Below the boiling point of a liquid, evaporation occurs. Solutions boil at a higher temperature than pure solvents. Theoretical and real refrigeration cycle
BOILING, the process of vaporization in a liquid, including the birth of vapor bubbles, their growth, movement and interaction; nonequilibrium phase transition of the 1st kind.
Boiling is caused by overheating of the liquid, the state of which falls into the region above the liquid-vapor equilibrium line (binodal 1, figure), or by a decrease in pressure below its value on the equilibrium line. On the phase diagram, the boiling process is described by a trajectory or a point inside the region of the metastable (overheated) state, bounded on the one hand by the binodal (dependence of the equilibrium boiling temperature on pressure), on the other hand, by the spinodal (the boundary of the thermodynamic stability of the liquid). The equilibrium boiling point at atmospheric pressure is usually given as one of the main physicochemical characteristics of a chemically pure substance. When a liquid is stretched, the phenomenon of cavitation, akin to boiling, is observed.
The vapor pressure in a quasi-equilibrium bubble p p is balanced by the liquid pressure p l and the interfacial tension σ and is related to the pressure saturated steam over a horizontal surface at the same Kelvin temperature by the equation. The critical radius of the bubble in accordance with Laplace's law is equal to
R cr = 2σ/(r p −r w),
and for R< R кp пузырьки схлопываются, при R >R kp - grow.
The process of birth of vapor bubbles in the volume of a homogeneous purified liquid occurs due to thermodynamic fluctuations in the density of the liquid. This process develops at high overheating of the liquid. In the volume of the crude liquid and at the boundaries with the solid phase (near the walls of the vessel), there are usually (or temporarily appear) zones of preferential birth of bubbles - boiling centers, which can be both fluctuating (poorly wetted areas, zones of increased concentration of a light-boiling component, zones of an exothermic reaction etc.), and ready-made, already present in the liquid (bubbles of undissolved gas, gas or steam bubbles in microcracks with incomplete wetting of a solid surface). With developed boiling, the boiling centers are renewed when the vapor is captured by microdepressions (pores) on the heated surface.
In industrial apparatuses, boiling is usually provided by ready-made centers and overheating above the equilibrium boiling point is small (at atmospheric pressure less than 10 K). At a high power of heat release in explosive processes, the achievable overheating of the liquid is much higher, and fluctuation bubble production regimes are realized.
Distinguish between volumetric and surface (wall) boiling. In surface boiling, the main source of vapor bubbles is the liquid layer adjacent to the heated surface. If the liquid volume has a temperature below the equilibrium temperature on the binodal (the so-called boiling with subcooling), then the vapor bubbles formed near the heated surface, falling into the cold layers of the liquid during migration, collapse. Bulk boiling occurs when the liquid is overheated in its entire volume (or when the pressure is reduced). In this case, vapor bubbles are born in the entire volume of the liquid.
The growth of bubbles during boiling has a mechanical (hydrodynamic) effect on the system as a whole. In particular, in a closed volume of a superheated liquid, as the vapor content increases, the pressure increases. In subsonic flows of boiling liquid limited by solid walls (for example, in pipes), an increase in the vapor content downstream is accompanied by a decrease in pressure. As vapor bubbles grow and collapse, they radiate acoustic waves - boiling noise occurs. The rapid increase in pressure during boiling, which occurs when a liquid is sufficiently quickly overheated (explosive boiling mode), can lead to the so-called steam explosion with the destruction of structures. Vapor bubbles floating up in the gravitational field cause additional convective flows, which contribute to the mixing of the liquid, and surface boiling causes turbulent motion of the near-wall layer of the liquid.
In surface nucleate boiling, with an increase in the heater temperature, heat removal from the surface increases until a boiling crisis occurs. The boiling crisis is a consequence of the transition of nucleate boiling to film boiling, when the bubbles on the heated surface are replaced by a vapor layer. The boiling crisis leads to a deterioration in heat removal and is dangerous for a number of energy devices.
