Entropy enthalpy internal energy what is the difference. Internal energy and enthalpy
BASICS OF CHEMICAL THERMODYNAMICS
Basic concepts of chemical thermodynamics. System, equilibrium state and thermodynamic process. Extensive and intensive properties. State functions and process functions. Can a thermodynamic quantity, which is, in the general case, a process function, acquire the properties of a state function? If yes, please provide examples.
First law of thermodynamics. Internal energy. Heat and work as forms of energy transfer. Interrelation of these values in isochoric and isothermal processes.
First law of thermodynamics, formulations of the 1st law of thermodynamics. Internal energy of the system. Heat and work as forms of energy transfer. 1st law of thermodynamics as applied to isothermal and isochoric processes.
Draw schematically on one graph in the coordinates of the state parameters Р=f(V) processes of reversible isothermal and reversible isobaric expansion of an ideal diatomic gas from the same initial state to a twofold increase in volume. Explain for which of the above processes the expansion work is greater?
The adiabatic plot (thick line) on the diagram for gas. - gas pressure; - volume.
In a particular case, when work is done through a change in volume, it can be defined in the following way: let the gas be enclosed in a cylindrical vessel tightly closed with an easily sliding piston, if the gas expands, it will move the piston and, when moving to a segment, do work
where F is the force with which the gas acts on the piston. Let's rewrite the equation:
where s is the area of the piston. Then the work will be
where is the gas pressure, is a small volume increment. Similarly, it can be seen that the equation is also valid for vessels with an arbitrary cross-sectional shape
Isochoric and isobaric molar heat capacities. Communication between them for ideal gas. Dependence of isobaric heat capacity on temperature for substances in crystalline, liquid and gaseous states.
When heating liquid and solids their volume practically does not change, and the work of expansion turns out to be equal to zero. Therefore, the entire amount of heat received by the body goes to change its internal energy. Unlike liquids and solids, a gas in the process of heat transfer can greatly change its volume and do work. Therefore, the heat capacity of a gaseous substance depends on the nature of the thermodynamic process. Usually, two values of the heat capacity of gases are considered: C V – molar heat capacity in an isochoric process (V= const) and C p – molar heat capacity in an isobaric process (p= const).
In the process at constant volume gas does no work A= 0. From the first law of thermodynamics for 1 mole of gas follows
where ∆ V- change in the volume of 1 mole of an ideal gas when its temperature changes by Δ T. This implies:
where R is the universal gas constant. At p= const
Thus, the relation expressing the relationship between molar heat capacities C p and C V, has the form ( Mayer's formula ):
|
C p = C V + R. |
|
During phase transitions(from one crystalline modification to another, from solid state into liquid, etc.) the heat capacity changes abruptly, while for most substances C V slightly more liquid at the melting point C V crystalline (Fig. 1.7). |
|||
|
| |||
|
| |||
|
Rice. 1.7. Heat capacity dependence HCl temperature T T F.P. - temperature phase transition; T PL. - melting temperature; T KIP. - boiling temperature | |||
|
3. The heat capacity of gaseous and liquid substances usually increases with increasing temperature (Fig. 1.8). | |||
|
| |||
|
The dependence of the heat capacity of substances on temperature in the range from 298 to T is usually described | |||
|
for organic substances by the empirical equation: C P = a + in T + c T 2 (1.22) |
| ||
6. Internal energy and enthalpy si, their relationship. Dependence of internal energy and enthalpy of matter on temperature. Integration of the corresponding equations.
internal energy system is called the sum of the potential energy of the interaction of all particles of the body with each other and kinetic energy their movements, i.e. internal energy system consists of the energy of translational and rotary motion molecules, the energy of intramolecular vibrational motion of atoms and atomic groups that make up molecules, the energy of rotation of electrons in atoms, the energy contained in the nuclei of atoms, the energy of intermolecular interaction and other types of energy.
The absolute value of the internal energy of a body is unknown, but to study chemical phenomena it is important to know only the change in internal energy during the transition of the system from one state to another. In many processes, energy can be transferred partly in the form of heat and partly in the form of work.
Thus, heat and work characterize qualitatively and quantitatively two different forms of energy transfer from one body to another; they are measured in the same units as energy.
Work or energy of any kind can be represented as the product of two factors: the intensity factor times the change in the capacitance factor, also called the extensiveness factor (if the intensity factor remains constant during the process). So, for example, normal work (mechanical) is equal to the product of the applied force and the path increment:
If heat Q is supplied to the system (substance or set of substances), then, according to the law of conservation of energy, it is generally spent on increasing the internal energy of the system U and on doing work A, i.e.
(1) where U is the change in the internal energy of the system during the transition from the initial state U BEGINNING to the final state U CON.
![]() In chemical reactions, work is mainly characterized by work against external pressure. In the first approximation, it is equal to the product of pressure and volume change V of the system:
In chemical reactions, work is mainly characterized by work against external pressure. In the first approximation, it is equal to the product of pressure and volume change V of the system:
(2) where V is the volume change in the process.
With an isochoric process, A = 0, because there is no change in the volume of the system ( V=0). Therefore, the transition of the system, suppose from state 1 to state 2, corresponds to the equality:
(3) Therefore, if the reaction proceeds at V=const, then the release and absorption of heat Q V is associated with a change in internal energy U.
