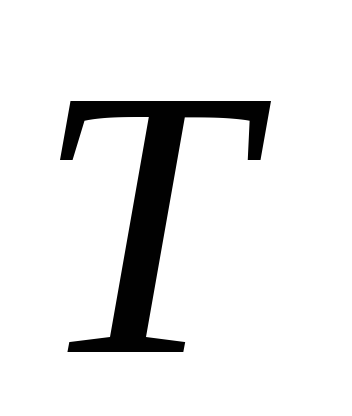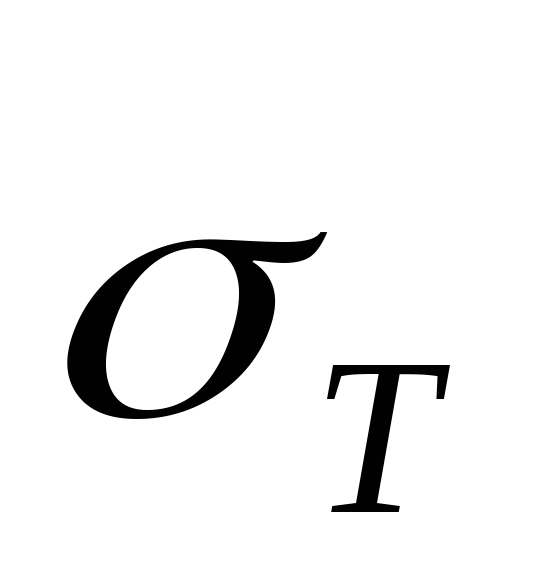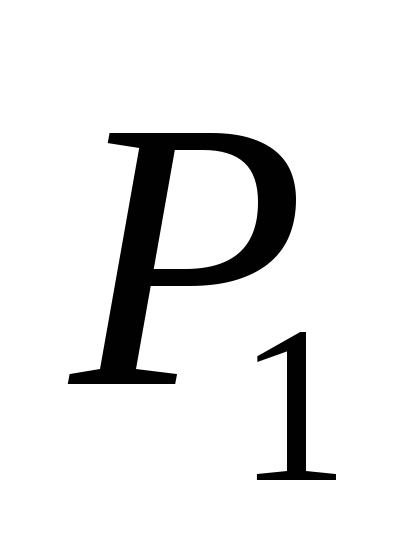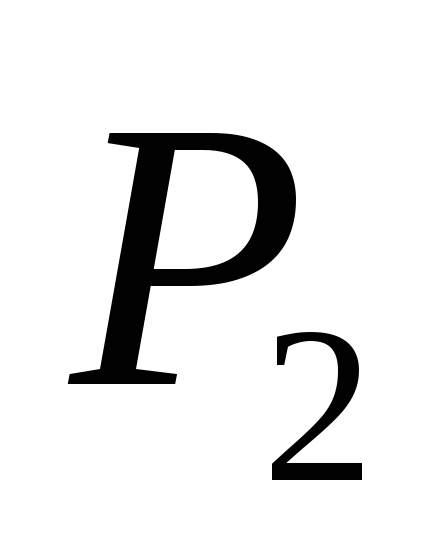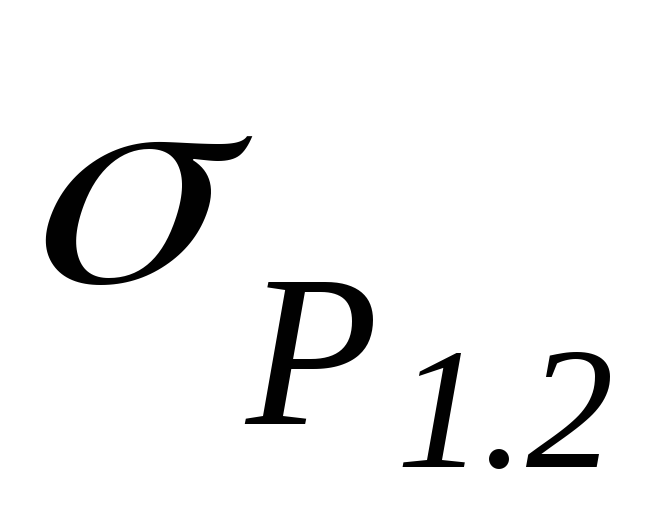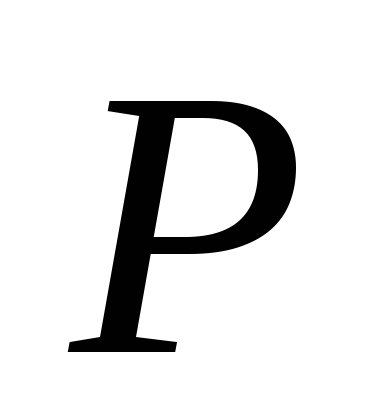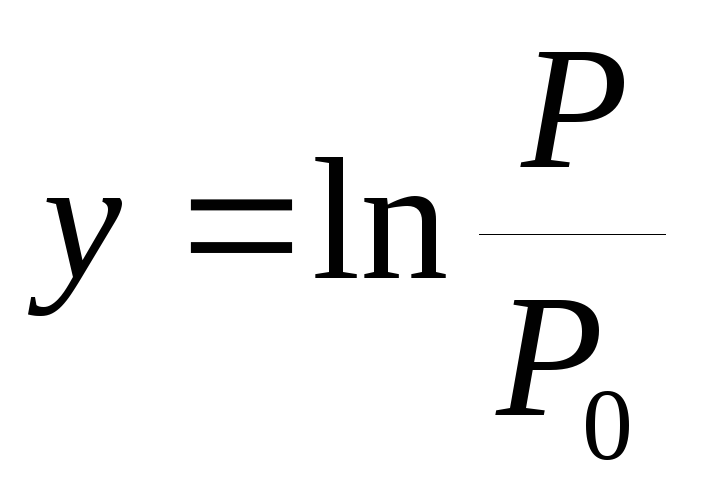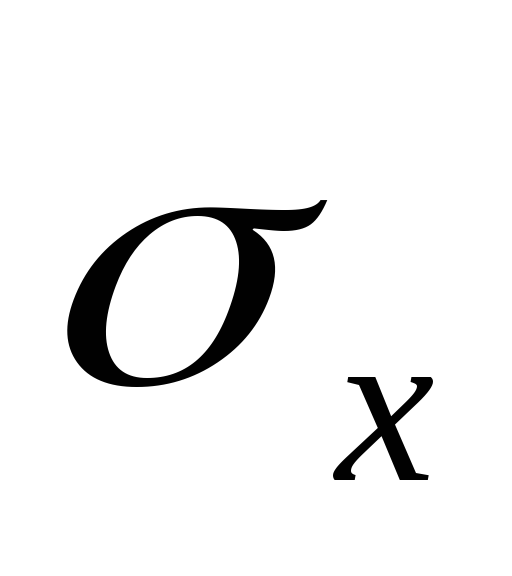Specific heat capacity of water vapor condensation. Determination of the specific heat of vaporization
Laboratory work №4.
Determination of the heat of vaporization of a liquid
1. Brief theory
Evaporation is the transition of a substance from a liquid to a gaseous state. During evaporation, molecules fly out from the surface of the liquid, forming vapor above it. Only the fastest molecules can fly out of the liquid into the surrounding space, since only they are able to overcome the attractive forces acting in the surface layer of the liquid. As a result of the departure of fast molecules, the liquid is cooled. To keep its temperature constant, a heat supply is required.
The amount of heat that must be imparted to a substance in order to change it from liquid state into gaseous constant temperature and constant pressure, is called the heat of vaporization (or heat of vaporization).
The heat of vaporization of liquids can be measured directly with a calorimeter. This method, however, does not provide accurate results due to uncontrolled heat loss, which is difficult to make small. In this paper, to determine the heat of evaporation, we use an indirect method based on the Clausius-Clapeyron equation:
 . (1)
. (1)
Here P
is the saturated vapor pressure of the liquid at temperature T
,T is the absolute temperature of the liquid and vapor, 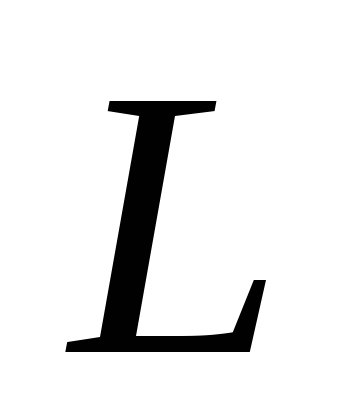 - specific heat of vaporization of liquid,
- specific heat of vaporization of liquid,  - specific volume of liquid,
- specific volume of liquid,  - specific volume of steam.
- specific volume of steam.