The use of boiling processes in everyday life, science and technology is diverse. Surface boiling is widely used for intensive surface cooling - heat removal (for example, in nuclear reactors, jet engines, when cooling elements of electronic equipment). Boiling is used to increase the evaporation surface in desalination plants, in steam boilers at thermal power plants, bubble chambers for visualization of tracks elementary particles, in refrigeration, rectification processes, various chemical technologies, food industry, etc.
Lit .: Skripov V.P. Metastable liquid. M., 1972; Prisnyakov VF Boiling. K., 1988; Labuntsov D. A. Physical foundations energy. M., 2000.
It is well known that every substance boils at certain values of temperature and external pressure.
Boiling is the process of evaporation of a liquid, accompanied by the rapid formation and growth of vapor bubbles that break through the surface of the liquid to the outside.
Consider the conditions under which the boiling process occurs.
A vapor bubble in a liquid can form if a region of low density forms at some point in the liquid. In principle, such a region can form, since due to the chaotic nature of thermal motions, random deviations from the average uniform distribution of particles in the volume are not only possible, but also inevitable. Such deviations from the mean, as already mentioned, are called fluctuations. The bubbles in the vapor and are formed in the order of fluctuations. However, the following must be kept in mind.
The density of a vapor at a temperature far from the critical one is thousands of times less than the density of a liquid. Therefore, in order for a bubble to form, a very significant fluctuation is needed: a region must accidentally form in which the density of particles is thousands of times less than in the rest of the volume! It is clear that the probability of such a fluctuation is very small. Moreover, it is unlikely that it will cover any significant volume. In the vast majority of cases, bubbles, if they form, are very small in size. But in this case, they do not have the conditions for growth. After all, a bubble is a vessel with saturated steam(because the vessel is closed), and the walls of the vessel are the concave surface of the liquid surrounding the bubble. Because of the curvature of the surface, there is a force directed towards the center of curvature. This force will crush the bubble, because at small bubble sizes it is very large.
It would seem that under such conditions, vapor bubbles in a liquid cannot actually form at all, which means that boiling is also impossible. However, the situation changes significantly if there is dissolved or absorbed (adsorbed) air (or some other gas) in the liquid itself or in the walls of the vessel in which it is enclosed. In this case, when the liquid is heated, gas bubbles are formed, and their formation is not associated with fluctuations in the density of the liquid. Therefore, they need not be too small from the outset, and the pressure associated with the curvature of the surface need not crush them.
Gas bubbles, therefore, play the same role in the phenomenon of vaporization (boiling) as dust particles or ions play in the phenomenon of condensation, i.e., the role of nuclei.
So, let, for one reason or another, a gas bubble form in the liquid. After its formation, it is immediately filled with saturated vapor of the surrounding liquid and will be in equilibrium with it. The vapor pressure inside the bubble is obviously determined by the temperature of the liquid. As long as the temperature of the liquid is such that the pressure of saturated vapor inside the bubble is less than the external pressure above the liquid, the bubble cannot grow, because even now there is no shortage of Forces striving to crush it. There is still a force associated with the curvature of the surface of the liquid in the bubble. In addition, the bubble is affected by the hydrostatic pressure of the liquid column above it. Finally, an external pressure acts on the bubble, under which the entire liquid is located, and it is this pressure that plays the main role. The remaining two forces only facilitate the crushing of the bubble by external pressure.
But when the temperature of the liquid reaches a value at which the elasticity of its saturated vapor becomes equal to the external pressure, the vapor pressure inside the bubble will also be equal to the external one (if we do not take into account the hydrostatic pressure and the pressure caused by the surface curvature). Then the slightest increase in temperature is enough for the vapor pressure inside the bubble to exceed the external one: under the influence of this pressure difference, the bubble will quickly inflate and it will eventually float up and break out;
This means that when boiling, the liquid evaporates not only from the surface of the liquid, but also from the surface of the bubbles inside the liquid. In order for the liquid to boil, it is necessary to bring its temperature to a value at which the elasticity saturated vapors of this liquid is equal to the external pressure (more precisely, somewhat more).
It is clear that the lower the external pressure, the lower the boiling point of the liquid. This explains well known fact that at high altitudes where Atmosphere pressure low, liquids boil at temperatures lower than at sea level. By measuring the boiling point of a liquid, you can determine the barometric pressure, and hence the height above sea level. The corresponding device is called a hypsothermometer.
