What is the formula of the substance. Chemical formulas of substances
2.1. Chemical language and its parts
Mankind uses many different languages. Except natural languages(Japanese, English, Russian - more than 2.5 thousand in total), there are also artificial languages e.g. Esperanto. Among the artificial languages are languages various Sciences. So, in chemistry, one uses its own, chemical language.
chemical language- a system of symbols and concepts designed for concise, concise and visual recording and transmission of chemical information.
A message written in most natural languages is divided into sentences, sentences into words, and words into letters. If we call sentences, words and letters parts of the language, then we can distinguish similar parts in the chemical language (Table 2).
Table 2.Parts chemical language
It is impossible to master any language at once, this also applies to the chemical language. Therefore, for now, you will only get acquainted with the basics of this language: learn some "letters", learn to understand the meaning of "words" and "sentences". At the end of this chapter, you will be introduced to titles chemicals are an integral part of the chemical language. As you study chemistry, your knowledge of the chemical language will expand and deepen.
CHEMICAL LANGUAGE.
1. What artificial languages do you know (except those named in the text of the textbook)?
2. How do natural languages differ from artificial ones?
3. Do you think it is possible to do without the use of chemical language when describing chemical phenomena? If not, why not? If so, what would be the advantages and disadvantages of such a description?
2.2. Symbols of chemical elements
The symbol for a chemical element denotes the element itself or one atom of that element.
Each such symbol is an abbreviated Latin name of a chemical element, consisting of one or two letters of the Latin alphabet (see Appendix 1 for the Latin alphabet). The symbol is capitalized. Symbols, as well as Russian and Latin names of some elements, are given in Table 3. Information about the origin of Latin names is also given there. general rule pronunciation of symbols does not exist, therefore, table 3 also shows the "reading" of a symbol, that is, how this symbol is read in a chemical formula.
It is impossible to replace the name of an element with a symbol in oral speech, but in handwritten or printed texts this is allowed, but not recommended. chemical elements, 109 of them have names and symbols approved by the International Union of Pure and Applied Chemistry (IUPAC).
Table 3 provides information on only 33 elements. These are the elements that you will encounter first when studying chemistry. Russian names (in alphabetical order) and symbols of all elements are given in Appendix 2.
Table 3Names and symbols of some chemical elements
Name |
||||
latin |
Writing |
|||
| - | Writing |
Origin |
- | - |
| Nitrogen | N itrogenium | From Greek. "giving birth to saltpeter" | "en" | |
| Aluminum | Al uminium | From lat. "alum" | "aluminum" | |
| Argon | Ar gon | From Greek. "inactive" | "argon" | |
| Barium | Ba rium | From Greek. " heavy" | "barium" | |
| Bor | B orum | From Arabic. "white mineral" | "bor" | |
| Bromine | Br omum | From Greek. "malodorous" | "bromine" | |
| Hydrogen | H hydrogenium | From Greek. "giving birth to water" | "ash" | |
| Helium | He lium | From Greek. " Sun" | "helium" | |
| Iron | Fe rrum | From lat. "sword" | "ferrum" | |
| Gold | Au rum | From lat. "burning" | "aurum" | |
| Iodine | I odum | From Greek. " violet" | " iodine" | |
| Potassium | K alium | From Arabic. "lye" | "potassium" | |
| Calcium | Ca lcium | From lat. "limestone" | "calcium" | |
| Oxygen | O xygenium | From Greek. "producer of acids" | " about" | |
| Silicon | Si licium | From lat. "flint" | "silicium" | |
| Krypton | kr ypton | From Greek. "hidden" | "krypton" | |
| Magnesium | M a g nesium | From the name peninsulas of Magnesia | "magnesium" | |
| Manganese | M a n ganum | From Greek. "purifying" | "manganese" | |
| Copper | Cu prum | From Greek. name about. Cyprus | "cuprum" | |
| Sodium | Na trium | From Arabic, "detergent" | "sodium" | |
| Neon | Ne on | From Greek. " new" | "neon" | |
| Nickel | Ni colum | From him. "copper of St. Nicholas" | "nickel" | |
| Mercury | H ydrar g yrum | Lat. "liquid silver" | "hydrargyrum" | |
| Lead | P lum b um | From lat. the name of the alloy of lead and tin. | "plumbum" | |
| Sulfur | S sulfur | From Sanskrit "flammable powder" | "es" | |
| Silver | A r g entum | From Greek. " light coloured" | "argentum" | |
| Carbon | C arboneum | From lat. " coal" | "ce" | |
| Phosphorus | P hosphorus | From Greek. "bringer of light" | "pe" | |
| Fluorine | F luorum | From lat. verb "to flow" | "fluorine" | |
| Chlorine | Cl orum | From Greek. "greenish" | "chlorine" | |
| Chromium | C h r omium | From Greek. " dye" | "chrome" | |
| Cesium | C ae s ium | From lat. "sky blue" | "cesium" | |
| Zinc | Z i n cum | From him. "tin" | "zinc" | |
2.3. Chemical formulas
Used to refer to chemicals chemical formulas.