If the dependence of the specific heat of evaporation is known 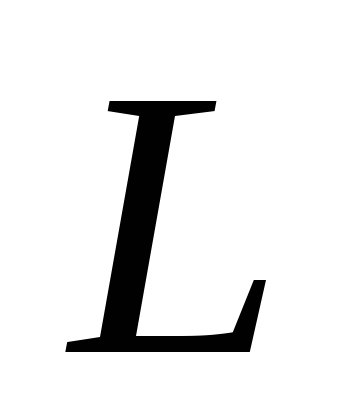 and specific volumes
and specific volumes 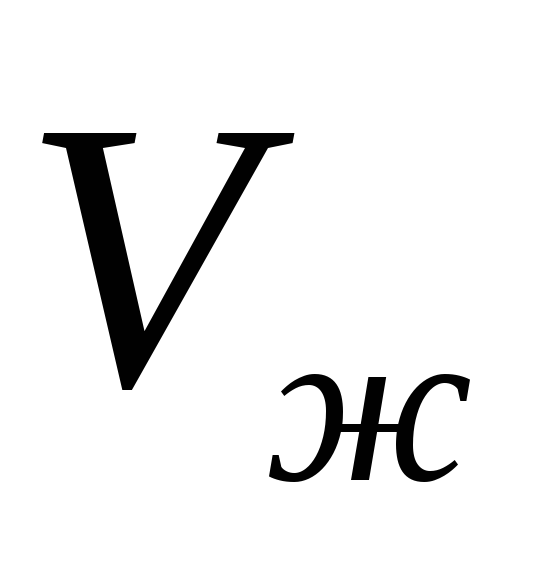 and
and 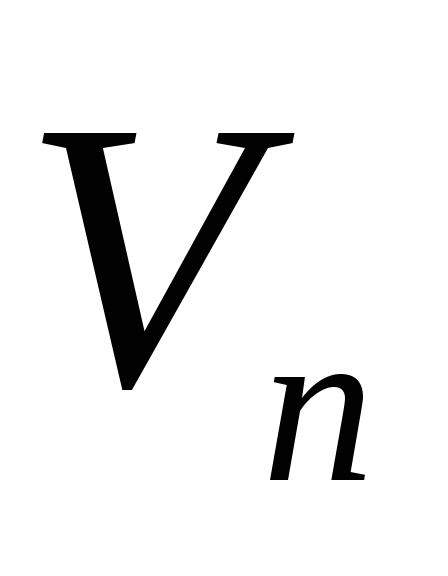 on temperature, then equation (1) can be integrated and the dependence of saturated vapor pressure on temperature can be found explicitly. In the roughest approximation, we can assume that the value
on temperature, then equation (1) can be integrated and the dependence of saturated vapor pressure on temperature can be found explicitly. In the roughest approximation, we can assume that the value 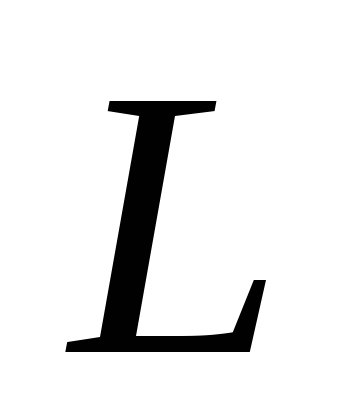 does not depend on temperature, and the specific volume of liquid compared to the specific volume of vapor can be neglected. In addition, we can assume that the equation of state of an ideal gas (the Mendeleev-Clapeyron equation) is applicable to the vapor, from which we express specific volume pair:
does not depend on temperature, and the specific volume of liquid compared to the specific volume of vapor can be neglected. In addition, we can assume that the equation of state of an ideal gas (the Mendeleev-Clapeyron equation) is applicable to the vapor, from which we express specific volume pair: 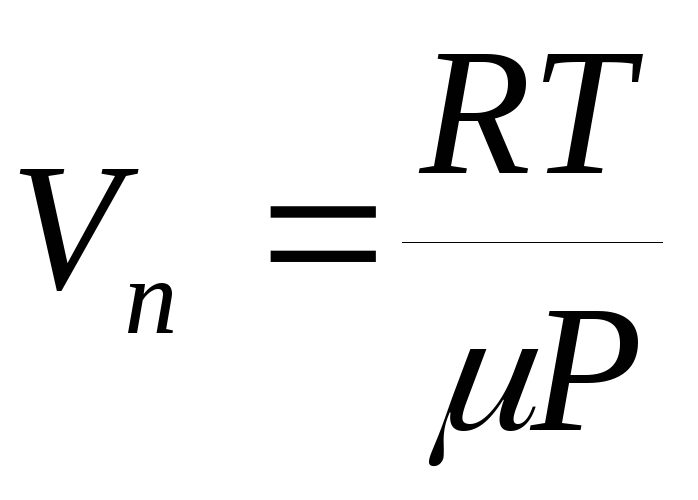 .
.
In the accepted approximation, equation (1) will turn into a differential equation:
 ,
,
integrating which, we get:
 , (2)
, (2)
where 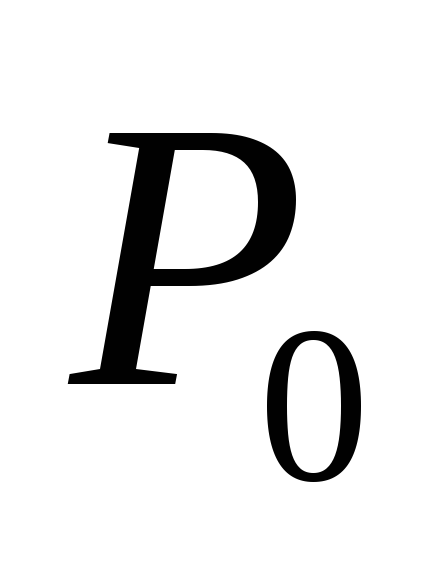
 .