Water, normally (i.e., at an external pressure of 1 atm) boiling at 100 ° C (it is at this temperature that the elasticity of saturated water vapor is 1 atm), can also boil at room temperature, if intensive pumping of air and vapors above its surface sufficiently reduce the external pressure above it.
It is easy to establish the dependence of the boiling point of a liquid on external pressure. Indeed, above we have
formula (105.6), which determines the dependence of the elasticity of saturated vapors of a liquid on temperature:
(Clapeyron-Clausius formula). Since at boiling the external pressure is just equal to the elasticity of the saturated vapor of the liquid, it is clear that if we invert the Clausius-Clapeyron equation, then we will get the dependence of the boiling temperature of the liquid on the external pressure. Therefore, this dependence looks like:
![]()
Liquid overheating. From what has been said above, it is clear that the phenomenon of boiling can only occur if gas bubbles can form in the liquid, and for this the liquid must contain gases dissolved in it. It can be said that, just as condensation is possible only in the presence of condensation centers (dust particles, ions), boiling, i.e., intense vaporization, requires the presence of vaporization centers, which are gas bubbles. (Phase transitions that occur only in the presence of nuclei are called phase transitions first kind.)
In the absence of condensation centers, it is possible, as we have seen, to obtain supersaturated vapor. In the same way, in the absence of dissolved gases in the liquid that can form bubbles, one can obtain a superheated liquid, i.e. a liquid whose temperature is higher than the boiling point at a given external pressure, but which, nevertheless, does not boil.
The possibility of liquid overheating is associated with the additional pressure that the liquid experiences under the curved surface. Indeed, the spherical surface of the liquid surrounding the bubble tends to contract. The resulting pressure
directed towards the center of the bubble, i.e., it is added to the external pressure crushing the bubble. And at small bubble sizes it reaches a very significant value. Therefore, if the liquid to be heated and the walls of the vessel in which it is contained are carefully cleaned of dissolved gases, the formation of bubbles in the liquid is so difficult that it can be heated without boiling to a temperature much higher than the boiling point. More precisely, the liquid can be superheated to a temperature at which the elasticity
of saturated vapor in the bubbles does not exceed the external pressure plus the pressure caused by the curvature of the surface of the smallest bubbles.
As we have seen, the formation of vapor bubbles at low temperatures is practically impossible due to the fact that this requires large density fluctuations, the probability of which is very small. But as the temperature rises, the difference in the densities of the liquid and vapor decreases (at the critical temperature, it disappears altogether). Therefore, the density fluctuations necessary for the formation of bubbles become less and less significant. Accordingly, their probability increases. When enough high temperature(high means close to the critical temperature) bubbles can form and grow even in the absence of nuclei in the form of gas bubbles. This means that in this case, boiling of a liquid is also possible, but only superheated. And the more thoroughly the liquid is cleaned, the stronger it can be overheated.
When superheated liquid nevertheless, in the end, it boils, then boiling occurs very violently, resembling an explosion. In this case, the liquid is rapidly cooled to the normal boiling point at a given pressure.
Overheating of the liquid and the subsequent violent boiling up are a great danger to the vessel in which the liquid is enclosed. Therefore, special measures are taken to prevent overheating. To do this, porous bodies are placed in the liquid, from which air is released in abundance when heated. Such bodies are ceramic tubes (unfired porcelain, for example) or trimmings of capillary tubes.
It is not difficult to determine the size of the bubbles, which guarantee against noticeable overheating of the liquid. Let's make such an assessment for water. In this case, we will assume that overheating of water at normal external pressure by 0.1 degrees is permissible.
What should be the size of the bubbles so that the superheat does not exceed this value?
To do this, it is necessary to determine how much the vapor pressure will increase when the temperature rises from 100 to. This can be done using the temperature dependence curve of water vapor pressure. From this curve it can be determined that a change in temperature by 0.1° causes a change in vapor pressure by . Therefore, in order for the overheating not to exceed 0.1°, it is necessary that the average radius of the bubbles be not less than
![]()
Let us also estimate the influence of the hydrostatic pressure experienced by the bubbles on the overheating of the liquid.