For an isobaric process, V is the difference between the sum of the volumes of the reaction products and the sum of the volumes of the starting substances (P=const).
![]() (4) For an isobaric process, the thermal effect Q P will be equal to:
(4) For an isobaric process, the thermal effect Q P will be equal to:
![]() (5)(6)
(5)(6)
Denote, (8) - Enthalpy
Enthalpy is equal to the sum of internal energy and the product of volume and pressure.
Enthalpy like internal energy, it is an extensive function of the state, depending on the nature of the substance, pressure and temperature. Within the temperature range where the phase state of the system does not change, the enthalpy is a monotonic function of the main parameters.
those. H is the thermal effect of the reaction Q p at p=const.
Enthalpy is a state function, i.e. its change is determined by the given initial and final states of the system and does not depend on the transition path.
dim [H]=[kJ] or [kJ/mol]
Thus, at isochoric process thermal effect of the reaction:
thermal effect(warmth chemical reaction) is the amount of heat (energy) released or absorbed by the system during the reaction under conditions of constant volume or pressure, and the resulting products have the same temperature as the original substance.
the internal energy of an ideal gas does not depend on volume and pressure, but is only a function of temperature, then, based on equation (2.7), after integrating it, we obtain for the internal energy at any temperature T:
U T = U o + With V (T – T o). (5.2) The enthalpy of an ideal gas, like the internal energy, also depends only on temperature. Since, by definition, H = U + pV, and for 1 mole of gas pV = RT and ( With R – With V)
=R, then H = U o + With R (T – T o). (5.3) Isochoric and isobaric potentials of an ideal gas at constant temperature are determined by integrating equations (4.28) and (4.34). For one mole of an ideal gas at T= const dF =
– pdV =
–  dV, (5. 4) whence after integration we obtain F = F(T)
– RT ln v.(5.5)
dV, (5. 4) whence after integration we obtain F = F(T)
– RT ln v.(5.5)
Thermochemistry. Hess's law and its thermodynamic substantiation. Relationship between the thermal effects of a chemical reaction at constant pressure and constant volume.
Hess' law- the basic law of thermochemistry, which is formulated as follows:
The thermal effect of a chemical reaction carried out under isobaric-isothermal or isochoric-isothermal conditions depends only on the type and state of the starting materials and reaction products and does not depend on the path of its occurrence..
In other words, the amount of heat released or absorbed in any process is always the same, regardless of whether the given chemical transformation proceeds in one or several stages (provided that the temperature, pressure and aggregate states of substances are the same) .

The figure shows a schematic representation of some generalized chemical process of transformation of the starting substances A 1, A 2 ... into reaction products B 1, B 2 ..., which can be carried out in various ways in one, two or three stages, each of which is accompanied by a thermal effect ΔH i. According to Hess's law, the thermal effects of all these reactions are related by the following relation.
THERMOCHEMICAL EQUATIONS
Hess' law
All qualitative calculations are made in accordance with the law of Hess:
The change in the enthalpy of the reaction depends only on the physical state and chemical nature of the starting materials and reaction products and does not depend on the intermediate stages of the reaction. (1840)
Consequences from the law of Hess:
1) The thermal effect of the forward reaction is equal in magnitude and opposite in sign to the thermal effect of the reverse reaction.
2) The heat effect of the reaction is equal to the difference between the sums of the enthalpies of formation of the reaction of the products and starting materials, multiplied by stoichiometric coefficients.
3) The heat effect of the reaction is equal to the difference between the sums of the enthalpies of combustion of the reaction of the initial substances and products, multiplied by stoichiometric coefficients.
Thermodynamic parameters
The thermodynamic parameters are physical quantities, which characterize the state and processes in thermodynamic systems.
They are also called thermodynamic properties. They are divided into intensive (which do not depend on the amount of substance) - this is temperature, pressure, concentration, and extensive (depend on the amount of substance) - this is mass, volume, energy. The set of values of thermodynamic properties characterizes thermodynamic state systems.
Thermodynamic properties are used to describe thermodynamic systems and are often presented quantitatively as a set of quantities.
Internal energy and enthalpy
Enthalpy is a generalized parameter that takes into account changes in the energy system due to the release of heat and the performance of work. Enthalpy is also called the heat content of the system, since the change in enthalpy at constant pressure is equal to the absorbed heat. This is an extensive property of the system.
If only liquids and solids, then the change in the enthalpy of the reaction is approximately equal to the change in internal energy. This is due to the fact that the change in volume is practically zero and it turns out that the work of expansion is not performed.
But if, as a result of a chemical reaction, gaseous substances are released or absorbed, then the equation is used in full. In the isothermal expansion of an ideal gas, the internal energy is zero. In the general case, the change in energy in the system occurs in connection with the exchange between the system and environment heat or work.
Entropy and its role in the description of processes occurring in isolated and closed systems
Entropy is a quantitative thermodynamic property that explains randomness or disorder in a system; in other words, lack of organization. The greater the disorder, the greater the value of entropy and vice versa. The entropy is proportional to the probability of the system S=klnW, where k is the Voltzmann constant. The entropy of the universe is always increasing. According to the second law of thermodynamics in isolated systems only such processes are possible in which there is an increase in entropy. When the entropy reaches the maximum possible level, equilibrium occurs in the system. The entropy of a pure, perfect crystal at 0 K is zero (the third law of thermodynamics).