.
Relation (2) makes it possible to determine the specific heat of vaporization, knowing the dependence of saturated vapor pressure on temperature. The present laboratory work is devoted to the determination of the specific heat of evaporation of water.
2. Experimental setup. Experimental technique
The experiment is carried out on the setup schematically shown in the figure. The setup consists of a thermostat filled with water, heating elements, a cooling system (not shown in the figure), two flasks with test gases, and measuring instruments.
H  heating elements H serve to increase the temperature in the system. For uniform heating of the system, the water is constantly mixed with air supplied by the compressor To. Turning on and off the heating elements and the compressor is carried out using the control panel PU. Before starting the cranes To 1
and To 2
open, then close. This is necessary so that the pressure in the flasks at the initial moment becomes equal to atmospheric pressure. Thermometer measures temperature T water in which flasks with dry and moist air are immersed. The installation parameters are such that the water is heated slowly and the gas in the flasks has time to warm up to the same temperature as the water in the system. Pressure gauges M 1
and M 2
show the excess pressure in the flasks with dry and moist air, respectively.
heating elements H serve to increase the temperature in the system. For uniform heating of the system, the water is constantly mixed with air supplied by the compressor To. Turning on and off the heating elements and the compressor is carried out using the control panel PU. Before starting the cranes To 1
and To 2
open, then close. This is necessary so that the pressure in the flasks at the initial moment becomes equal to atmospheric pressure. Thermometer measures temperature T water in which flasks with dry and moist air are immersed. The installation parameters are such that the water is heated slowly and the gas in the flasks has time to warm up to the same temperature as the water in the system. Pressure gauges M 1
and M 2
show the excess pressure in the flasks with dry and moist air, respectively.
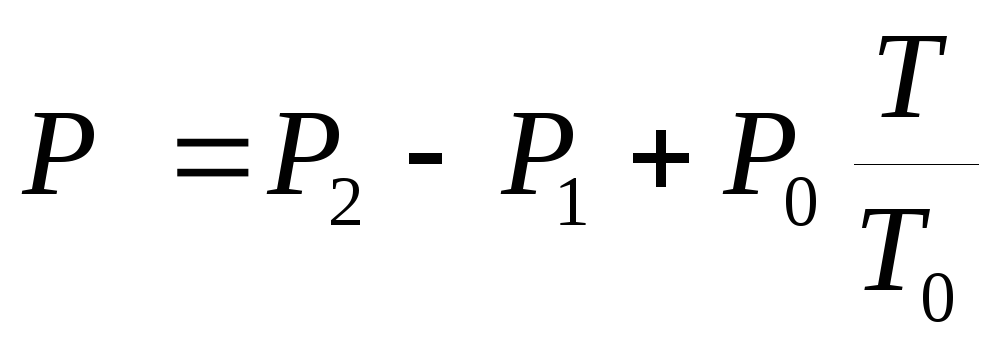 , (3)
, (3)
where P 1
and P 2
- indications of manometers M 1
and M 2
respectively, T– temperature in the system 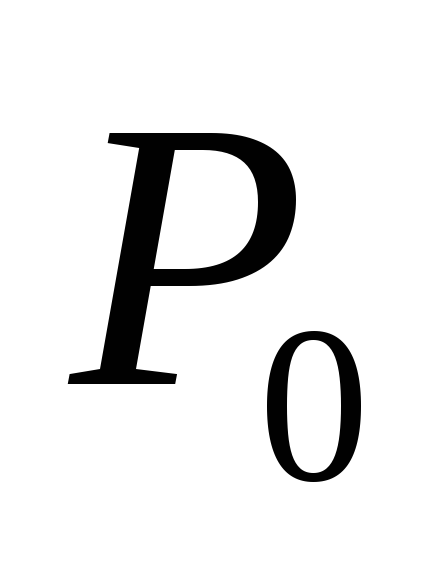 saturated steam pressure at initial temperature
saturated steam pressure at initial temperature  (this value is considered known).
(this value is considered known).