At a depth below the liquid surface, a gas bubble experiences pressure
It is added to the external pressure (as well as the pressure due to the curvature of the surface of the bubble), and by this amount the vapor pressure inside the bubble must be increased in order for it to float. From the same curve it can be found how much the temperature of water at boiling changes with depth below the surface. See, for example,
This corresponds to an increase in the boiling point by
The temperature of boiling water is not, as we see, a well-defined value. Due to the reasons we have just considered, it fluctuates within small limits. Not boiling water itself, but water vapor above it, has a quite definite temperature, since, no matter what happens inside the liquid, the steam coming out of the bubbles bursting on its surface has a quite definite temperature - the temperature at which the elasticity of saturated vapors is equal to the external pressure . That is why, when calibrating thermometers, they are placed not in boiling water, but in vapors above it.
bubble chamber. In the cloud chamber, as we have seen, supersaturation of vapor and condensation of supersaturated vapor on ions as condensation centers are used. Similarly, and for the same purposes, i.e., to detect fast particles, the phenomenon of overheating of a liquid with the subsequent formation of bubbles in it can also be used. This principle is the basis for the operation of the so-called bubble chamber, which has recently become widespread in research in the field of nuclear physics and high energy particle physics.
The bubble chamber is a vessel with a liquid that can be superheated using a heater. This vessel is connected to a device that allows you to create an increased pressure above the liquid and quickly remove this pressure. If you first heat the liquid and compress it with external pressure, and then remove the external pressure, then the liquid, of course, will be overheated, but during the time sufficient for the experiment (several tens of seconds), it does not boil. If at this moment a fast particle flies into the chamber, then on its way into the liquid it will lose part of its energy, which will be converted mainly into heat. Since the liquid is overheated, this additional heat is sufficient for the intensive formation of bubbles in the path of the particle. The resulting bubbles grow rapidly to sizes at which they
become visible, so that the chain of bubbles - the trail of the particle - can be photographed.
If a cloud chamber filled with loose vapor can penetrate through a particle, then in a bubble chamber filled with a dense substance - a liquid, the particle passes all its way without leaving the chamber, which facilitates the study of the properties of this particle. This is one of the important advantages of the bubble chamber.
Details Category: Molecular-kinetic theory Posted on 09.11.2014 21:08 Views: 8345In a liquid state, a substance can exist in a certain temperature range. At a temperature below the lower value of this interval, the liquid turns into a solid. And if the temperature value exceeds the upper limit of the interval, the liquid passes into gaseous state.
We can observe all this in the example of water. In a liquid state, we see it in rivers, lakes, seas, oceans, a water tap. Solid state water is ice. It turns into it when, at normal atmospheric pressure, its temperature drops to 0 o C. And when the temperature rises to 100 o C, water boils and turns into steam, which is its gaseous state.
The process of changing a substance into vapor is called vaporization. The reverse process of changing from vapor to liquid is condensation .
Vaporization occurs in two cases: during evaporation and during boiling.
Evaporation
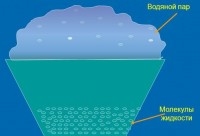
Evaporation is the phase process of the transition of a substance from liquid state into gaseous or vaporous, occurring on the surface of the liquid .
As with melting, heat is absorbed by a substance during evaporation. It is spent on overcoming the cohesive forces of particles (molecules or atoms) of the liquid. Kinetic energy molecules with the highest speed exceeds their potential energy interactions with other liquid molecules. Due to this, they overcome the attraction of neighboring particles and fly out from the surface of the liquid. The average energy of the remaining particles becomes smaller, and the liquid gradually cools down if it is not heated from the outside.
Since particles are in motion at any temperature, evaporation also occurs. at any temperature. We know that puddles dry up after rain, even in cold weather.
But the rate of evaporation depends on many factors. One of the most important - substance temperature. The higher it is, the greater the speed of particles and their energy, and the greater their number leaves the liquid per unit time.