If the system is not isolated, then the sum of changes in the entropies of the system and the environment during irreversible processes is always positive. This sum is equal to zero only in thermodynamically reversible processes.
In closed systems, only with the help of entropy it is impossible to determine the direction and equilibrium; they use generalized parameters: the Helmholtz energy and the Gibbs energy.
Helmholtz energy is used in processes where V and T are constant. dA=dU-TdS
Gibbs energy is used in processes where P and T are constant. dG=dH-TdS 298K
Qualitative determination of the change in enthalpy and entropy
Change free energy in the system, its role in describing the processes occurring in closed system
The value of G characterizes the ability of the system to perform useful work
Gibbs energy can be used to determine the direction of the process.
If G<0, то процесс протекает самопроизвольно.
If G=0, then equilibrium occurs.
CHEMICAL EQUILIBRIUM
Factors Affecting Chemical Equilibrium
The chemical equilibrium of the system is influenced by concentration, pressure (for gaseous substances), temperature, as well as catalysts.
Equilibrium constant
The equilibrium constant is equal to the ratio, the numerator of which includes the product of the equilibrium concentrations of the reaction products, and the denominator is the product of the concentrations of the starting substances, while all concentration values are taken in powers equal to the stoichiometric coefficients in the reaction equation. And also the equilibrium constant is equal to the ratio of the rate constants of the forward and reverse reactions.
Shift in chemical equilibrium
The position of chemical equilibrium depends on the following reaction parameters: temperature, pressure and concentration. The influence that these factors have on a chemical reaction is subject to a pattern that was expressed in general terms in 1885 by the French scientist Le Chatelier.
Factors affecting the chemical balance:
1) temperature
As the temperature increases, the chemical equilibrium shifts towards an endothermic (absorption) reaction, and as it decreases, towards an exothermic (isolation) reaction.
CaCO3=CaO+CO2 -Q t →, t↓ ←
N2+3H2↔2NH3 +Q t ←, t↓ →
2) pressure
When the pressure increases, the chemical equilibrium shifts towards a smaller volume of substances, and when it decreases, towards a larger volume. This principle only applies to gases, i.e. if solids are involved in the reaction, they are not taken into account.
CaCO3=CaO+CO2 P ←, P↓ →
1mol=1mol+1mol
3) concentration of starting substances and reaction products
With an increase in the concentration of one of the starting substances, the chemical equilibrium shifts towards the reaction products, and with an increase in the concentration of the reaction products, towards the starting substances.
S2+2O2=2SO2 [S],[O] →, ←
Catalysts do not affect the shift of chemical equilibrium!
10. Principle LE - CHATELIER (consider an example)
If the system is in equilibrium, then when one of the conditions (temp., conc., pressure) changes, the equilibrium shifts in the direction of the reaction that counteracts the change. N2(g)+3H2(g)<->2NH3(g) +Q
Catalysts
A catalyst is a substance that changes the rate of a chemical reaction but is not part of the final products. Catalysts do not affect the composition of the equilibrium reaction mixture, but speed up the reaction, which reduces the time required to reach chemical equilibrium. There are catalysts (positive catalysts) and inhibitors (negative catalysts).
Homogeneous and heterogeneous catalysis
In homogeneous catalysis, the reactants and the catalyst form a single-phase system - gas or liquid, there is no interface between the catalyst and the reactants. For homogeneous catalysis, it has been found that the rate of a chemical reaction is proportional to the catalyst concentration.
In heterogeneous catalysis, the reactants and the catalyst form a system of different phases. In this case, there is an interface between the catalyst and the reactants. The catalyst is usually a solid and the reactants are gases or liquids. The activity of a solid catalyst depends on the properties of its surface (size, chemical composition, structure, and state). The action of positive catalysts is reduced to a decrease in the activation energy of the reaction, in other words, to a decrease in the height of the energy barrier. This forms an activated complex with a lower energy level, and the reaction rate increases greatly.
STRUCTURE OF THE ATOM AND THE PERIODIC LAW
Isotopes, isobars, type of nuclei?
Isotopes were first discovered in the experiments of JJ Thomson in 1912, when he observed the deflection of charged particles in an electric and magnetic field. Isotopes were called varieties of atoms of the same chemical element, having the same nuclear charges, but different mass numbers. Elements in nature consist of mixtures of isotopes, for example, natural carbon 12 6 C and 13 6 C. But the mass of atoms is very small, therefore relative units, called atomic mass units, are 1/12 of the mass of carbon 12.
Isobars are varieties of atoms of different chemical elements that have the same mass number, but different atomic numbers.
Core types:
Schrödinger equation
For an electron moving in one-dimensional space in a field of forces with potential energy V has the following form:
-(h^2/8pi^2m)d^2horns/dx^2+Vhorns=Ehorns
In this equation, m is the electron mass, x is the coordinate for the electron along the x axis, E is the total energy, and horns are the wave function. The second derivative of the wave function with respect to the x-coordinate.
wave numbers
quantity related to the wavelength λ by the relation: k= 2π/λ (the number of waves at a length of 2π). In V. spectroscopy, the quantity reciprocal to the wavelength (1/λ) is often called.
Pauli principle
Each orbital can contain only two electrons, and their spins are opposite. An atom cannot have two electrons with the same four quantum numbers.