For molecular substances, the chemical formula can also denote one molecule of this substance.
Information about a substance can be different, so there are different types of chemical formulas.
Depending on the completeness of information, chemical formulas are divided into four main types: protozoa,
molecular, structural and spatial.
Subscripts in the simplest formula do not have a common divisor.
Index "1" is not put in formulas.
Examples of the simplest formulas: water - H 2 O, oxygen - O, sulfur - S, phosphorus oxide - P 2 O 5, butane - C 2 H 5, phosphoric acid - H 3 PO 4, sodium chloride (table salt) - NaCl.
The simplest formula of water (H 2 O) shows that the water contains the element hydrogen(H) and element oxygen(O), and in any portion (a portion is a part of something that can be divided without losing its properties.) of water, the number of hydrogen atoms is twice more number oxygen atoms.
Number of particles, including number of atoms, denoted by the Latin letter N. Denoting the number of hydrogen atoms - N H , and the number of oxygen atoms is N O , we can write that
Or N H: N O=2:1.
The simplest formula of phosphoric acid (H 3 PO 4) shows that phosphoric acid contains atoms hydrogen, atoms phosphorus and atoms oxygen, and the ratio of the numbers of atoms of these elements in any portion of phosphoric acid is 3:1:4, that is
NH: N P: N O=3:1:4.

The simplest formula can be drawn up for any individual chemical substance, and for a molecular substance, in addition, molecular formula.
Examples of molecular formulas: water - H 2 O, oxygen - O 2, sulfur - S 8, phosphorus oxide - P 4 O 10, butane - C 4 H 10, phosphoric acid - H 3 PO 4.
Nonmolecular substances do not have molecular formulas.
The sequence of writing the symbols of elements in the simplest and molecular formulas is determined by the rules of the chemical language, which you will learn as you study chemistry. The sequence of characters does not affect the information conveyed by these formulas.
Of the signs reflecting the structure of substances, we will use so far only valence stroke("dash"). This sign shows the presence between the atoms of the so-called covalent bond(what kind of connection is this and what are its features, you will soon find out).
In the water molecule, the oxygen atom is connected by simple (single) bonds with two hydrogen atoms, and the hydrogen atoms are not connected to each other. This is what clearly shows structural formula water. ![]()
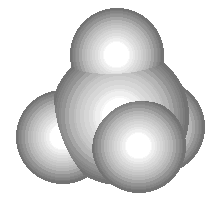
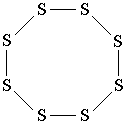
Another example: the sulfur molecule S 8 . In this molecule, 8 sulfur atoms form an eight-membered cycle in which each sulfur atom is connected to two other atoms by simple bonds. Compare the structural formula of sulfur with the three-dimensional model of its molecule shown in fig. 3. Please note that the structural formula of sulfur does not convey the shape of its molecule, but only shows the sequence of connecting atoms by covalent bonds.
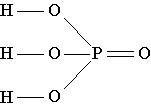
The structural formula of phosphoric acid shows that in the molecule of this substance one of the four oxygen atoms is connected only with the phosphorus atom by a double bond, and the phosphorus atom, in turn, is connected with three more oxygen atoms by simple bonds. Each of these three oxygen atoms, in addition, is connected by a simple bond with one of the three hydrogen atoms present in the molecule./p>
Compare the following three-dimensional model of the methane molecule with its spatial, structural and molecular formula:
|
|
|
In the spatial formula of methane, wedge-shaped valence strokes, as if in perspective, show which of the hydrogen atoms is "closer to us" and which is "farther from us".