According to the experience, a dependency graph is built  from
from 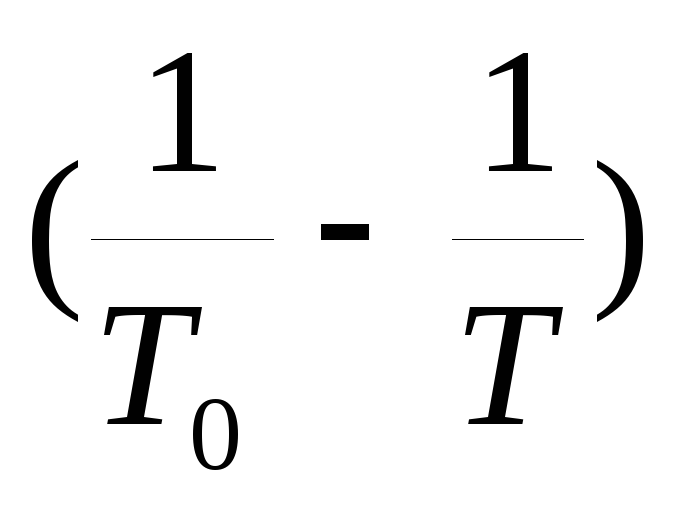 .
.
According to equation (2), the experimental points must lie on a straight line passing through the origin. According to the graph, the slope of this line is determined  and the specific heat of vaporization of water is found.
and the specific heat of vaporization of water is found.
Equation (2) shows that  and hence:
and hence: 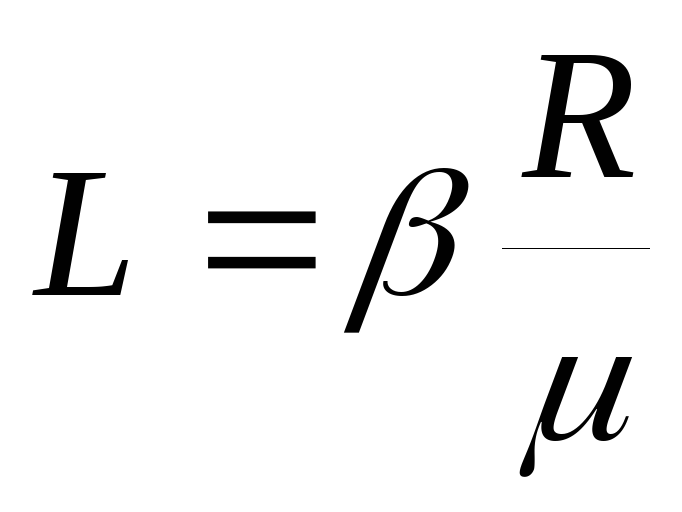 .
.
3. Measurements. Processing of measurement results
1. Familiarize yourself with the installation device and its elements.
2. Remove the dependence of the pressure gauges P 1 and P 2 on temperature, and calculate for each temperature the saturation vapor pressure P using formula (3). Make a table based on your experience:
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
|
3. Plot saturation vapor pressure versus temperature P(T) and a graph of the logarithm of the pressure ratios y=  from the value x =
from the value x =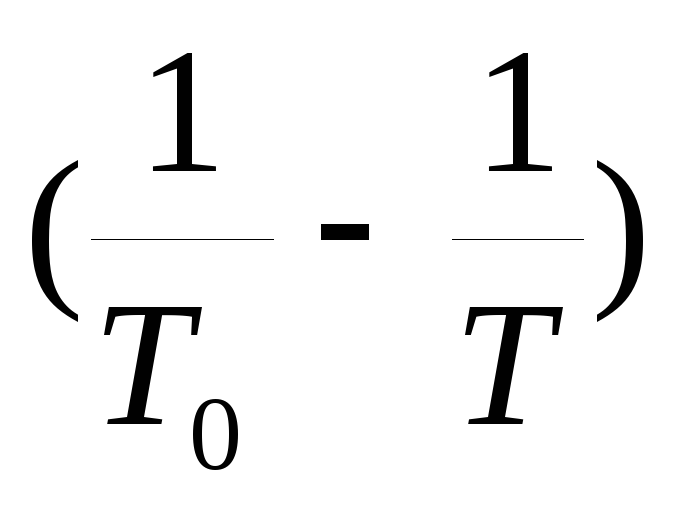 .
.
To build the last graph, you need to fill in the table:
|
| ||||||
|
| ||||||
|
| ||||||
|
|
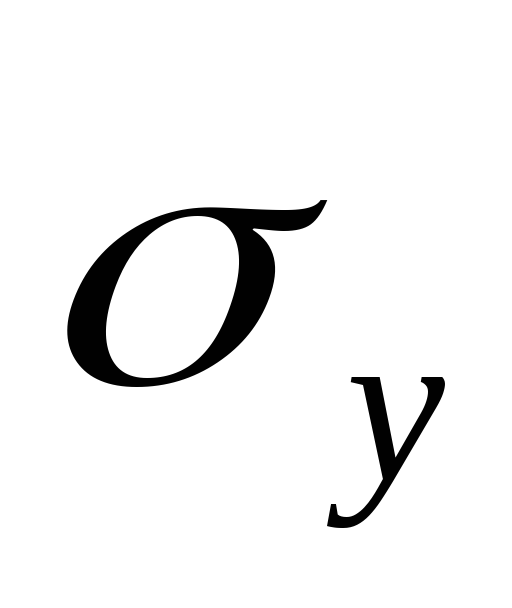
By angular coefficient graph determine the heat of vaporization L and estimate the error of the result.
