Water vapor and its types. Water vapor is the gaseous state of water
3. Water vapor and its properties
3.1. Water vapor. Basic concepts and definitions.
One of the most common working fluid in steam turbines, steam engines, nuclear power plants, coolant in various heat exchangers is water vapor. Steam - a gaseous body in a state close to a boiling liquid. vaporization The process of changing a substance from a liquid state to a vapor state. Evaporation - vaporization, which always occurs at any temperature from the surface of the liquid. At a certain certain temperature, depending on the nature of the liquid and the pressure under which it is located, vaporization begins in the entire mass of the liquid. This process is called boiling . The reverse process of vaporization is called condensation . It also runs at a constant temperature. Transition process solid directly into steam called sublimation . The reverse process of transition of vapor to a solid state is called desublimation . When a liquid evaporates in a limited space (in steam boilers), the opposite phenomenon occurs simultaneously - steam condensation. If the rate of condensation becomes equal to the rate of evaporation, then dynamic equilibrium sets in. The vapor in this case has a maximum density and is called saturated steam . If the steam temperature is higher than the temperature saturated steam the same pressure, then such steam is called overheated . The difference between the temperature of superheated steam and the temperature of saturated steam at the same pressure is called degree of overheating . Since the specific volume of superheated steam is greater than the specific volume of saturated steam, the density of superheated steam is less than the density of saturated steam. Therefore, superheated steam is unsaturated steam . At the moment of evaporation of the last drop of liquid in a limited space, without changing temperature and pressure, a dry saturated steam . The state of such steam is determined by one parameter - pressure. The mechanical mixture of dry and tiny droplets of liquid is called wet steam . Mass fraction of dry steam in wet steam called degree of dryness –X.
X\u003d m cn / m ch,
m cn - the mass of dry steam in wet; m vp - mass of wet steam. The mass fraction of liquid in wet steam is called degree of humidity –at.
at= 1 – .
For a boiling liquid at saturation temperature = 0, for dry steam – = 1.
3.2 Humid air. Absolute and relative humidity.
Atmospheric air is widely used in technology: as a working fluid (in air refrigeration units, air conditioners, heat exchangers and dryers) and as an integral part for fuel combustion (in internal combustion engines, gas turbine plants, steam generators).
Dry air called air that does not contain water vapor. Atmospheric air always contains some water vapor.
humid air is a mixture of dry air and water vapour.
In heat engineering, some gaseous bodies are called steam. So, for example, water in a gaseous state is called water vapor, ammonia - ammonia vapor.
Let us consider in more detail the thermodynamic properties of water and steam. (1-6).
The formation of vapor from the liquid of the same name occurs through evaporation and boiling . There is a fundamental difference between these processes. Evaporation of the liquid occurs only from the open surface. Individual molecules with a high speed overcome the attraction of neighboring molecules and fly out into the surrounding space. The rate of evaporation increases with the temperature of the liquid. The essence of boiling is that steam generation occurs mainly in the volume of the liquid itself due to its evaporation inside the vapor bubbles. There are the following states of water vapor:
wet steam;
dry saturated steam;
superheated steam.
Atmospheric air (moist air) can be:
supersaturated moist air;
saturated moist air;
unsaturated moist air.
oversaturated Moist air is a mixture of dry air and moist water vapour. A natural phenomenon is fog. Saturated Moist air is a mixture of dry air and dry saturated water vapour. unsaturated Moist air is a mixture of dry air and superheated water vapor.
It should be noted fundamentally different meanings of the term “wet” in relation to steam and air. Steam is called wet if it contains a finely dispersed liquid. Humid air in all cases of interest to technology contains superheated or dry saturated water vapor. In the general case, moist air may also contain moist water vapor (for example, clouds), but this case is not of technical interest and is not considered further.
In atmospheric (humid) air, each component is under its own partial pressure, has a temperature equal to the temperature of moist air and is evenly distributed throughout the volume.
