The heat engine does. Heat engine: cycle, work, efficiency. Ecological problems of thermal machines. What is the ideal heat engine?
For one cycle of operation of an ideal Carnot engine, 1000 kJ of heat was transferred to the working fluid from the heater and 800 kJ of heat was given to the refrigerator. What is the refrigerator temperature if the heater temperature is 800 K? A) 600 K B) 640 K C) 340 K D) 300 K Help
Answer:
Efficiency \u003d (Qload-Qcold) / Qload \u003d 200/1000 \u003d 0.2. (800-Thol)/800=0.2, Thol/800= 0.8, Thol=640K. (B)
Similar questions
- It would be a pity if his slender waist had never been pulled down by a military uniform, and if he had spent his youth bent over stationery. Grammatical basis, scheme and characteristics
- Masha is in the sixth grade. In this class, there are 2 times fewer boys than girls. Masha has 8 more classmates than her classmates. How many students are in the class?
- Select three paragraphs from the text and read it aloud. Write a sentence from each paragraph that expresses the main idea. What synonyms can be used when speaking about the Russian language? Which of them is used by D. Likhachev. Russian literature is one of the peaks of world culture, the most valuable asset of all mankind The names of A. S. Pushkin, M. N. Tolstoy, F. M. Dostoevsky, A. P. Chekhov and other great Russian writers are known to all cultural world. How did this literature come about? On a thousand-year experience of the culture of the word. The outstanding literary scholar Dmitry Sergeevich Likhachev wrote:<Рождению русской литературы способствовал превосходный, гибкий и лаконичный русский язык, достигший ко времени возникновения русской литературы высокого уровня развития... Это был язык с обширным словарным составом, с развитой терминологией -- юридической, военной, феодальной, технической; обильный синонимами, способными отразить различные эмоциональные оттенки...>
In production led to the emergence of thermal engines.
The device of heat engines
Heat engine (heat engine) - a device for converting internal energy into mechanical.
Any heat engine has a heater, a working fluid (gas or steam), which, as a result of heating, performs work (turns the turbine shaft, moves the piston, and so on) and a refrigerator. The figure below shows a diagram of a heat engine.
Fundamentals of heat engines
Each heat engine operates thanks to the engine. To do the job, he needs to have a pressure difference on either side of the engine piston or turbine blades. This difference is achieved in all heat engines as follows: the temperature of the working fluid rises by hundreds or thousands of degrees in comparison with the ambient temperature. In and in engines internal combustion(ICE) there is an increase in temperature due to the fact that the fuel burns inside the engine itself. The refrigerator can be an atmosphere or a special-purpose device for condensing and cooling the exhaust steam.
Carnot cycle
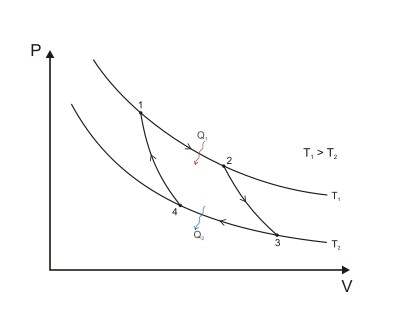
Cycle ( circular process) - a set of changes in the state of the gas, as a result of which it returns to its original state (it can do work). In 1824, the French physicist Sadi Carnot showed that the heat engine cycle (Carnot cycle), which consists of two processes, isothermal and adiabatic, is beneficial. The figure below shows a graph of the Carnot cycle: 1-2 and 3-4 are isotherms, 2-3 and 4-1 are adiabats.
In accordance with the law of conservation of energy, the work of heat engines performed by the engine is equal to:
A \u003d Q 1 - Q 2,
where Q 1 is the amount of heat that is received from the heater, and Q 2 is the amount of heat that is delivered to the refrigerator.
The efficiency of a heat engine is the ratio of the work A that the engine performs to the amount of heat that is received from the heater:
η \u003d A / Q \u003d (Q 1 - Q 2) / Q 1 \u003d 1 - Q 2 / Q 1.