Fill 2 glasses with the same amount of water. We put one in the sun, and the other we leave in the shade. After a while, we will see that there is less water in the first glass than in the second. It was heated by the sun's rays, and it evaporated faster. Puddles after rain also dry up much faster in summer than in spring or autumn. In extreme heat, rapid evaporation of water from the surfaces of reservoirs occurs. Ponds and lakes are drying up, the beds of shallow rivers are drying up. The higher the temperature environment, the higher the evaporation rate.
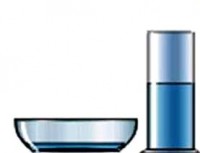
With the same volume, the liquid in a wide plate will evaporate much faster than the liquid poured into a glass. It means that evaporation rate depends on the surface area of evaporation . The larger this area, the greater the number of molecules flies out of the liquid per unit time.
With the same external conditions evaporation rate depends on the type of substance . Fill the glass flasks with the same volume of water and alcohol. After a while, we will see that there is less alcohol left than water. It evaporates at a faster rate. This happens because alcohol molecules interact more weakly with each other than water molecules.
affect the rate of evaporation and presence of wind . We know that things after washing dry much faster when they are blown by the wind. The jet of hot air in a hair dryer can quickly dry our hair.
The wind carries away the molecules that have flown out of the liquid, and they do not return back. Their place is taken by new molecules leaving the liquid. Therefore, they become less in the liquid itself. Therefore, it evaporates faster.
Sublimation

Evaporation takes place in solids Oh. We see how the frozen, ice-covered linen gradually dries out in the cold. Ice turns into steam. We smell the pungent odor produced by evaporation solid matter naphthalene.
Some substances do not have a liquid phase at all. For example, elemental iodineI 2 - a simple substance, which is black-gray crystals with a purple metallic luster, under normal conditions immediately turns into gaseous iodine - purple vapor with a pungent odor. The liquid iodine that we buy in pharmacies is not its liquid state, but a solution of iodine in alcohol.
The transition process of solids into a gaseous state, bypassing the liquid stage, is called sublimation, or sublimation .
Boiling

Boiling This is also the process of liquid turning into vapor. But vaporization during boiling occurs not only on the surface of the liquid, but throughout its entire volume. Moreover, this process is much more intense than during evaporation.
Put a kettle of water on the fire. Since there is always air dissolved in water, when heated, bubbles appear on the bottom of the kettle and on its walls. These bubbles contain air and saturated water vapor. First they appear on the walls of the teapot. The amount of steam in them increases, and they themselves increase in size. Then, under the influence of the buoyant force of Archimedes, they will break away from the walls, rise up and burst on the surface of the water. When the water temperature reaches 100 ° C, bubbles will form throughout the entire volume of water.
Evaporation occurs at any temperature, and boiling occurs only at a certain temperature, which is called boiling point .
Each substance has its own boiling point. It depends on the amount of pressure.
At normal atmospheric pressure, water boils at a temperature of 100 o C, alcohol - at 78 o C, iron - at 2750 o C. And the boiling point of oxygen is minus 183 o C.
As the pressure decreases, the boiling point decreases. In the mountains, where atmospheric pressure is lower, water boils at a temperature of less than 100 ° C. And the higher above sea level, the lower the boiling point will be. And in a pressure cooker, where increased pressure is created, water boils at a temperature above 100 o C.
Saturated and unsaturated steam
If a substance can simultaneously exist in a liquid (or solid) phase and a gaseous one, then its gaseous state is called ferry . Vapor is made up of molecules escaping from a liquid or solid during evaporation.
Pour the liquid into the vessel and close it tightly with a lid. After a while, the amount of liquid will decrease due to its evaporation. Molecules leaving the liquid will concentrate above its surface in the form of vapor. But when the vapor density becomes quite high, some of them will begin to return to the liquid again. And there will be more and more such molecules. Finally, a moment will come when the number of molecules leaving the liquid and the number of molecules returning to it will be equal. In this case, they say that liquid is in dynamic equilibrium with its vapor . This pair is called rich .
If, during vaporization, more molecules fly out of the liquid than return, then such vapor will be unsaturated . unsaturated steam formed when the evaporating liquid is in an open vessel. The molecules leaving it are scattered in space. Not all of them return to the liquid.