The thermodynamic properties of moist air as a gaseous mixture of dry air and water vapor are determined according to the laws characteristic of ideal gases.
The calculation of processes with moist air is usually carried out under the condition that the amount of dry air in the mixture does not change. The variable is the amount of water vapor contained in the mixture. Therefore, the specific values characterizing moist air refer to 1 kg of dry air.
Humid air pressure is determined by Dalton's law:
Р=Рв+Рп, (3.1)
Where Rv - partial pressure dry air, kPa; Pp is the partial pressure of water vapor, kPa.
Let's write the Clapeyron - Mendeleev equation
wet air PV=MRT; (3.2)
dry air P B V=M B R B T; (3.3)
water steam P P V=M P R P T, (3.4)
where V is the volume of moist air, m 3; M, M V, M P - mass of moist, dry air and water vapor, respectively, kg; R, R V, R P – gas constant of moist, dry air and water vapor, respectively, kJ/(kgK); T - absolute temperature moist air, K.
Absolute air humidity - the amount of water vapor contained in 1 m 3 of moist air. It is denoted by P and is measured in kg / m 3 or g / m 3. In other words, it represents the density of water vapor in the air: P \u003d R P / (R P T). It's obvious that
P \u003d M P / V, where V is the volume of moist air with mass M.
Relative humidity is the ratio of the absolute humidity of the air in a given state to absolute humidity saturated air (H) at the same temperature.
Two characteristic states of air can be noted in terms of the value :<100 %, при этом Р П <Р Н и водяной пар перегретый, а влажный воздух ненасыщенный;=100 %, при этом Р П =Р Н и водяной пар сухой насыщенный, а влажный воздух насыщенный. Температура, до которой необходимо охлаждать ненасыщенный влажный воздух, чтобы содержащийся в нем перегретый пар стал сухим насыщенным, называется температурой точки росы t Н.
3.3 id - diagram of humid air
For the first time id - chart for humid air was proposed by prof. OK. Ramzin. Currently, it is used in the calculations of air conditioning, drying, ventilation and heating systems. Vid - the diagram along the abscissa shows the moisture content d, g / kg of dry air, and along the ordinate - the specific enthalpy of moist air i, kJ / kg of dry air. For a more convenient arrangement of individual lines drawn on the id - diagram, it is built in oblique coordinates, in which the abscissa axis is drawn at an angle of 135 ° to the y-axis.
With this arrangement of the coordinate axes, the lines i=const, which should be parallel to the x-axis, go obliquely. For the convenience of calculations, the values of d are taken down to the horizontal coordinate axis.
The lines d=const are in the form of straight lines parallel to the y-axis, i.e. vertically. In addition, isotherms t C =const, t M =const (dashed lines in the diagram) are plotted on the id.-diagram in the line of constant relative humidity values (starting from .=5% to =100%). Lines of constant values of relative humidity =const are built only up to the isotherm 100 °, i.e. until the partial pressure of vapor in the air P P is less than atmospheric pressure P. At the moment when P P becomes equal to P, these lines lose physical meaning, which can be seen from equation (10), in which, at P P = P, the moisture content is d=const.
The curve of constant relative humidity =100% divides the entire diagram into two parts. That part of it that is located above this line is an area of unsaturated moist air in which the steam is in a superheated state. The part of the diagram below the line =100% is the area of saturated moist air.
Since at =100% the readings of dry and wet thermometers are the same, t C =t M , then the isotherms t C =t M =const intersect at the line =100%..
To find a point on the diagram corresponding to the state of a given humid air, it is enough to know two of its parameters from those shown in the diagram. When conducting an experiment, it is advisable to use those parameters that are easier and more accurately measured in the experiment. In our case, these parameters are the temperature of dry and wet bulbs.
Knowing these temperatures, one can find the intersection point of the corresponding isotherms on the diagram. The point found in this way will determine the state of humid air, and from the id - diagram, you can determine all other air parameters: moisture content - d; relative humidity -, air enthalpy -i; partial vapor pressure - R P, dew point temperature - t M.
