskaya N.A., Zakharchenko T.I., Medyanskaya A.V., Ryabusova T.A. Laboratory diagnostics in medical practice: clinical, biochemical and microbiological research methods. Toolkit. - Omsk, 1996.
2. Ermolaeva M.V., Ilyicheva L.P. Biological chemistry: Textbook. – M.: Medicine, 1989.
3. Pustovalova L.M. Fundamentals of biochemistry. - Rostov-on-Don: Phoenix, 2003.
- Berezov T.T., Korovkin B.F. Biological chemistry. – M.: Medicine, 2002.
- Biochemistry / ed. E.S. Severin.– M.: GEOTAR-MED, 2003.
- Dolgov V., Morozova V. and others. Clinical and diagnostic significance of laboratory parameters. – M.: Center, 1995.
- Kamyshnikov V.S. Handbook of clinical and biochemical laboratory diagnostics: in 2 T. T.1,2. - Minsk: Belarus, 2002.
- Clinical biochemistry / ed. V.A. Tkachuk. - M.: GEOTAR-MED, 2002.
- Murray R. and others. Human biochemistry. In 2 volumes. – M.: Mir, 1993.
- Medical laboratory diagnostics (programs and algorithms). Handbook / Under. ed. professors A.I. Karpishchenko. - St. Petersburg: Intermedica, 1997.
- Medical laboratory technologies. Directory: in 2 vols. Vol. 1. / under. ed. professors A.I. Karpishchenko. - St. Petersburg: Press, 2002.
- Medical laboratory technologies. Directory: in 2 vols. Vol. 2. / under. ed. professors A.I. Karpishchenko. - St. Petersburg: Intermedica, 1999.
- Nikolaev A.Ya. Biological chemistry. – M.: MIA, 2001.
- Quality assurance of laboratory research: Reference manual / ed. V.V. Menshikov. – M.: LABINFORM, 1999.
- Tsyganenko A.Ya., Zhukov V.I. et al. Clinical Biochemistry ( Tutorial for medical students). - M.: Triada-X, 2002.
- Quality management of clinical laboratory research: Regulatory documents / under. ed. V.V. Menshikov. – M.: Labpress, 2000.