In "Thoughts on driving force fire and about machines that are capable of developing this force "(1824) Carnot described a heat engine called "an ideal heat engine with an ideal gas, which is a working fluid." Thanks to the laws of thermodynamics, it is possible to calculate the efficiency (maximum possible) of a heat engine with a heater that has a temperature T 1 and a refrigerator with a temperature T 2. The Carnot heat engine has an efficiency:
η max \u003d (T 1 - T 2) / T 1 \u003d 1 - T 2 / T 1.
Sadi Carnot proved that any real heat engine that works with a heater with temperature T 1 and a refrigerator with temperature T 2 is not capable of having an efficiency that would exceed the efficiency of a heat engine (ideal).
Internal combustion engine (ICE)
A four-stroke internal combustion engine consists of one or more cylinders, a piston, a crank mechanism, intake and exhaust valves, and spark plugs.
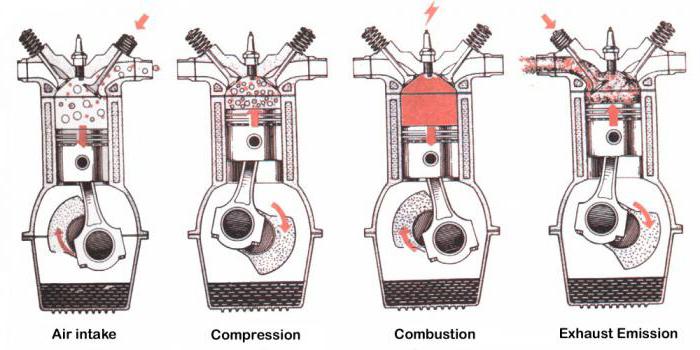
The work cycle consists of four cycles:
1) suction - the combustible mixture enters the cylinder through the valve;
2) compression - both valves are closed;
3) working stroke - explosive combustion of a combustible mixture;
4) exhaust - the release of exhaust gases into the atmosphere.
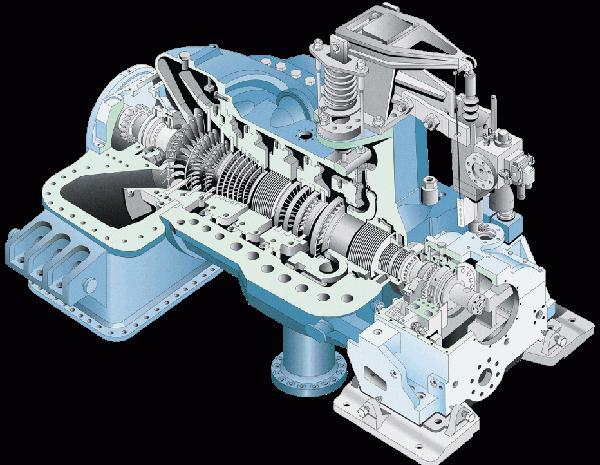
Steam turbine
In a steam turbine, energy conversion occurs due to the difference in pressure of water vapor at the inlet and outlet.
The power of modern steam turbines reaches 1300 MW.
Some technical parameters of a 1200 MW steam turbine
- Steam pressure (fresh) - 23.5 MPa.
- Steam temperature - 540 °C.
- Steam consumption by the turbine - 3600 t/h.
- Rotor speed - 3000 rpm.
- The vapor pressure in the condenser is 3.6 kPa.
- Turbine length - 47.9 m.
- Turbine weight - 1900 tons.
The heat engine consists of an air compressor, a combustion chamber and a gas turbine. Principle of operation: air is adiabatically sucked into the compressor, so its temperature rises to 200 ° C or more. Then it enters the combustion chamber, where at the same time under great pressure liquid fuel is supplied - kerosene, photogen, fuel oil. When fuel is burned, the air heats up to a temperature of 1500-2000 ° C, expands, and its speed increases. Air moves from high speed, and the combustion products are sent to the turbine. After the transition from stage to stage, the combustion products give their kinetic energy to the turbine blades. Part of the energy received by the turbine goes to the rotation of the compressor; the rest is spent on the rotation of the rotor of the electric generator, the propeller of an aircraft or a sea vessel, the wheels of a car.