Sometimes the spatial formula indicates the bond lengths and the values of the angles between the bonds in the molecule, as shown in the example of the water molecule.

Nonmolecular substances do not contain molecules. For the convenience of chemical calculations in a non-molecular substance, the so-called formula unit.
Examples of the composition of the formula units of some substances: 1) silicon dioxide (quartz sand, quartz) SiO 2 - formula unit consists of one silicon atom and two oxygen atoms; 2) sodium chloride (common salt) NaCl - the formula unit consists of one sodium atom and one chlorine atom; 3) iron Fe - a formula unit consists of one iron atom. Like a molecule, a formula unit is the smallest portion of a substance that retains its chemical properties.
Table 4
Information Conveyed by Different Types of Formulas
Formula type |
The information passed by the formula. |
|
| Protozoa Molecular Structural Spatial |
|
|
Let us now consider, with examples, what information formulas of different types give us.
1. Substance: acetic acid. The simplest formula is CH 2 O, the molecular formula is C 2 H 4 O 2, the structural formula
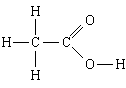
The simplest formula tells us that
1) acetic acid contains carbon, hydrogen and oxygen;
2) in this substance, the number of carbon atoms is related to the number of hydrogen atoms and to the number of oxygen atoms, as 1:2:1, that is N H: N C: N O = 1:2:1.
Molecular formula adds that
3) in a molecule of acetic acid - 2 carbon atoms, 4 hydrogen atoms and 2 oxygen atoms.
Structural formula adds that
4, 5) in the molecule, two carbon atoms are linked by a single bond; one of them, in addition, is associated with three hydrogen atoms, with each single bond, and the other with two oxygen atoms, with one double bond, and with the other a single bond; the last oxygen atom is also linked by a simple bond to the fourth hydrogen atom.
2. Substance: sodium chloride.
The simplest formula is NaCl.
1) Sodium chloride contains sodium and chlorine.
2) In this substance, the number of sodium atoms is equal to the number of chlorine atoms.
3. Substance: iron.
The simplest formula is Fe.
1) The composition of this substance includes only iron, that is, it is a simple substance.
4. Substance: trimetaphosphoric acid . The simplest formula is HPO 3, the molecular formula is H 3 P 3 O 9, the structural formula
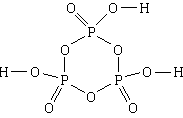
1) The composition of trimetaphosphoric acid includes hydrogen, phosphorus and oxygen.
2) N H: N P: N O = 1:1:3.
3) A molecule consists of three hydrogen atoms, three phosphorus atoms and nine oxygen atoms.
4, 5) Three phosphorus atoms and three oxygen atoms, alternating, form a six-membered cycle. All links in the cycle are simple. Each phosphorus atom, in addition, is associated with two more oxygen atoms, with one - a double bond, and the other - a simple one. Each of the three oxygen atoms linked by simple bonds to phosphorus atoms is also linked by a simple bond to a hydrogen atom.
| Phosphoric acid - H 3 PO 4(another name is phosphoric acid) - transparent colorless crystalline substance molecular structure, melting at 42 o C. This substance is very soluble in water and even absorbs water vapor from the air (hygroscopically). Phosphoric acid is produced in large quantities and is used primarily in the production of phosphate fertilizers, as well as in the chemical industry, in the production of matches, and even in construction. In addition, phosphoric acid is used in the manufacture of cement in dental technology, is part of many medicines. This acid is cheap enough that in some countries, such as the United States, very pure phosphoric acid, highly diluted with water, is added to refreshments to replace expensive citric acid. |
| Methane - CH 4. If you have a gas stove at home, then you come across this substance every day: the natural gas that burns in the burners of your stove is 95% methane. Methane is a colorless and odorless gas with a boiling point of -161 o C. When mixed with air, it is explosive, which explains the explosions and fires that sometimes occur in coal mines (another name for methane is firedamp). The third name of methane - swamp gas - is due to the fact that bubbles of this particular gas rise from the bottom of swamps, where it is formed as a result of the activity of certain bacteria. In industry, methane is used as a fuel and raw material for the production of other substances. Methane is the simplest hydrocarbon. This class of substances also includes ethane (C 2 H 6), propane (C 3 H 8), ethylene (C 2 H 4), acetylene (C 2 H 2) and many other substances. |
Table 5.Examples of formulas of different types for some substances-