Gund's rule
Among several possible orbitals of the same energy, the most stable configuration is the one with the most unpaired electrons.
quantum numbers
The principal quantum number n determines the total energy of the electron. Each number corresponds to an energy level. n=1,2,3,4…or K,L,M,N…
The orbital quantum number l determines the sublevels on the energy level. The quantum number l determines the shape of the orbitals (n-1) 0,1,2…
The magnetic quantum number ml determines the number of orbitals in a sublevel. …-2,-1,0,+1,+2… The total number of orbitals in the sublevel is 2l+1
The spin quantum number ms refers to two different orientations +1/2 -1/2 There can only be two electrons in each orbital with opposite spins.
The oxidation states of the elements
The oxidation state (oxidation number, formal charge) is an auxiliary conditional value for recording the processes of oxidation, reduction and redox reactions, the numerical value of the electric charge attributed to an atom in a molecule on the assumption that the electron pairs that carry out the connection are completely shifted towards more electronegative atoms.
Ideas about the degree of oxidation form the basis for the classification and nomenclature of inorganic compounds.
The oxidation state corresponds to the charge of an ion or the formal charge of an atom in a molecule or in a chemical formal unit, for example:
The oxidation state is indicated above the element symbol. Unlike indicating the charge of an atom, when indicating the degree of oxidation, the sign is put first, and then the numerical value, and not vice versa:
The degree of oxidation,
Charges.
The oxidation state of an atom in a simple substance is zero, for example:
The algebraic sum of the oxidation states of atoms in a molecule is always zero:
The concept of the degree of oxidation is quite applicable to nonstoichiometric compounds (KS 8 , Mo 5 Si 3 , Nb 3 B 4 , etc.). For example, in the well-known pyrite roasting reaction:
4FeS 2 + 11O 2 \u003d 2Fe 2 O 3 + 8SO 2
it is most convenient to take the oxidation state of iron +3 in the original compound (although in reality the iron atom displaces 2 electrons from itself, that is, the oxidation state of iron is +2), and for sulfur it is −3/2 (!), which does not at all contradict the definition of the oxidation state , as a conventional unit and allows you to equalize the reaction as simply as in the case of other redox processes.
The total oxidation state of atoms in a molecule is always zero.
CHEMICAL BOND
Communication order
The bond order in the theory of molecular orbitals is determined by the expression
![]()
where and are the total numbers of electrons in the bonding and loosening orbitals, respectively.
Types of chemical bond
It is known that atoms can combine with each other to form both simple and complex substances. In this case, various types of chemical bonds are formed: ionic, covalent (non-polar and polar), metallic and hydrogen. One of the most essential properties of the atoms of elements, which determines what kind of bond is formed between them - ionic or covalent, is electronegativity, i.e. the ability of atoms in a compound to attract electrons to itself.
The type of chemical bond depends on how large the difference in the electronegativity values of the connecting atoms of the elements is. The more the atoms of the elements forming the bond differ in electronegativity, the more polar the chemical bond is. It is impossible to draw a sharp boundary between the types of chemical bonds. In most compounds, the type of chemical bond is intermediate; for example, a highly polar covalent chemical bond is close to an ionic bond. Depending on which of the limiting cases is closer in nature to the chemical bond, it is referred to as either an ionic or a covalent polar bond.
Electron hybridization
Hybridization of orbitals is a hypothetical process of mixing different (s, p, d) orbitals of the central atom of a polyatomic molecule with the appearance of the same number of orbitals, which are equivalent in their characteristics. Types of hybridization
[edit] sp hybridization
sp hybridization
Occurs when mixing one s- and one p-orbitals. Two equivalent sp-atomic orbitals are formed, located linearly at an angle of 180 degrees and directed in different directions from the nucleus of the carbon atom. The two remaining non-hybrid p-orbitals are located in mutually perpendicular planes and participate in the formation of π-bonds, or are occupied by lone pairs of electrons.
[edit] sp 2 hybridization

sp 2 hybridization
Occurs when mixing one s- and two p-orbitals. Three hybrid orbitals are formed with axes located in the same plane and directed to the vertices of the triangle at an angle of 120 degrees. The non-hybrid p-atomic orbital is perpendicular to the plane and, as a rule, participates in the formation of π-bonds
[edit] sp 3 hybridization
![]()
sp 3 hybridization
Occurs when mixing one s- and three p-orbitals, forming four sp3-hybrid orbitals of equal shape and energy. They can form four σ-bonds with other atoms or be filled with lone pairs of electrons.
The axes of sp3-hybrid orbitals are directed to the vertices of a regular tetrahedron. The tetrahedral angle between them is 109°28", which corresponds to the lowest electron repulsion energy. Sp3 orbitals can also form four σ-bonds with other atoms or be filled with unshared pairs of electrons.
Hybridization and molecular geometry
Ideas about the hybridization of atomic orbitals underlie the Gillespie-Nyholm theory of repulsion of electron pairs. Each type of hybridization corresponds to a strictly defined spatial orientation of the hybrid orbitals of the central atom, which allows it to be used as the basis of stereochemical concepts in inorganic chemistry.