The gas turbine can be used, in addition to the rotation of the wheels of a car and the propellers of an aircraft or ship, as jet engine. Air and combustion products are expelled from the gas turbine at high speed, therefore jet thrust, which occurs during this process, can be used for the movement of air (aircraft) and water (ship) ships, railway transport. For example, An-24, An-124 ("Ruslan"), An-225 ("Dream") aircraft have turboprop engines. So, "Dream" at a flight speed of 700-850 km / h is capable of transporting 250 tons of cargo over a distance of almost 15,000 km. It is the largest transport aircraft in the world.
Environmental problems of thermal engines
The state of the atmosphere, in particular the presence of carbon dioxide and water vapor, has a great influence on the climate. Thus, a change in the content of carbon dioxide leads to an increase or decrease in the greenhouse effect, in which carbon dioxide partially absorbs the heat that the Earth radiates into space, retains it in the atmosphere and thereby increases the temperature of the surface and lower layers of the atmosphere. The phenomenon of the greenhouse effect plays a decisive role in climate mitigation. In his absence average temperature the planet would not be +15 °С, but lower by 30-40 °С.
There are now more than 300 million different kind vehicles, which create more than half of all air pollution.
 For 1 year, 150 million tons of sulfur oxides, 50 million tons of 50 million tons of ash, 200 million tons of carbon monoxide, 3 million tons of pheon are released into the atmosphere from thermal power plants as a result of fuel combustion.
For 1 year, 150 million tons of sulfur oxides, 50 million tons of 50 million tons of ash, 200 million tons of carbon monoxide, 3 million tons of pheon are released into the atmosphere from thermal power plants as a result of fuel combustion.
The composition of the atmosphere includes ozone, which protects all life on earth from the harmful effects of ultraviolet rays. In 1982, J. Farman, an English researcher, discovered an ozone hole over Antarctica - a temporary decrease in the ozone content in the atmosphere. At the time of maximum development of the ozone hole on October 7, 1987, the amount of ozone in it decreased by 2 times. The ozone hole probably arose as a result of anthropogenic factors, including the use of chlorine-containing freons (freons) in industry, which destroy ozone layer. However, research in the 1990s did not support this view. Most likely, the appearance of the ozone hole is not associated with human activity and is a natural process. In 1992, an ozone hole was discovered over the Arctic.
If all atmospheric ozone is collected in a layer near the Earth's surface and condensed to the density of air at normal atmospheric pressure and a temperature of 0 °C, the thickness of the ozone shield will be only 2-3 mm! That's the whole shield.
A bit of history...
- July 1769. In the Parisian park Meudon, military engineer N. J. Cugno on a “fire cart”, which was equipped with a two-cylinder steam engine, drove several tens of meters.
- 1885 Karl Benz, a German engineer, built the first 0.66 kW Motorwagen four-stroke three-wheeled petrol car, for which he received a patent on January 29, 1886. The speed of the car reached 15-18 km / h.
- 1891 Gottlieb Daimler, a German inventor, made a cargo trolley with a 2.9 kW (4 horsepower) engine from a passenger car. the car reached 10 km / h, the carrying capacity in various models ranged from 2 to 5 tons.
- 1899 The Belgian K. Zhenatzi in his car "James Content" ("Always dissatisfied") for the first time overcame the 100-kilometer speed limit.

Examples of problem solving
Task 1. An ideal heat engine has a heater temperature of 2000 K, and a refrigerator temperature of 100 °C. Determine efficiency.
Solution:
The formula that determines the efficiency of a heat engine (maximum):
ŋ \u003d T 1 -T 2 / T 1.
ŋ \u003d (2000K - 373K) / 2000 K \u003d 0.81.
Answer: Engine efficiency - 81%.
Task 2. In the heat engine during the combustion of fuel, 200 kJ of heat was received, and 120 kJ of heat was transferred to the refrigerator. What is the engine efficiency?
Solution:
Formula for definitions of efficiency looks like this:
ŋ = Q1 - Q2 / Q1.
ŋ \u003d (2 10 5 J - 1.2 10 5 J) / 2 10 5 J \u003d 0.4.
Answer: The efficiency of the heat engine is 40%.