Steam condensation

The reverse transition of a substance from a gaseous state to a liquid state is called condensation. During condensation, some of the vapor molecules return to the liquid.
Steam begins to turn into a liquid (condense) at a certain combination of temperature and pressure. This combination is called critical point . Maximum temperature , below which condensation begins is called critical temperature. Above the critical temperature, the gas will never turn into a liquid.
AT critical point the liquid-vapor interface is blurred. disappears surface tension liquid, the densities of the liquid and its saturated vapor are equalized.
At dynamic equilibrium, when the number of molecules leaving the liquid and returning to it is equal, the processes of evaporation and condensation are balanced.
When water evaporates, its molecules form water vapor , which is mixed with air or other gas. The temperature at which such vapor in the air becomes saturated, begins to condense upon cooling and turns into water droplets, is called dew point .

When there is a large amount of water vapor in the air, it is said that its humidity is increased.
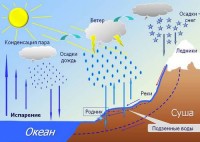
We observe evaporation and condensation very often in nature. Morning fog, clouds, rain - all this is the result of these phenomena. FROM earth's surface moisture evaporates when heated. The molecules of the resulting vapor rise up. Encountering cool leaves or blades of grass on its way, the steam condenses on them in the form of dew drops. A little higher, in the surface layers, it becomes fog. And high in the atmosphere at low temperatures, the cooled vapor turns into clouds consisting of water droplets or ice crystals. Subsequently, rain or hail will fall from these clouds to the earth.
But water droplets form during condensation only when the smallest solid or liquid particles are in the air, which are called condensation nuclei . They can be products of combustion, spraying, dust particles, sea salt over the ocean, particles formed as a result of chemical reactions in the atmosphere, etc.
desublimation

Sometimes a substance can go from a gaseous state immediately to a solid state, bypassing the liquid stage. Such a process is called desublimation .
Ice patterns that appear on glasses in cold weather are an example of desublimation. During frosts, the soil is covered with hoarfrost - thin ice crystals into which water vapor has turned from the air.
Two important consequences follow from Raoult's law:
1) Solutions boil at a higher temperature than a pure solvent;
2) Solutions freeze at a lower temperature than a pure solvent.
Let's consider them in more detail.
Boiling is the physical process by which a liquid changes into a gaseous state or vapor, in which gas bubbles form throughout the volume of the liquid.
A liquid boils when its pressuresaturated vapor becomes equal to the external pressure. If the external pressure (for example, atmospheric) does not change, and theliquid is an individual and chemically pure substance, then its boiling in an open heated vessel occurs at a constant temperature until the liquid phase completely disappears.
So, at an atmospheric pressure of 101.325 kPa, the boiling point of purified (distilled) water is 100 o C or 373.16K.
If some non-volatile substance is dissolved in H 2 O, then the pressure of its saturated vapor will decrease. In order for the resulting solution to boil, it is necessary to heat it to a temperature higher than 373.16K, because only under such conditions will the pressure of the saturated vapor of the solvent again become equal to atmospheric.
Freezing or crystallizationis a physical phenomenon, accompanied by the transformation of a liquid into a solid. Moreover, crystalline structures are formed in the entire volume of the liquid.
The freezing process begins if the saturation vapor pressure over a liquid becomes equal to the saturation vapor pressure over its solid crystals.
If the external (atmospheric) pressure remains constant, andthe liquid does not contain foreign impurities, then during the crystallization process the temperature of the cooled liquid will remain constant until the liquid phase completely turns into a solid.
At an atmospheric pressure of 101.325 kPa, distilled water freezes at 0°C (273.16K). The pressure of saturated water vapor over ice and liquid in this case is 613.3 Pa.
For an aqueous solution, the saturated vapor pressure of the solvent at 0 ° C will be less than 613.3 Pa, but over ice remains unchanged. The ice lowered into such a solution will quickly melt due to the condensation of an excess amount of steam above it.