The table shows examples of the correspondence between the most common types of hybridization and the geometric structure of molecules, assuming that all hybrid orbitals participate in the formation of chemical bonds (there are no unshared electron pairs).
| Type of hybridization | Number of hybrid orbitals | Geometry | Structure | Examples |
| sp | Linear | | BeF 2 , CO 2 , NO 2 + | |
| sp 2 | triangular |  | BF 3 , NO 3 – , CO 3 2– | |
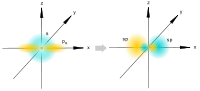
| sp 3 | tetrahedral | 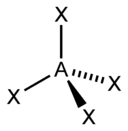 | CH 4, ClO 4 –, SO 4 2–, NH 4 + | |
| dsp2 | flat square |  | Ni(CO) 4 , XeF 4 | |
| sp 3 d | Hexahedral | 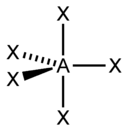 | PCl 5 , AsF 5 | |
| sp 3 d 2 | Octahedral | 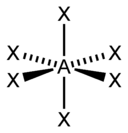 | SF 6 , Fe(CN) 6 3– , CoF 6 3– |
Sigma and pi connection
Sigma- and pi-bonds (s- and p-bonds), covalent chemical bonds, characterized by a definite, but different spatial symmetry of the electron density distribution. As is known, a covalent bond is formed as a result of the socialization of electrons of interacting atoms. The resulting s-bond electron cloud is symmetrical with respect to the communication line, i.e., the line connecting the nuclei of the interacting atoms. Simple bonds in chemical compounds are usually (t-bonds (see. Simple bond). The p-bond electron cloud is symmetrical about the plane passing through the bond line (Fig. 1, b), and in this plane (called the nodal) the electron density is equal to The use of the Greek letters s and p is associated with their correspondence to the Latin letters s and p in the designation of the electrons of the atom, with the participation of which for the first time it becomes possible to form s- and p-bonds, respectively. p z) are symmetrical about the corresponding axes of Cartesian coordinates (x, y, z), then if one p-orbital, for example p z, takes part in the formation of an s-bond (the z-axis is a communication line), the two remaining p-orbitals (p x, p y) can take part in the formation of two p-bonds (their nodal planes will be yz and xz, respectively; see Fig. 2) d- (see Fig. 1) and f can also take part in the formation of s and p-bonds -electrons of an atom.If between atoms in a molecule both s- and p-bonds hiccup at the same time, then the resulting bond is a multiple (see Fig. multiple bonds, double bonds, triple bonds, and valency).  Rice. 1. Schematic representation of the spatial orientation of orbitals during the formation of an s-bond as a result of s - s-, s - p s - , p s - p s -interactions (a) and p-bonds as a result of p p - , p p - , d p - d p - interactions ( b). Rice. 1. Schematic representation of the spatial orientation of orbitals during the formation of an s-bond as a result of s - s-, s - p s - , p s - p s -interactions (a) and p-bonds as a result of p p - , p p - , d p - d p - interactions ( b). 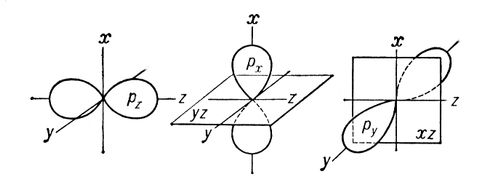 Rice. 2. Schematic representation of clouds p x -, p y -, p z - electrons. Shown are the axes of Cartesian coordinates and the nodal planes p x - and p y -orbitals. Rice. 2. Schematic representation of clouds p x -, p y -, p z - electrons. Shown are the axes of Cartesian coordinates and the nodal planes p x - and p y -orbitals. |
Dipole moment
Single and multiple connection
Relationships σ and π. Single and multiple bonds
Two atoms can also form multiple bonds with each other, that is, double and triple bonds. In this case, the component formed first will always be a σ-bond (it has the highest strength and determines the geometric shape of the molecule).
The second and third components are called π-bonds, they are formed by lateral overlap of any orbitals, except s-orbitals: 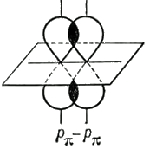

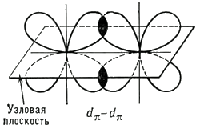
For example, 2 p-orbitals of two carbon atoms can form between themselves single, double and triple connections. In the first case, the skeleton of the molecule is formed ethane C2H6.
At double binding carbon atoms first 2 p-orbitals create a σ-bond, and the second - a π-bond; in this case, the skeleton of the molecule is formed ethylene C2H4.
At triple bonding (one σ-bond, two π-bonds) the backbone of the molecule is formed acetylene C2H2.
Such multiple bonds are always shorter and stronger than single bonds and are more difficult to break. Often this explains chemical inertness substances - such as nitrogen N 2 (:N≡N:) and carbon dioxide CO 2 (O=C=O).
Examples of particles with multiples bonds are also molecules SO 3, SO 2, NO 2 and anions CO 3 2−, SO 4 2−, SO 3 2−
Ionic bond and its properties
Formed by the complete transfer of one or more electrons between atoms. An atom that donates an electron(s) becomes a cation, while an atom that receives it becomes an anion. Ionic arises as a result of electrostatic forces of attraction between oppositely charged ions. An ionic bond is characteristic of compounds and elements whose atoms have a large difference in electronegativity values; it occurs between the atoms of alkali metals (electropositive elements) and halogens (electronegative elements). PROPERTIES
Structure of ionic compounds
The structure of an ideal ionic compound, due to the maximum attraction between unlike ions and the minimum repulsion of like ions, is largely determined by the ratio of the ionic radii of cations and anions. This can be shown by simple geometric constructions.