Task 3. What is the efficiency of a heat engine if the working fluid, after receiving an amount of heat of 1.6 MJ from the heater, performed 400 kJ of work? How much heat was transferred to the refrigerator?
Solution:
The efficiency can be determined by the formula
ŋ \u003d 0.4 10 6 J / 1.6 10 6 J \u003d 0.25.
The amount of heat transferred to the refrigerator can be determined by the formula
Q 1 - A \u003d Q 2.
Q 2 \u003d 1.6 10 6 J - 0.4 10 6 J \u003d 1.2 10 6 J.
Answer: the heat engine has an efficiency of 25%; the amount of heat transferred to the refrigerator is 1.2 10 6 J.
5.196. An ideal heat engine operating according to the Carnot cycle performs work A = 2.94 kJ in one cycle and transfers the amount of heat H2 = 13.4 kJ to the refrigerator in one cycle. Find efficiency cycle.
5.197. An ideal heat engine operating on the Carnot cycle performs work A \u003d 73.5 kJ in one cycle. Heater temperature t1 = 100 ° C, refrigerator temperature t2 = 0 ° C. Find the cycle efficiency, the amount of heat Q1 received by the machine in one cycle from the heater, and the amount of heat Q2 given in one cycle to the refrigerator.

5.198. An ideal heat engine operates according to the Carnot cycle. At the same time, 80% of the amount of heat received from the heater is transferred to the refrigerator. The machine receives from the heater the amount of heat Q1 = 6.28 kJ. Find the efficiency of the cycle and the work A done in one cycle.
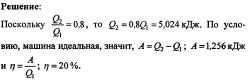
5.199. An ideal heat engine operates according to the Carnot cycle. Air at pressure p1 = 708 kPa and temperature t1 = 127°C occupies a volume V1 = 2 liters. After isothermal expansion, the air occupied the volume V2 = 5 l; after adiabatic expansion, the volume became equal to V3 = 8 l. Find: a) the coordinates of the intersection of isotherms and adiabats; b) work A performed at each section of the cycle; in) full work A, performed for the entire cycle; cycle efficiency; e) the amount of heat Q1 received from the heater in one cycle; f) the amount of heat Q2 given to the refrigerator in one cycle.


5.200. Quantity v = 1 kmol ideal gas completes a cycle consisting of two isochores and two isobars. In this case, the gas volume changes from V1 = 25 m3 to V2 = 50 m3 and the pressure changes from p1 = 100 kPa to p2 = 200 kPa. How many times the work done in such a cycle is less than the work done in the Carnot cycle, the isotherms of which correspond to the highest and lowest temperatures of the cycle under consideration, if the volume increased by 2 times during isothermal expansion?
![]()
1. Maxwell distribution. Experimental verification of Maxwell's distribution law
2. Definition of wave number and wave vector
3. The heat engine performs 3 kJ of work in one cycle and gives the refrigerator an amount of heat equal to 12 kJ. Determine the efficiency of a heat engine
Maxwell distribution
Velocity (or momentum) distribution of molecules in a system in thermodynamic equilibrium. Assuming that a certain number of molecules will have a certain speed and the proportion of fast and slow molecules is not large, we can
determine what proportion of molecules ∆! has the speed included in def. interval | ||||||||
!!!! | ||||||||
Experimental verification of Maxwell's distribution law
Estherman's experience. A beam of cesium atoms flew out of the furnace through hole 1, moved along a parabola under the action of gravity. Some motion trajectories passed through slit 2, and then were captured by detector 3, with varying height h, where h depended on the speed of the atoms. That is, the detector counted how many atoms flew into the slot (and only those that had a certain speed flew) So the distribution of velocities of Cesium atoms was obtained. They confirmed Maxwell's formula.
Wave number definition
The number of waves in one centimeter; numerically equal to the number of wave periods that fit into a segment of 2π meters. This is the spatial analogue of the circular frequency; defines
spatial period and direction of wave propagation. = 2 = phases
Wave vector definition
Where is a unit vector directed perpendicular to the wave surface, in the direction of wave propagation.