4. Compare the result with the table value.
4. Security questions
What is the specific heat of vaporization of a liquid called?
Derive the Clausius-Clapeyron equation.
What assumptions were made when deriving relation (2)? Derive this ratio.
Azeotropic- a term used to designate a mixture of liquids, the liquid and gas phases of which, under conditions of thermodynamic equilibrium, have the same composition. The boiling point of the mixture is constant.
Azeotropy point- the temperature at which a mixture of liquids boils and vapor of the same composition as the liquid mixture is formed.
Vacuum(or rarefied gas) - the state of a gaseous medium in which its pressure is significantly lower than atmospheric pressure.
Dephlegmator - heat exchanger for partial condensation of steam. In distillation columns, it serves to form reflux necessary for irrigation of the contact elements of the column.
Evaporation Vaporization occurs from the free surface of a liquid. The molecules of a liquid at the same temperature move at different speeds. If a fast enough molecule is at the surface of the liquid, then it can overcome the attraction of neighboring molecules and fly out of the liquid. Molecules escaping from the surface of the liquid form vapor. Simultaneously with evaporation, molecules are transferred from vapor to liquid. The phenomenon of the transformation of vapor into a liquid is called condensation.
If there is no influx of energy to the liquid from outside, then the evaporating liquid is cooled. The condensation of vapor is accompanied by the release of energy.
The rate of evaporation of a liquid depends on the type of liquid and on its temperature, on its surface area, on the movement of air masses (wind) over the surface of the liquid.
Boiling- the process of vaporization occurring in the entire volume of the liquid. When boiling, evaporation occurs into air bubbles dissolved in the liquid. With increasing pressure in the bubbles, they float to the surface and burst. These bubbles contain not only air, but also water vapor, as the liquid evaporates inside these bubbles.
Boiling Center Formation- the appearance and growth of vapor bubbles on the heated surface in contact with the liquid.
Boiling point is the boiling point at normal pressure.
vaporization - phase transition first kind; the transition of a substance from a liquid or solid state into a gaseous state.
In a closed volume, vaporization continues until the space above the liquid or solid is filled with vapor having an equilibrium pressure at a given temperature (saturation pressure). The largest part of the heat of vaporization is spent on breaking the bonds between the particles, some of it goes to the work done during the expansion of the steam.
Boiling temperature is the temperature at which the liquid boils.
Boiling temperature:
Depends on the nature of the liquid and the external pressure; and
Located between triple point and critical temperature.
At constant pressure, each liquid has its own boiling point.
With an increase in atmospheric pressure, the boiling point of a liquid rises, and the specific heat of vaporization decreases.
Heat of evaporation- the amount of heat that must be imparted to a substance in order to transfer it from a liquid state to a gaseous state at a constant temperature.
Heat of vaporization- the amount of heat that must be imparted to a substance at constant pressure and temperature in order to transfer it from a liquid state to a gaseous state.
In SI, the heat of vaporization is measured in J.
Specific heat of vaporization- the amount of heat that must be imparted to 1 kg of a substance in order to transfer it from a liquid state to a gaseous state at a constant temperature.
The specific heat of vaporization is measured in J/kg.
Specific heat of vaporization is the amount of heat required to turn 1 kg of liquid into vapor at a constant temperature. This value is denoted by the letter L or the letter r and is expressed in joules per kilogram (J / kg).
With increasing temperature, the value specific heat vaporization is reduced.
Specific heat of vaporization - the heat of vaporization, calculated per unit mass of a substance.
The specific heat of vaporization decreases with increasing temperature and becomes zero at the critical temperature.
The specific heat of vaporization shows how much energy needs to be imparted to one kilogram of liquid taken at the boiling temperature in order to completely turn it into steam at this temperature (for condensation: how much energy is released by one kilogram of steam taken at the condensation temperature, completely turning into a liquid).
At the same pressure, the boiling point and the condensation point of the same substance are the same.
Steam condensation - the process of returning vapor molecules to a liquid is called vapor condensation.
Capacitor- a heat exchanger in which vapors are condensed, while giving off heat to the cooler.
water condenser A condenser that uses water as a coolant.
Condenser PIPE IN PIPE- a condenser consisting of two concentric tubes, with the steam most often circulating in the annular gap, and the coolant in the central tube.
Heat exchange or heat transfer- transition process internal energy from one body to another as a result of thermal contact without doing work.