Water vapor has high pressure and relatively low temperature, it is close to the state
liquid, therefore, it is impossible to neglect the cohesion forces between its molecules and their volume, as in ideal gases. Therefore, it is not possible to use the equations of state of ideal gases to determine the parameters of the state of water vapor, i.e., for
pair pv ≠ RT.
What is the difference between boiling and evaporation processes?
A liquid can turn into vapor when it evaporates and boils. by evaporation called vaporization, occurring only from the surface of the liquid and at any temperature. The rate of evaporation depends on the nature of the liquid and its temperature. Evaporation of a liquid can be complete if there is an unlimited space above the liquid. In the process of evaporation, vaporization occurs only on the free surface of the liquid. This is a two-way process, in which, along with the departure of a part of the molecules from the liquid, a partial return of the molecules back to the liquid occurs. In the process boiling vapor is formed throughout the mass of the liquid. When a liquid is heated, the solubility of gases in it decreases, as a result of which bubbles form on the bottom and walls of the vessel in which water is located. In the process of heating inside the bubbles, the liquid begins to evaporate, and at a certain temperature, the pressure of saturated vapor inside the bubbles becomes equal to the external pressure. At this point, the bubbles break off and the liquid begins to boil. Thus, if evaporation occurs from the surface of a liquid at any temperature, then boiling occurs at one, quite definite for a given pressure and temperature, called boiling point or saturation temperature.
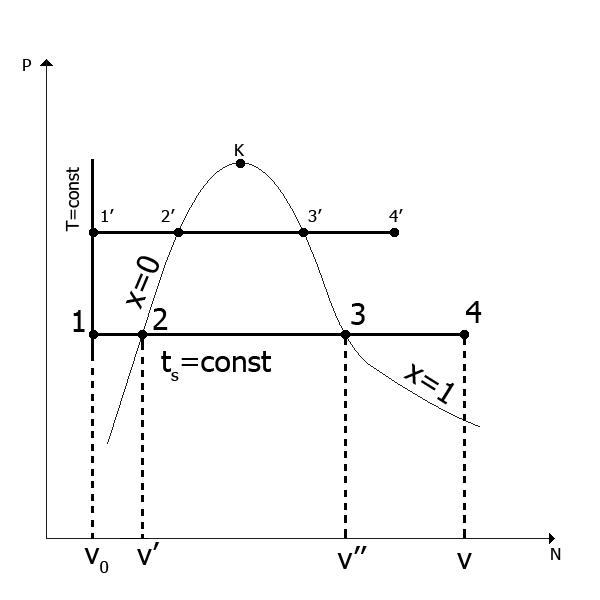
Depict the process of vaporization in p-V coordinates.
For the initial temperature of water at any pressure, take the temperature t=0°С. Thus, line I in Fig. 1 corresponds to the states of the so-called cold liquid at different pressures, which has a temperature 0°C(cold liquid isotherm). The specific volume of water at t=0°С taken equal to 0.001 m3/kg. Due to the slight compressibility of water, line I turns out to be almost a vertical straight line. To the left of this straight line is the area of equilibrium coexistence of water and ice. For the origin u, i and s for water it is customary to consider the triple point TT (p 0 =611 Pa, t 0 =0,01 0 C, v 0 =0.00100 m 3 /kg). Neglecting the influence of pressure on the change in the volume of water, it is considered for all states on line I v 0 \u003d 0.00100 m 3 / kg, u 0 =0, i 0 =0 and s 0 =0. The final state of the water in the heating stage (point b) is determined by the achievement at a given pressure of the boiling point, which depends on the pressure. From pv- diagram shows that with increasing pressure, the boiling point increases. This dependence is established empirically. The states of boiling water for various pressures will correspond to line II, which is called the lower boundary curve. It depicts the dependence of the specific volumes of boiling water on pressure. On the lower boundary curve, the degree of dryness X= 0. The parameters of boiling water are given in the tables depending on their pressure or temperature.