1. Statistical substantiation of the second law of thermodynamics. Boltzmann formula for statistical entropy.
2. Definition of the equation of state of matter.
3. Determine the ratio of root-mean-square velocities of helium and nitrogen molecules at the same temperatures. Relative atomic mass helium is 4, and nitrogen 14.
Statistical substantiation of the second law of thermodynamics.
Let there be six gas molecules in the vessel. Mentally divide the vessel into three equal parts. Chaotically moving, the molecules create certain macrodistributions. In theoretical physics, it is proved that the thermodynamic probability, i.e., the number N of particles according to
P states (six particles in three parts of the vessel), is determined by the formula
= ! !! !. .! !
The uniform distribution has the highest thermodynamic probability, it can be carried out largest number ways. All processes in nature proceed in a direction, leading to an increase in the probability of a state
Boltzmann formula for statistical entropy.
The connection between entropy and thermodynamic probability was established by Boltzmann - entropy is proportional to the logarithm of thermodynamic probability: = . The statistical meaning of the concept of entropy is that an increase in the entropy of an isolated system is associated with the transition of this system from a less probable state to a more probable one.
Definition of the equation of state of matter.
Describes the relationship between the thermodynamic (macroscopic) parameters of the system (pressure, volume, temperature).
Where =! =!

1. Entropy increase law. Third law of thermodynamics
2. Definition of an ideal gas.
3. How many times kinetic energy particle is less than its rest energy if the particle moves at a speed of 0.8C, where C \u003d 3 * 10^8 m / s is the speed of light
Entropy increase law.
"In an isolated system, entropy does not decrease." If at some point in time closed system is in a non-equilibrium macroscopic state, then at subsequent times the most likely consequence will be a monotonic increase in its entropy. If at some point in time the entropy of a closed system is different from the maximum, then at subsequent moments the entropy does not decrease - it increases or, in the limiting case, remains constant.
Let us consider heat transfer between two parts of the system A1 and A2 having temperatures T1 and T2. Let T1 In this case, the entropy of the body A1 will change by the value ΔQ1/T1 , and the entropy of the body A2 will change by the value Valid only for equilibrium systems. When a system tends to absolute zero, its entropy tends to a constant, taken as zero. The heat capacity also tends to zero. Consequences: it is impossible to reach a state with absolute zero; ur. Mend-clap. not applicable to describe an ideal gas as → 0 Definition of an ideal gas. A mathematical model of a gas, in which it is assumed that: 1) the potential energy of the interaction of molecules can be neglected in comparison with their kinetic energy; 2) the total volume of gas molecules is negligible. Forces do not act between molecules attraction or repulsion, collisions of particles between themselves and with the walls of the vessel are absolutely elastic, and the time of interaction between molecules is negligible compared to the average time between collisions. 1.
Carnot cycle. Carnot's theorem. efficiency of an ideal heat engine. 2.
Determination of the average kinetic energy of an atom. 3.
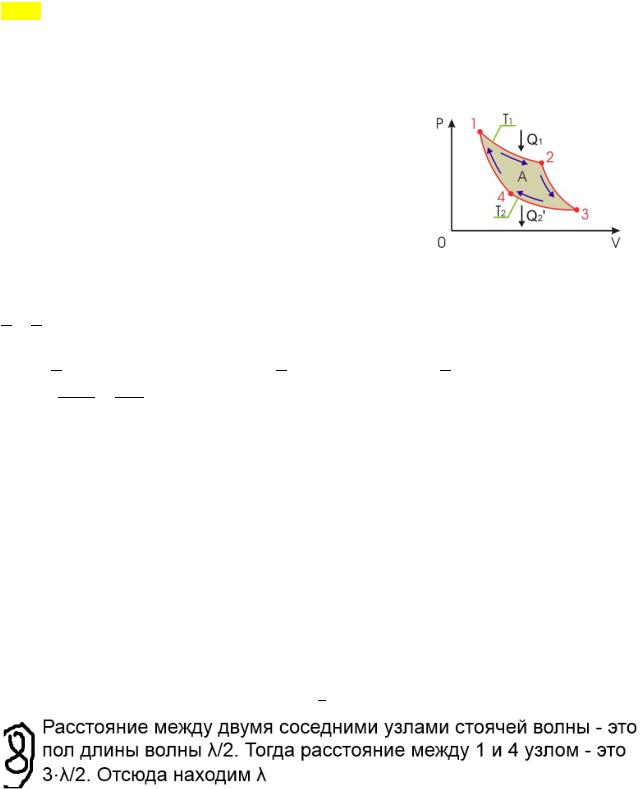
Determine the wavelength of the standing wave if the distance between the first angle and the fourth angle of the standing wave is 18 cm Carnot cycle. Closed cycle. For heat transfer to occur, a temperature difference is required. 1-2-isothermal process: the gas receives heat (!) from the heater, expanding at a constant temperature T! . 2-3-adiabatic: the gas expands without heat exchange 3-4-isothermal: the gas gives off heat to the refrigerator (the refrigerator takes ′! =! ), contracts at a constant temperature T! 4-1-adiabatic: the gas is compressed without heat exchange. efficiency of an ideal heat engine. Using the adiabatic equation, we write the processes 2-3 4-1: Let's divide the first by the second: Since processes 1-2 and 3-4 are isothermal, the change in internal energy = 0, !