Thermal equilibrium- the state of the system in which its macroscopic parameters do not change over time. At thermal equilibrium, the volume and pressure do not change, there is no heat exchange, there are no mutual transformations of liquids and gases, liquids and solids, stop chemical reactions and others. In a state of thermal equilibrium, the temperature is the same in all parts of the system.
Specific heat- (small letter c) - a characteristic of a substance, showing how much heat is needed to heat 1 kg of a substance by 1 degree (or released when 1 kg of a substance cools by 1 degree). It is measured in J/kg K or J/kg 0C. Table data.
heat exchanger- an apparatus designed to transfer heat between two media separated from each other.
Shell and tube heat exchanger- a heat exchanger consisting of a bundle of pipes placed in a casing, one of the fluids flowing through the pipes, and the other in the space between the pipes and the inner surface of the casing.
heat pipe- a closed volume, usually in the form of a tube, partially filled with a liquid and its vapors and used to transfer heat between its two extreme sections by evaporating the liquid in the hot section and condensing the vapor in the cold one. The condensed liquid is returned to the hot spot by gravity or capillary forces through an appropriate device acting as a WICK
Partial pressure- (partialis - partial) - the pressure that a gas that is part of a gas mixture would have if it alone occupied a volume equal to the volume of the mixture of the same temperature.
Dew point The temperature at which water vapor in the air becomes saturated.
Phase- the equilibrium state of matter, which differs in physical properties from other possible states of the same substance. A substance has phases: one is gaseous, one is liquid and several solid phases (for example, ice can be in five different modifications, which means it has five solid phases).
phase transition- the transition of a substance from one phase to another.
The physical meaning of the temperature gradient is the maximum rate of temperature increase over distance. This is a vector directed in the direction of increasing temperature, numerically equal to the first derivative of temperature with respect to distance. The temperature gradient is measured in degrees per meter. The temperature gradient is non-zero if there is a temperature difference.
Ejector- a device that increases the flow rate of one medium in a narrowing section to create reduced pressure and thereby causing an influx of another medium there.
Syrup Usually a solution of sugar in water.
Brine- usually a solution of salt in water.
Introduce students to the phenomenon of boiling. To teach to explain the process of boiling on the basis of molecular - kinetic theory. Consider the physical features of boiling. Determine the method for calculating heat in the process under study.
Download:
Slides captions:
The presentation was prepared by the teacher of physics Starkova Evgenia Evgenievna MOU Burevestnikovskaya secondary school
Lesson topic:
What process is called vaporization?
What is evaporation?
At what temperature does evaporation occur?
1. Why does water evaporate faster from a plate than from a bowl?
2. Why was the balance of the scales disturbed?
3. Why did the levels of different fluids become different after a few days?
What process is called condensation?
Is energy absorbed or released during condensation?
Boiling is an intense transition of a liquid into vapor, which occurs with the formation of vapor bubbles throughout the volume of the liquid at a certain temperature.
Boiling point of some substances, 0C (at normal atmospheric pressure)
Hydrogen
-253
Water
100
Oxygen
-183
Mercury
357
Milk
100
Lead
1740
Ether
35
Copper
2567
Alcohol
78
Iron
2750
The boiling point depends on the pressure exerted on the free surface of the liquid
As pressure decreases, the boiling point of a liquid decreases.
As pressure increases, the boiling point of a liquid increases.
Specific heat of vaporization.
Physical quantity, showing how much heat is needed to convert a liquid of mass 1 kg into vapor without changing the temperature, is called the specific heat of vaporization.
The specific heat of vaporization is denoted L, measured in J / kg
Specific heat of vaporization of certain substances, J / kg (at the boiling point and normal atmospheric pressure)
To turn water with a 1 kg mask into steam at a temperature of 100 0C, 2.3 * 106 J of energy is required.
Condensing, the steam gives off the amount of energy that went into its formation
Calculation of the amount of heat required to turn a liquid into steam
To calculate the amount of heat required to vaporize a liquid of any mass, taken at the boiling point, you need to multiply the specific heat of vaporization by the mass
Q=lm
Q – amount of heat, J L – specific heat of vaporization, J/kg m – body weight, kg
The amount of heat released by steam, condensing at the boiling point, is determined by the same formula
Q
3 *106 J
?
4.5*107 J
L
2.3*106 J/kg
2.3*106 J/kg
?
m
?
0.8kg
19 kg
1.3 kg
1.84 * 106 J
2.3 * 106 J/kg
The graphs show the processes of heating and boiling of identical masses of water and alcohol.
20
40
60
80
100
BUT
AT
FROM
To
M
t, 0c
t, s
1. Indicate the graph of heating and boiling built for alcohol
2. Calculate the amount of heat that is absorbed during the MC process. The mass of water is assumed to be 5 kg.
Textbook A.V. Peryshkin§18, §20Ex. 10 No. 3.4
Preview:
Physics lesson in 8th grade.