51. Donor-acceptor bond. Its properties, give examples
The donor-acceptor mechanism (otherwise the coordination mechanism) is a method for the formation of a covalent chemical bond between two atoms or a group of atoms, carried out due to the lone pair of electrons, the donor atom and the free orbital of the acceptor atom.
The terms "donor-acceptor bond" or "coordination bond" are incorrect, since this is not a type of chemical bond, but only a theoretical model describing the peculiarity of its formation. The properties of a covalent chemical bond formed by the donor-acceptor mechanism do not differ in any way from the properties of bonds formed by the exchange mechanism (for example, N-H bonds in the ammonium ion NH 4 + or O-H bonds in the hydroxonium ion H 3 O +).

Formation of the adduct of ammonia and boron trifluoride
Donors are usually atoms of nitrogen, oxygen, phosphorus, sulfur, etc., which have unshared electron pairs in small valence orbitals. The role of an acceptor can be performed by an ionized hydrogen atom H +, some p-metals (for example, aluminum in the formation of the AlH 4 - ion) and, in particular, d-elements that have unfilled energy cells in the valence electron layer.
It is from the standpoint of the donor-acceptor mechanism that the formation of localized covalent bonds in molecules and molecular ions of complex (coordination) compounds is described: the bond is formed due to the lone pair of electrons of the ligand and the free orbital of the complexing atom. The donor-acceptor mechanism also describes the formation of reaction intermediates, such as charge-transfer complexes.
The model of the donor-acceptor mechanism exists only within the framework of the concept of valence as the localization of electron density during the formation of covalent bonds (the method of valence schemes). Within the framework of the molecular orbital method, there is no need for such representations.
Hydrogen bond
This is a type of intermolecular interactions. These bonds are weak permanent forces between an H atom covalently bonded to a very electronegative A atom and an electronegative B atom capable of providing a free pair of electrons to form the bond. A hydrogen bond is represented by three dots. -A-N ... B-
Hydrogen bonds form only with atoms of the most electronegative elements. The most important of them are F, O, N, Cl. ADDITIONAL
53. Intermolecular forces of interaction, their properties (van der Waals forces)
Van der Waals forces include several types of interactions: orientational dipole-dipole, induction and dispersion.
1) If two molecules of the same substance or different substances are permanent dipoles, then they are attracted to each other by oppositely charged edges and are oriented in space accordingly. FORMULA (p. 172).
2) Inductive interaction is that a polar molecule causes polarization (induces a dipole) of a neighboring non-polar molecule. Further, they are oriented relative to each other in space. The final result is the interaction: dipole - induced dipole. FORMULA (p. 172).
3) Dispersion interactions are weak attractive forces between neutral atoms, such as noble gas atoms, or molecules, including non-polar molecules. FORMULA (p. 173).
SOLUTIONS
What is a solution
Solutions are thermodynamically stable homogeneous systems of variable composition containing two or more components. A substance whose concentration is greater than all other substances is called a solvent, and other substances are called dissolved. The phase state of the solvent in solution does not change, for example, water remains liquid in solution. Solutions are liquid, solid and gaseous.
Water
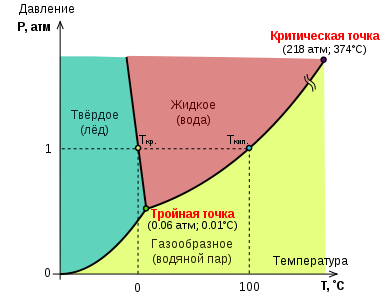
Water is one of the most common solvents on Earth. The water molecule has an angular structure: (105 degrees). The chemical bond O-H is covalent, but the common electron pair is drawn to the O atom. A positive charge appears on each H atom, and a negative charge appears on O. These partial charges create the polarity of the molecule, which gives water as a solvent its special properties. Pure water has a low concentration of O and OH and is a poor conductor of electricity. Intermolecular hydrogen bonds have a significant effect on the properties of water. Water molecules bound by hydrogen bonds form an openwork spatial grid. Water can be in three states of aggregation: liquid, solid (ice) or gaseous (steam). Evaporation of water and sublimation of ice occur at any temperature.
Association of water molecules
Water molecules, which are permanent dipoles, can, due to mutual
the attraction of oppositely charged poles can be combined in twos, threes, etc.
However, the attractive forces acting in this case are small, and in the case of water, a similar
dipole association plays only a minor role.
Of primary importance for the association of water molecules is the formation of
called hydrogen bonds. The latter arise due to the attraction of hydrogen
one water molecule to oxygen another according to the following scheme:
H
?
O-N O-N
.
.
H
The possibility of such an attraction is consistent with the assumption that there are significant
effective charges for both hydrogen (?H = ?0.33) and oxygen (?O = ?0.66) in the molecule
water.
Water Status Diagram
Solutions of non-electrolytes
Non-electrolytes are substances whose aqueous solutions and melts do not conduct electric current, since their molecules do not dissociate into ions.