!!
! then according to the first law of thermodynamics and the work of the isothermal process ( !" =
! ! ), we get:! We use ! (!)
!!
!!!
!!
!!!!
Carnot's theorem. 1.
The efficiency of any reversible heat engine operating according to the Carnot cycle does not depend on the nature of the working fluid and the design of the machine, but is a function of only the temperature of the heater! and refrigerator! : arr = 1 − Ф(! ,! ) 2.
The efficiency of any heat engine operating on an irreversible cycle is less than the efficiency of a machine with a reversible Carnot cycle, provided that the temperatures of their heaters and refrigerators are equal: nebr<
обр Determination of the average kinetic energy of an atom. =
!
<
!
>
3
The kinetic energy of the translational motion of atoms and molecules, averaged over a huge number of randomly moving particles, is a measure of what is called temperature. If the temperature T is measured in degrees Kelvin (K), then its relationship with ! is given by the ratio: 1.
barometric formula. Boltzmann distribution. 2.
Definition of the atomic mass unit. 3.
Determine the adiabatic exponent of a diatomic gas. Using the well-known Poisson equation, write down the adiabatic equation for this gas in P-T variables. barometric formula. Allows you to calculate the atmospheric pressure depending on the altitude or, by measuring the pressure, find the altitude. Let an ideal gas be in an external gravity field. Consider the equilibrium of a small volume of gas: − − − = !"#
! =
!" By setting the pressure at where = = !
!
!!
!" => !
Divide by Avogadro's number: ! ! − Boltzmann constant Boltzmann distribution. !" , but ! =
! - distribution Boltzmann. ! !"
Definition of a.m.u. Carbon unit - an off-system unit of mass used for the masses of molecules, atoms, atomic nuclei and elementary particles. The atomic mass unit is expressed in terms of the mass of the carbon nuclide 12 C and is equal to 1/12 of the mass of this nuclide. 1.
Clausius inequality. Thermodynamic entropy. The second law of thermodynamics. 2.
Determination of the relative atomic mass of an atom. 3.
Determine the number of hydrogen molecules per unit volume of a vessel, the pressure in which is 270 Pa, if the root mean square velocity of its molecules is 2.4 km/s Clausius inequality. The total amount of reduced heat in any circuit. cycle for any TermSist cannot be greater than zero: !! ! ≤ 0, where is the amount of heat reported to the system (or removed from it: -) in an infinitely small section of the cycle; T - abs. temp-pa resp. environment element;!! ! − elementary reduced heat. irreversible (at least one section) the cycle corresponds to inequality, the cycle consisting only of reversible processes - the equal sign (Clausius equality). Depends only on the initial and final states. Thermodynamic entropy. A measure of the irreversible dissipation of energy. = (diff form), ! − ! = ! ! !! ! (integr form), where ! ,! − entropy of the final and initial state. The second law of thermodynamics. According to Clasius: - heat in itself, without a change in the surrounding bodies, cannot pass from a less heated body to a more heated one. According to Thomson: in nature, a circular process is impossible, the only result of which would be mechanical work performed by removing heat from a thermal reservoir. Heat cannot pass from a cold body to a hot one without any other changes in the system (energy dissipation). Relative atomic mass. A "tmonaya mass, relative atomic mass (old name - atomic weight ) is the value of the mass of an atom, expressed in atomic mass units. At present, the atomic mass unit is taken equal to 1/12 of the mass of a neutral atom of the most common isotope of carbon 12
C, so the atomic mass of this isotope is, by definition, exactly 12. 1.