Topic: Boiling. Specific heat of vaporization and condensation.
The purpose of the lesson: Introduce students to the phenomenon of boiling. To teach to explain the process of boiling on the basis of molecular-kinetic theory. Consider the physical features of boiling. Determine the method for calculating heat in the process under study.
Demos:1 .Observation of the process of heating and boiling water in a glass flask. (Video)
2 . Boiling water at reduced pressure (video).
3 . Observation of the condensation process (video).
Equipment: computer, multimedia projector.
Lesson steps:
- E Tap to update knowledge
- Motivational stage
- The stage of creating new knowledge
- The stage of applying the acquired knowledge
- Homework
Lesson structure
- The stage of updating knowledge.
Org.moment.
Examination homework in the form of an oral survey:
In the last lesson we learned two physical phenomena These are evaporation and condensation. Let's remember what they are.
Slide 2. What process is called vaporization?
What is evaporation?
At what temperature does evaporation occur?
slide 3. The rate of evaporation depends on several factors. Let's clarify them by answering the following questions:
Therefore, the rate of evaporation depends on:
- from surface area.
- on the temperature of the liquid.
- From a kind of liquid.
slide 4. What do you see on the screen? ( dew and clouds).
What process resulted in the formation of dew and clouds?
What process is called condensation?
Is energy absorbed or released during condensation? ( stands out).
- motivational stage.
Well done! Now let's look at the images presented on the screen:
Slide 5. Tell me what do you see? ( over water steam)
slide 6. What do we see here? (boiling water, steam above the water)
Slide 7. What unites image data? ( steam )
Good! In the last lesson, we learned that there are two ways for a liquid to pass into gaseous state, evaporation and boiling.
Today we will consider the second method of steam formation - boiling.
- The stage of creating new knowledge.
slide 8. I demonstrate a video with the experience "Heating and boiling water in a glass flask."
When heated, the evaporation of water from the surface increases, sometimes you can even notice fog above it. This water vapor condenses in the air as it cools.
With a further increase in temperature, we will notice the appearance of numerous small bubbles in the water. Their size is gradually growing. These are air bubbles that are dissolved in water. When heated, excess air is released from the water in the form of bubbles. They contain saturated steam as the water evaporates into these air bubbles.
As the water warms up further, the bubbles become larger and more numerous. As the size of the bubbles increases, the Archimedean force also increases, pushing them out of the water, and they float. At this point, a noise is heard, usually preceding boiling. At a certain temperature, as the surface of the liquid is approached, the volume of the bubbles increases sharply. On the surface, they burst, and the saturated steam in them escapes into the atmosphere - the water boils.
We remove the spirit lamp from under the flask. What are we seeing? (boiling stops)
To continue boiling, it is necessary to supply energy to the boiling liquid.
slide 9. Boiling is an intense transition of a liquid into vapor, which occurs with the formation of vapor bubbles throughout the volume of the liquid at a certain temperature. (writing in a notebook)
The temperature at which a liquid boils is called the boiling point. (writing in a notebook)
slide 10. During boiling, the temperature of the liquid does not change.
Different liquids have different boiling points.
Slide 11. The boiling point depends on the pressure exerted on the free surface of the liquid.
I demonstrate a video with the experience "Boiling under reduced pressure"
As pressure decreases, the boiling point of a liquid decreases
(In mountainous areas at high altitudes, at low atmospheric pressure, water boils at temperatures lower than 100 degrees Celsius. It takes longer to wait until such a dinner is cooked.)
As pressure increases, the boiling point of a liquid increases
(When cooking, the pressure inside the pot - "pressure cooker" - is about 200 kPa, and the soup in such a pot will cook much faster).
slide 12. Specific heat of vaporization.
slide 13. The specific heat of vaporization varies from substance to substance.
slide 14. In contact with a cold object, water vapor condenses. In this case, the energy absorbed during the formation of steam is released.
I demonstrate a video with the experience "Observation of the condensation process."
Condensing, the steam gives off the amount of energy that went into its formation.
slide 15. Calculation of the amount of heat required to turn a liquid into steam.
- The stage of applying the acquired knowledge.
(After presenting new material, it is advisable to conduct reinforcing tasks)
slide 16.
Work with a formula to calculate the amount of heat required to turn a liquid of any mass into vapor
Q = Lm.
Answers:
slide 17. Working with charts.
Outcome . Today at the lesson we got acquainted with the phenomenon of boiling. We learned to explain the boiling process on the basis of molecular-kinetic theory. We considered the physical features of boiling and determined the method for calculating heat in the studied process.
- Homework
(The teacher notes the most active students, grades)