Further heat supply to the boiling water, which is carried out in the evaporative circuit of the steam generator, is accompanied by rapid vaporization inside the liquid and the transition of part of the water into steam. Thus, the site b-c will correspond to the equilibrium state of the mixture of liquid and steam (wet saturated steam). At each point of this process, water will be characterized by the mass fraction of dry saturated steam contained in it (degree of dryness X).
The final state in this stage is characterized by the complete transformation of the liquid into vapor, which will have a temperature equal to the saturation temperature ( t c =t n) at a given pressure. Such steam, as already mentioned, is called dry saturated steam.
Vaporization process b-c is simultaneously isobaric ( p=p 1 =const) and isothermal ( T=T 1 =const). In this case, the expended heat is not spent on raising the temperature, but only on overcoming the forces of attraction between the molecules and on the work of vapor expansion.
Considering that between the saturation temperature t n and pressure R there is an unambiguous relationship, the state of dry saturated steam will be determined by only one parameter - pressure or temperature.
The states of dry saturated steam at different pressures will correspond to line III, which is called the upper boundary curve. It is quite obvious that on the upper boundary curve at each point the degree of dryness x=1.
It should be noted that in the process of vaporization, the specific volume of water increases sharply. So, for water R= 0.1 MPa specific volume of boiling water v\u003d 0.001043 m 3 /kg, while the specific volume of dry saturated steam is 1.696 m 3 /kg. With increasing pressure, this difference also decreases at the critical point To specific volumes of water and steam are equal to 0.00326 m 3 /kg. Wherein t kr =374,15 0 FROM, a p kr=221.29 bar. At high critical pressures and temperatures, the process of vaporization is absent. There is a transition of water to steam when crossing the isobar T kr .
What is wet and dry saturated steam?
Water heated to its saturation temperature is called a saturated liquid. The mixture of liquid and vapor at the boiling point is called wet saturated steam.With further heat supply to wet saturated steam, its volume will increase, and the temperature will remain constant. There will come a moment when all the liquid will turn into vapor - dry saturated steam. The state of dry saturated steam is extremely unstable, since a slight removal of heat from it at constant pressure is associated with the transformation of dry steam into wet steam, and a slight influx of heat turns it into superheated steam.
What is steam content?
What is the heat of vaporization?
The heat of vaporization of a substance- the amount of heat required to transfer 1 mole of a substance to a state of vapor at the boiling point. Measured in Joules.
What is superheated steam?
If heat is continued to be supplied to dry saturated steam, there is a further increase in the volume of steam and its temperature - superheated steam. The state of superheated steam is relatively stable (practical use).
What happens to water at a critical point?
Critical point- a combination of temperature and pressure values at which the difference in the properties of the liquid and gaseous phases of the substance disappears (i.e., at this point, the density and other properties of liquid and gaseous water coincide). The critical point for water is reached with great difficulty at a temperature of 374.2 ° C and a pressure of 21.4 MPa. At the moment of reaching the critical point, water is characterized by extremely low viscosity, opacity, a sharp drop in the speed of propagation of sound waves and three times lower density than under normal conditions. The supercritical state is a cross between a liquid and a gas. Water in the supercritical state can compress like a gas, and at the same time, is able to dissolve solids, which is not typical for gases.
What is enthalpy? How is the internal energy of steam determined in terms of enthalpy?
Enthalpy is a function of the H state of a thermodynamic system, equal to the sum of the internal energy of the system U and the product of the pressure p and the volume V of the system.
Consequently,
In an isobaric process (p = const), the enthalpy increment is equal to the amount of heat imparted to the system.
How is the specific volume, specific enthalpy, internal energy and entropy of wet saturated steam determined?