The first law of thermodynamics in integral and differential forms records. 2.
Determination of the interval of events in the special theory of relativity. 3.
What number of gas atoms does a unit volume of a vessel contain at a temperature of 20 degrees Celsius and a pressure of 0.5 atm. Determine the mean square velocity of the atoms of this gas. The relative amino acid of this gas is 4. First law of thermodynamics. The change in the internal energy of a thermodynamic system (body) can be carried out "in two ways: by making mechanical work and by heat transfer. = ∆ − ′, where is the amount of heat transferred to the system, ∆ is the change in the internal energy of the system, ! − work done on the system. = − . The first law forbids the existence of a perpetual motion machine without the supply of external energy. Determining the interval of events in SRT. It is: ∆ ! =! ∆! −∆! −∆! −∆! , where: ∆ =! −! , ∆ =! −! , ∆ =! −! , ∆ = ! −! - difference of times and coordinates of two events. That is, this is the distance between two events in the space of time, which is a generalization of the Euclidean space between two points. 1.
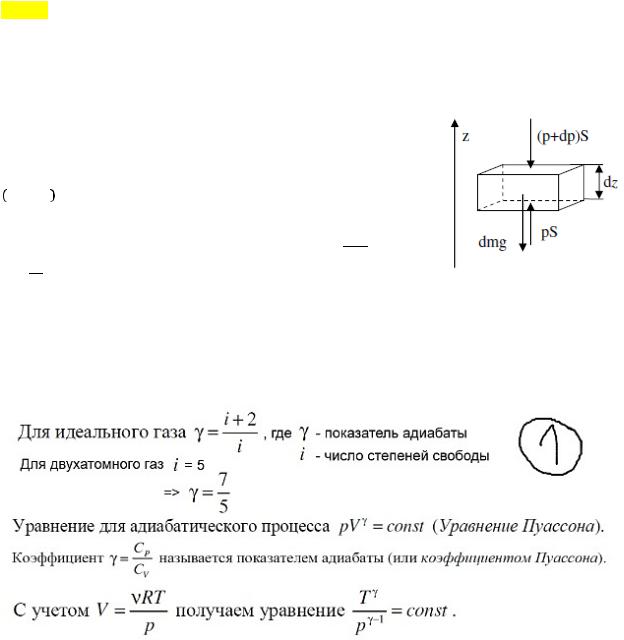
adiabatic process. Poisson equation. The adiabatic equation in P-T coordinates 2.
Determination of the relative molecular weight of a molecule. 3.