Only when the temperature is lowered can the saturation vapor pressure over the liquid and solid phases be equalized again and the crystallization process be initiated.
Empirically, it was found that an increase in the boiling point ( t bale ) and lowering the freezing point solution ( t deputy ) compared to a pure solvent, is directly proportional to the molar concentration of the solute. Mathematically, this can be written as follows:
t b.p. p-ra –t kip. p-calf \u003d t kip. =em
t r-tel -t deputy. p-ra \u003d t deputy. =Km
wherem– molal concentrationsolute;EandK, respectively, ebullioscopic (lat.ebbulio- boil away) and cryoscopic (Greek "cryos" - cold) constants, the values of which depend only on the naturesolvent (Table 7).
Table 7 Ebullioscopic E and cryoscopic K constants of some solvents (deg/mol)
Ebullioscopic and cryoscopic constants solvent show how many degrees it rises boiling point and freezing point solution obtained by dissolving one mole of non-electrolyte in one kilogram of solvent ( m = 1 mol/kg).
To determine the values of E and K, first experimentally determine Dt kip. andDt deputy. highly dilute solutions (m<< 1), а затем полученные данные пересчитывают или экстраполируют для растворов сm= 1 моль/кг.
whereRis the universal gas constant;Tis the boiling point of the solvent; is the specific heat of evaporation of the solvent.
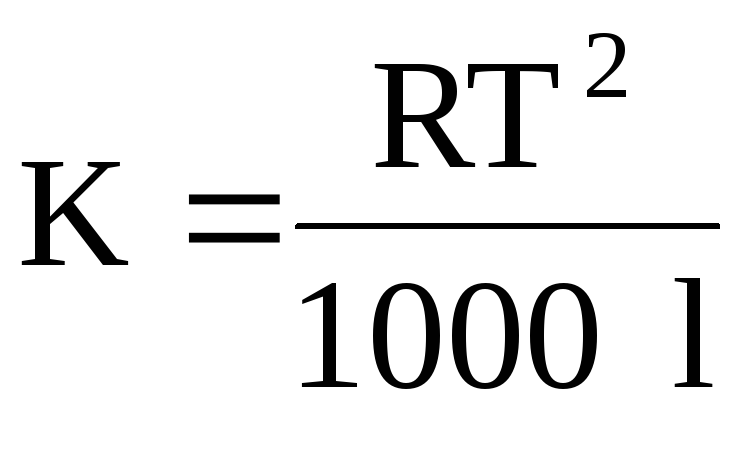
whereT- melting temperaturesolutionclient;l– specific heat of fusionsolvent.
In this way, solutions of substances of different nature, but with the same molar concentration will boil and freeze at the sametemperature.
Attention should be drawn to an important differencesolution from a pure solvent. If the latter boils and freezes at a constanttemperature, thensolutions do this in the intervaltemperatures, i.e. in the process of their boiling away, the temperature rises all the time, and when it freezes, it decreases. This is due to the fact that the removal from the liquid phasesolvent in the form of steam or solid crystals leads to an increase in the molar concentration of the solution, tk. the dissolved substance in the process of boiling and freezing remains unchanged in the liquid phase (up to its complete boiling or freezing), and the mass of the liquid solvent decreases.
In practical measurements freezing or boiling point solution, the moment of the appearance of the first solid crystals in it is fixed (for t deputy ) or gas bubbles (for t bale ).
The property of solutions to lower the freezing point allows them to be used as coolants.
So, solutions of some organic and inorganic substances are used as antifreezes for cooling internal combustion engines when they operate in a variety of climatic conditions.
downgrade freezing temperature solutions is of great importance for living organisms. So, the liquid in their cells is a solution of various inorganic and organic substances. Hisfreezing temperature is below 0 about C (273.16 K ), so cells do not die under supercooling conditions.
Thanks to this phenomenon, the plants are preserved in the winter. Moreover, the higher the concentration of substances in the cell fluid, the lower external temperatures the plant can tolerate.
At the same time, to lower the freezing point of the solution in cooled cells, the process of hydrolysis of higher molecular weight compounds to low molecular weight compounds (for example, carbohydrates to glucose) is intensified.