65. Raoult's first and second laws!
Raoult's first law relates the saturation vapor pressure over a solution to its composition; it is formulated as follows:
The partial pressure of the saturated vapor of a solution component is directly proportional to its mole fraction in the solution, and the coefficient of proportionality is equal to the saturated vapor pressure over the pure component.
Book 2 section 1.6
Electrolyte solutions
Since strong electrolytes almost completely decompose into ions in aqueous solutions, the concentration of ions can be high. In concentrated solutions of strong electrolytes, the ions approach so much that the interaction between them becomes very significant. Due to interionic interaction, the mobility of ions decreases, and with the participation of ions in chemical reactions, the effect of reducing the concentration is created. Therefore, ions enter into chemical reactions not in accordance with their true concentration, but in accordance with their apparent concentration - activity. There is a relationship between activity and concentration of an ion in a solution: ai=yi*ci .
Isotonic ratio
Allows you to take into account the influence of non-ideal solutions on their physical properties. Molecules of dissolved substances can dissociate, which is typical for electrolyte solutions, but along with dissociation, association of molecules can also occur. To take into account the change in the number of particles in the solution due to the processes of their dissociation and association, the isotonic coefficient i is used.
The isotonic coefficient expresses the ratio of the number of particles of a solute to the number of its particles in the initial state. For solutions of non-electrolytes, the isotonic coefficient is 1, the dissociation process is i.k. greater than 1, association less than 1. Experimental determination of the isotonic coefficient allows one to calculate the degree of dissociation or association of a solute.
Amphoteric hydroxides
Amphoteric hydroxides are chemicals that behave like bases in an acidic environment and like acids in an alkaline environment.
Within each period, elements with the properties of metals are replaced by elements that exhibit the properties of both metals and non-metals. Compounds of these elements are called amphoteric. The element aluminum exhibits the properties of a metal and a non-metal in compounds. Similar properties have elements of A-groups - Be, Ga, Ge, Sn, Pb, Sb, Bi and others, as well as most elements of B-groups - Cr, Mn, Fe, Zn, Cd and others. Almost all of them are insoluble in water and are weak electrolytes.
When heated, the compounds decompose. In most cases, the interaction of a metal hydroxide produces a hydroxosalt of the corresponding acid: for example, this is how the interaction proceeds for Al(3+), Cr(3+), Zn(2+) and many other metals. This reaction is reversible, the equilibrium position depends on the nature of the metal pH of the medium and partly on temperature. Ions with a lower coordination number of the metal can also exist in solution.
Ion activity
Activity (of ions) - effective concentration, taking into account the electrostatic interaction between ions in solution. Activity differs from concentration by some amount. The ratio of activity (a) to the concentration of a substance in solution (c, in g-ion / l) is called the activity coefficient: γ \u003d a / c.
Activity factor
The activity coefficient is the ratio of the activity of a given component of a solution to its concentration, which characterizes the deviation of the properties of real solutions from the properties of ideal solutions. In ideal solutions and at infinite dilution K. and. is equal to one. Approximate values of K. a. calculated by the Debye-Hückel equation.
Water dissociation
Dissociation of water is the decomposition of water into its constituent chemical elements, sometimes occurring with the creation of new elements that are not initially contained in the decomposing solution, or are contained before the start of decomposition in a smaller amount than after the completion of the dissociation process.
The dissociation of water is an endothermic reaction (see endothermic reaction), i.e. going with the absorption of heat from the environment.
Known ways to dissociate water:
1. Electrolysis of aqueous electrolyte solutions. - the least effective method of water decomposition known today, since in this case the energy is spent mainly on heating the conductor - electrolyte, so much so that the dissipated solution not only does not cool, but, on the contrary, is subjected to significant heating. In the industry of the 20th century, it was this method that was most widely used, due to the fact that it provides demand and allows maintaining high prices for such goods as non-renewable energy resources from which electricity is obtained, such as oil, gas, coal, etc.
2. Model of the process of water decomposition in a centrifugal field For example, a heated electrolyte is poured into a rotating drum, in which, during rotation, as a result of an incipient electrochemical process, water is decomposed into hydrogen and oxygen. This process decomposes water using the kinetic energy of an external source and the thermal energy of the heated electrolyte. Based on this process, there are a number of patents, one of which (RU 98/00190 (06/22/1998)) is the authors - Kudymov G.I. and Studennikov V.V. they are positioned, among other things, like a heat pump that absorbs the heat of the environment, thus, the production of a hydrogen-oxygen mixture is carried out here, to a large extent due to the energy of the environment or due to the usually irretrievably lost heat, for example, exhaust gases of internal combustion engines .
Ionic product of water
The equilibrium value of the product of the concentration of water ions is called the ion product of water. It is designated Kv and is equal to 10 -14 Kv \u003d (H +) (OH -)
Hydrogen index (pH)
pH is the negative decimal logarithm of the equilibrium concentration of hydrogen ions. The pH value can be used to determine the medium of the solution. The pH value is determined using a special device - a pH meter, as well as using indicators. Also, pH can be determined through the equilibrium concentration of hydroxide ions pH=14-pOH.
Solubility product
In a saturated solution of a poorly soluble ionic compound, the product of the concentration of its ions at a given temperature is a constant value and is called the solubility product. PR applies only to poorly soluble strong electrolytes, which means that in solution they must completely decompose into ions and should not participate in secondary reactions.