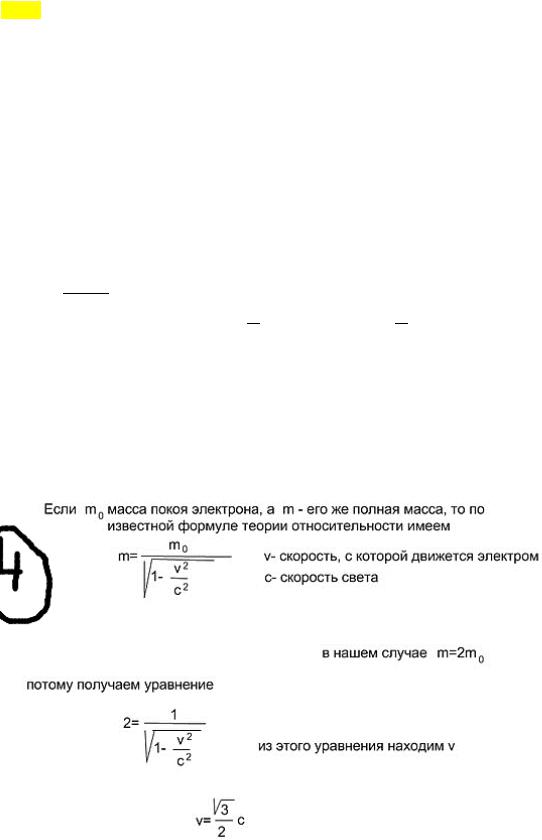
At what speed is the mass of a moving electron twice its rest mass? adiabatic process. A process in which there is no heat exchange (= 0 ) between the system and environment. All fast processes can be considered as adiabatic processes. From the first law of thermodynamics: − = , i.e. outside work is performed due to a change in the internal energy of the system.ad \u003d 0. Poisson equation. The first law of thermodynamics. + + - ur. Mendeleev-Klap. ! =! + - ur. Mayer Having carried out the transformations (we express dT from the 2nd, substitute into the first, also from the third R into the first), we get: ! percent!! ! + = 0 is the equation of some polytropic process. For Perc!! ! adiabats (proc = hell = 0), we get:! ! + = 0 , where! ! = - adiabatic exponent, then !
!!
! integrate + = 0: !" ! +!" ! = 0 => ln + ln = ln() => ln(! ) = ln() =>! = - Poisson equation. Adiabatic equation in P-T coordinates. With the help of ur. Mend-Clap is excluded from! = :!!! = Determination of the relative molecular weight of a molecule. This is the ratio of the mass of a molecule of a substance to 1/12 of the mass of an atom 12 C (carbon). 1.
Standing wave equation. Knots and antinodes of a standing wave. 3.
Carbon dioxide CO2 weighing 8 g was heated to ∆t = 20 degrees Celsius under conditions of free expansion. Find the work of expanding the gas and the change in its internal energy. The relative atomic mass of carbon is 12, and oxygen is 16 Standing wave equation. Standing wave - oscillations in distributed oscillatory systems with a characteristic arrangement of alternating maxima (antinodes) and minima (nodes) of the amplitude. In practice, such a wave arises during reflections from obstacles and inhomogeneities as a result of the superposition of the reflected wave on the incident one. Standing wave equation: = (−) Derivation of the standing wave equation. Consider two waveforms with three identical frequency ranges, which When two waves are superimposed on each other: Knots and antinodes of a standing wave. Antinodes - sections of a standing wave, where the amplitude of oscillations is maximum. =±. Similarly, nodes are sections of a standing wave with a minimum amplitude. = ! ! ± Definition of Thermodynamic System (TS).. A thermodynamic system is a physical system, consisting of a large number of particles, capable of exchanging with the environment, energy, matter. It is also usually assumed that such a system obeys statistical regularities. For thermodynamic systems, the laws of thermodynamics are valid. Isolated vehicle definition An isolated system (closed system) is a thermodynamic system that does not exchange either matter or energy with the environment. In thermodynamics it is postulated (as a result of the generalization of experience) that isolated system gradually comes to a state of thermodynamic equilibrium, from which it cannot spontaneously exit (zero law of thermodynamics). 1.
Energy flow of an elastic wave. Calculation of the energy flow using the Umov vector. 2.
Definition absolute scale temperature and its relation to the Celsius temperature scale. 3.
Determine the adiabatic exponent for a monatomic gas. Using the known Poisson equation, write down the adiabatic equation in V – T variables. Energy flow of an elastic wave. The energy flux of an elastic wave is the amount of energy carried by a wave through a certain surface per unit time. F = Measured in watts. Calculation of the energy flow using the Umov vector. The Umov vector is the flux density vector. Mean:< > = < > = . It is different at different points in space, changes in time according to the law of the square of the sine. Determination of the absolute temperature scale and its relationship with the Celsius temperature scale. Absolute temperature scale - the measure of the base of the lower limit of such a scale is absolute 0, below which the temperature cannot fall. 0K corresponds to -273 . Accordingly, the freezing point of water in Kelvin is 273 degrees, and the boiling point is 373K. 1 = 1K