The specific volume of wet steam vx with the degree of dryness X is determined taking into account the following conditions. If the volume of dry steam v"" and 1 kg of wet steam with a degree of dryness X contains X parts of dry steam, then the volume occupied by it is v""X. The rest (1 - X) is occupied by water, the volume of which is equal to v"(1 - X), where v" – specific volume of water. Thus, the specific volume of wet steam
v x= v"" X + v"(1 - X).
Since when 1 > X > 0 usually v"" >>v", then we can write
v x= v"" X.
Similarly, the specific enthalpy of wet steam is
h x =h" + (h"-h")x = h" + rx,
specific internal energy of wet steam
u x = u´+ (u´´-u´)x
specific entropy of wet steam
In this article, we will consider water vapor, which is the gaseous state of water.
The gaseous state refers to the three main states of aggregation of water found in nature under natural conditions. This issue is considered in detail in the material.
water vapor
Clean water vapor has no color or taste. The greatest accumulation of steam is observed in the troposphere.
Water vapor is water contained in the atmosphere in a gaseous state. The amount of water vapor in the air varies greatly; its largest content is up to 4%. Water vapor is invisible; what is called steam in everyday life (steam from breathing in cold air, steam from boiling water, etc.) is the result of the condensation of water vapor, like fog. The amount of water vapor determines the most important characteristic for the state of the atmosphere - air humidity.
Geography. Modern illustrated encyclopedia. - M.: Rosman. Under the editorship of prof. A.P. Gorkina. 2006.
How water vapor is formed
Water steam formed as a result of vaporization. Vaporization occurs as a result of two processes - evaporation or boiling. During evaporation, vapor is formed only on the surface of the substance, while boiling vapor is formed throughout the entire volume of the liquid, as evidenced by the bubbles that actively rise upward during the boiling process. Boiling water occurs at temperatures that depend on the chemical composition of the aqueous solution and atmospheric pressure, the boiling point remains unchanged throughout the process. Steam, resulting from boiling, is called saturated. Saturated steam in turn is subdivided into saturated dry and saturated wet steam. Saturated wet steam consists of suspended droplets of water, the temperature of which is at the level of boiling, and, accordingly, the steam itself, and saturated dry steam does not contain water droplets.
There is also "superheated steam", which is formed by further heating of wet steam, this type of steam has a higher temperature and lower density.
Water vapor is an indispensable element of such an important process for our planet as.
We constantly encounter steam in daily life, it appears - above the spout of the kettle when water boils, when ironing, when visiting a bath ... However, do not forget that, as we noted above, clean water vapor has no color or taste. Due to its physical properties and qualities, steam has long since found its practical application in human economic activity. And not only in everyday life, but also in solving large global problems. For a long time, steam has been the main driving force behind progress, both literally and figuratively. It was used as the working body of steam engines, the most famous of which is the steam locomotive.
Use of steam by man
Steam is still widely used in household and industrial needs:
- for hygiene purposes;
- for medicinal purposes;
- to extinguish fires;
- the thermal properties of steam are used (steam as a heat carrier) - steam boilers; steam jackets (autoclaves and reactors); heating of "freezing" materials; heat exchangers; heating systems; steaming of concrete products; in a special kind of heat exchangers ...;
- use the transformation of steam energy into motion - steam engines ...;
- sterilization and disinfection - food industry, agriculture, medicine ...;
- steam as a humidifier - in the production of reinforced concrete products; plywood; in the food industry; in the chemical and perfume industries; in woodworking industries; in agricultural production ...;
Summing up, we note that, despite all its "invisibility", water vapor is not only an important element of the Earth's global ecosystem, but also a very useful substance for human economic and economic activities.
At the word "steam", I remember the times when I was still in elementary school. Then, coming home from school, the parents would start preparing dinner and put a pot of water on the gas stove. And after ten minutes, the first bubbles began to appear in the saucepan. This process has always fascinated me, it seemed to me that I could look at it forever. And then, some time after the appearance of the bubbles, the steam itself began to flow. Once, I asked my mother: "Where are these white clouds coming from?" (That's what I used to call them). To which she answered me: "It all happens because of the heating of the water." Although the answer did not give a complete picture of the process of steam formation, in the lessons of school physics I learned everything I wanted about steam. So...