If the chemical formula of the compound includes stoichiometric coefficients other than unity, then the ion concentrations are taken in powers equal to their stoichiometric coefficients.
Three cases of hydrolysis
There are three options for hydrolysis:
1) Cation hydrolysis is the hydrolysis of a salt containing a weak base cation and a strong acid anion.
2) Anion hydrolysis is the hydrolysis of a salt containing a strong base cation and a weak acid anion.
3) Hydrolysis by cation and anion is the hydrolysis of a salt containing a cation of a weak base and an anion of a weak acid.
buffer solutions
The internal energy of a substance (or system) is the total energy of the particles that make up this substance (see also § 54). It is composed of the kinetic and potential energies of the particles. Kinetic energy is the energy of the translational, vibrational and rotational motion of particles; potential energy is due to the forces of attraction and repulsion acting between particles.
Internal energy depends on the state of matter. The change in the internal energy of the system AU in a particular process can be determined. Let, as a result of some process, the system passes from the initial state 1 to the final state 2, while doing work A and absorbing heat from the external environment.
It is clear that the internal energy of the system will decrease by A, increase by Q, and in the final state will be equal to
where and is the internal energy of the system in the initial (1) and final (2) states. If we denote the difference by , then the equation can be represented as:
![]()
This equation expresses the law of conservation of energy, according to which the change in internal energy does not depend on the way the process is carried out, but is determined only by the initial and final states of the system. However, what part of the energy will go to do work, and what will turn into heat - depends on the way the process is carried out: the ratio between work and heat can be different. In particular, if no work is done during the process, including the work of expansion against external pressure, i.e., if the volume of the system does not change, then
where is the heat absorbed by the system under conditions of constant volume.
The last equation makes it possible to determine the change in internal energy in various processes. For example, in the case of heating a substance at a constant volume, the change in internal energy is determined by the heat capacity of this substance:
Here is the molar heat capacity of a substance at a constant volume; n is the amount of substance; is the difference between the final and initial temperatures.
In the case of a chemical reaction proceeding without a change in the volume of the system, the change in internal energy is equal to the heat effect of this reaction, taken with the opposite sign.
where n is the amount of substance; Cp is the molar heat capacity of a substance at constant pressure.
With changes in the state of aggregation of a substance and with allotropic transitions, the change in enthalpy is equal in magnitude, but opposite in sign, to the heat of the corresponding transformation (melting, boiling, transformation from one modification to another). Finally, in the case of a chemical reaction, the change in enthalpy is equal to the heat effect of the reaction carried out at constant temperature and constant pressure, taken with the opposite sign.
Enthalpy, like internal energy, characterizes the energy state of a substance, but includes the energy expended to overcome external pressure, i.e., the work of expansion. Like internal energy, enthalpy is determined by the state of the system and does not depend on how this state is reached. In the case of gases, the difference between and during a given process can be significant. In the case of systems that do not contain gases, the changes in internal energy and enthalpy accompanying the process are close to each other. This is explained by the fact that volume changes during processes undergone by substances in condensed (i.e., in solid or liquid) states are usually very small, and the value is small compared to .

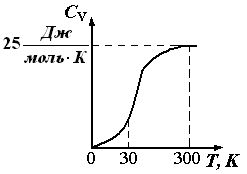 Rice. 1.6. The dependence of the heat capacity of crystalline substances on temperature
Rice. 1.6. The dependence of the heat capacity of crystalline substances on temperature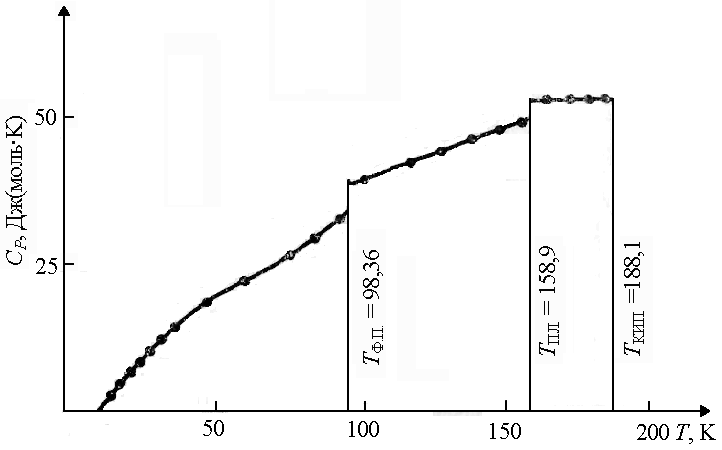
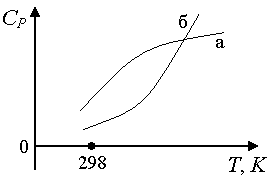 Rice. 1.8. The dependence of the heat capacity of gases on temperature: a) if the curve at low ^
T has a greater curvature than at high ones, it is preferable to describe it with an empirical power series of the form: C P
= a + inT + c'/T 2; b) curve b power series of the form: C P = a + inT + cT 2
Rice. 1.8. The dependence of the heat capacity of gases on temperature: a) if the curve at low ^
T has a greater curvature than at high ones, it is preferable to describe it with an empirical power series of the form: C P
= a + inT + c'/T 2; b) curve b power series of the form: C P = a + inT + cT 2