What is water vapor
From a scientific point of view, water vapor is simply one of the three physical states of water itself. It is known to occur when water is heated. Like herself, steam has no color, no taste, no smell. But not everyone knows that steam clubs have their own pressure, which depends on its volume. And it is expressed in Pascals(in honor of the notorious scientist).
Water vapor surrounds us not only when we cook something in the kitchen. It is constantly contained in the street air and atmosphere. And its content percentage is called "absolute humidity".

Facts about water vapor and its features
So here are some interesting points:
- the higher the temperature, which acts on water, the faster the evaporation process;
- Besides, evaporation rate increases with area size the surface on which the water is located. In other words, if we start heating a small layer of water on a wide metal cup, then the evaporation will take place very quickly;
- Plants need not only liquid water, but gaseous water as well.. This fact can be explained by the fact that vapors are constantly coming from the leaves of any plant, cooling it. Try to touch a leaf of a tree on a hot day - and you will notice that it is cool;
- the same applies to humans, the same system works with us as with plants above. Evaporation cools our skin on a hot day. Surprisingly, even with small loads, our body leaves about two liters of fluid per hour. What can we say about increased loads and hot summer days?

This is how you can describe the essence of steam and its role in our world. I hope you have discovered a lot of interesting things!
Water vapor is used as a working fluid in various processes, for example, to rotate a steam turbine.
Steam is usually produced by boiling a liquid. If heat is supplied to a liquid at constant pressure, the temperature of the liquid rises to a certain value Tbp. With further heating, the temperature remains constant - steam is formed during the boiling of the liquid.
Boiling- this is the process of vaporization in the bulk of the liquid, while evaporation occurs only from the free surface of the liquid, provided that the partial vapor pressure over the liquid is less than the saturated vapor pressure.
The boiling process in the p - V diagram is depicted isobar, which is also isotherm.
Specific heat of vaporization (r, J/kg) - is the amount of heat required to convert 1 kg of liquid into vapor.
According to the first law of thermodynamics:
q = r = (u // - u /) + p(v // - v /), (69)
where u // , v // - specific internal energy and specific volume of dry saturated steam; u / , v / – the same for water.
(u // - u /) is the change in internal energy associated with overcoming the forces of attraction between molecules during the transition of liquid into vapor. p(v // - v /) is the work of steam expansion.
Steam can be wet, dry, saturated and overheated.
Wet steam is a mixture of boiling liquid and dry saturated steam. The ratio of the mass of vapor to the mass of the mixture is called the degree of dryness of the steam.
Another value (1-x) is also used, called degree of steam humidity:
![]() (71)
(71)
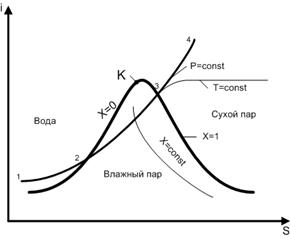 To analyze the process of vaporization and determine the parameters of water vapor, i - S diagrams of water vapor are used.
To analyze the process of vaporization and determine the parameters of water vapor, i - S diagrams of water vapor are used.
|
The main curve on the i–S diagram is saturation curve with a critical point indicated on it To, which divides the curve into two lines. To the left of point K is the boiling line of water. On this line, x = 0, that is, there is no steam. To the right of the critical point is the condensation line, for which x = 1, which corresponds to the absence of water. The saturation curve, together with the critical point on it, divides the entire diagram into three regions. Under the saturation curve there is an area of wet steam with a degree of dryness of 0< x < 1. Над кривой насыщения слева от точки К имеет место состояние воды. Справа от критической точки над кривой насыщения расположена область сухого пара. Кроме кривой насыщения на i – S диаграмме проводятся изобары (p = const), изотермы (t = const) и линии постоянной степени сухости пара (x = const). Под кривой насыщения изотермы и изобары совпадают.
Consider the process of heating water at constant pressure, moving along the isobar 1-2-3-4 (Fig. 12). In section 1-2, water is heated to the boiling point. The amount of heat that needs to be supplied for this is determined by the difference between the enthalpies i 2 and i 1:
q water \u003d i 2 - i 1
In section 2-3, water boils at a constant temperature. The enthalpy difference here determines the specific heat of vaporization:
In section 3-4, dry steam is overheated. The temperature in this area is rising. The increase in enthalpy determines the heat costs for steam overheating:
q overheating = i 4 - i 3
i - S diagram allows you to determine the total cost of heat required to produce superheated steam:
q = q water + r + q superheat = ∆i water + ∆i steam + ∆i overheating
End of work -
This topic belongs to:
Basic concepts of thermodynamics. The subject of thermodynamics. Basic parameters of the state of a thermodynamic system
On the website site read: Lecture notes Discipline according to the curriculum of the direction of preparation: 260901 Technology of garments. Omsk CONTENTS...
If you need additional material on this topic, or you did not find what you were looking for, we recommend using the search in our database of works:
What will we do with the received material:
If this material turned out to be useful for you, you can save it to your page on social networks:
| tweet |
All topics in this section:
History reference
Thermodynamics as a science began to develop starting from the 18th century after the appearance of the first steam engines. In 1824, the French engineer Sadi Carnot published the first work on the theory of thermal
Energy of a thermodynamic system
The total energy of a system is the sum of its internal and external energy, which is mechanical energy. E = U + Emech. mechanical energy
State equations
The functional relationship between the parameters of the state of a thermodynamic system - pressure p, volume V and temperature T - is called the equation of state. This one for
Ideal gas equation of state
An ideal gas is a gas that consists of molecules that have negligible dimensions, the interaction forces between which can be neglected. The equation
Laws of thermodynamics
The first law (first law) of thermodynamics. This is the law of conservation of energy as applied to thermodynamic processes. It is formulated as follows: The amount of heat
Total Differential Condition
It is known from mathematical analysis that the differential of a function of several variables F(x1, x2, x3, ...) is expressed as:
Reversible and irreversible processes
The definition of reversible and irreversible processes is associated with the concepts of equilibrium and non-equilibrium processes. Since equilibrium processes are ideal processes that do not really exist in nature, for
Conditions of existence and properties of equilibrium processes.
1. Infinitely small difference between acting and opposing forces. 2. Making maximum work in the direct process. 3. The infinitely slow course of the process associated with infinite
Specific heat capacity of gases
It has been experimentally established that the amount of heat required to heat the body is proportional to the body mass and the difference between the final and initial temperatures. Q ~ m (T2 - T1
Relationship between heat capacities at constant pressure and constant volume.
Let's take the internal energy as a function of volume and temperature: U = f (V, T) Let's write the total differential of this function
From the first law of thermodynamics
δQ = δL = p dV An adiabatic process is a process without heat exchange with the environment. (δQ = 0) From the first law of thermodynamics: δQ = m
Second law of thermodynamics
The first law of thermodynamics allows solving many thermodynamic problems. However, he does not consider the question of the direction of ongoing processes. From the point of view of the first law, any
Entropy calculation. Gibbs paradox.
Let us write down from expression (48) the expression for the entropy differential: (48) From the equation of state id
The second law of thermodynamics for non-static processes
The existence of a single-valued state function, entropy, in an equilibrium system expresses the second law of thermodynamics for quasi-static processes. Let us formulate this law in relation to the nonstatic
Third law of thermodynamics
When bodies are heated and when the state of aggregation changes from solid → liquid → gaseous entropy increases. Consequently, the minimum entropy will have a body in the solid state at
Thermal cycles
For continuous production of useful work in heat engines, it is necessary to have periodic stages of expansion of the working fluid. This is possible only if during the operation of the heat engine the working fluid