What does "critical point" mean?
What are the critical points of steel
Critical points of steel or Chernoff points are critical temperatures at which a change in the phase state and structure of steel occurs when it is heated or cooled in solid form. Established by Dmitry Konstantinovich Chernov in 1868.
Critical points are denoted by the letter A. The lower critical point corresponds to the PSK line of the iron-carbon state diagram. This point is called A1 and corresponds to the transformation of austenite into pearlite upon cooling or pearlite into austenite upon heating. The upper critical point is called A3. The critical point A3 for hypoeutectoid steels lies on the line GS of the iron-carbon diagram and corresponds to the beginning of ferrite precipitation upon cooling or the end of its dissolution upon heating. The critical point A3 for hypereutectoid steels lies on the SE line and corresponds to the beginning of secondary cementite precipitation upon cooling or the end of its dissolution upon heating.
Depending on whether the critical point is determined during heating or cooling, the index “c” is added to the letter A during heating (from the French word chauffage - heating) and the index “r” (from the French word refroidissement - cooling) during cooling, leaving the number characterizing this transformation.
Thus, for example, the heating of hypoeutectoid steel above the corresponding point on the GS line is denoted as heating above the point Ac3. When this steel is cooled, the first transformation should be designated as Ar3, the second (on the PSK line) - as Ar1. Point A3 for hypereutectoid steels is usually denoted Acm.
Dot Mn in the table denotes the temperature of the beginning of martensitic transformation.
In the heat treatment of steels, critical point values are most often used to determine the heating temperature for hardening.
How to choose the heating temperature for hardening
For hypoeutectoid steels (carbon content in steel is less than 0.8%), the quenching temperature is usually chosen according to the formula Ac3+30...50°C. In practice, there are cases when hypoeutectoid steels are hardened from the temperature range between Ac1 and Ac3. In this case, the steel structure will consist of martensite and undissolved ferrite. Such a process is called incomplete hardening and is theoretically a marriage. In practice, such a scheme is used to reduce warping of parts or to eliminate cracking in high-alloy steels.
Hypereutectoid steels are usually heated for hardening to temperatures Ac1+30...50°C. After hardening, the structure of the steel will be composed of martensite and secondary undissolved cementite, which increases the hardness and wear resistance of products.
critical point
depicts the critical state of matter on state diagrams. The critical point ends, for example, the liquid-vapour phase equilibrium curve in the coordinate system: temperature T, pressure p.
Critical point
point on the state diagram corresponding to critical condition. K. t. two-phase equilibrium liquid ≈ vapor is the end point on the equilibrium curve and is characterized by critical values of temperature Tk, pressure pk and volume Vk (table). K. t. is special case points phase transition and is characterized by a loss of thermodynamic stability in terms of density or composition of the substance. On one side of the C. t., the substance is homogeneous (usually at T > Tk), and on the other, it separates into phases. For mixtures or solutions, one should distinguish between the equilibrium temperature of liquid ≈ vapor and the equilibrium temperature of phases different composition located in one state of aggregation(liquid ≈ liquid, gas ≈ gas). In this regard, K. t. mixtures (solutions) is additionally characterized by a critical concentration of cc. As a result of an increase in the number of parameters that determine the state of the system, mixtures do not have a critical temperature, but a critical curve, the points of which differ in the values of Tk, pk, Vk, and xk. In the neighborhood of K, m, critical phenomena are observed. Critical point parameters liquid ≈ vapor of some substances
Substance
Oxygen
carbon dioxide
Ethanol)
* 1 amm = 1.01×105 n/m2.
Wikipedia
Critical point
Critical point- ambiguous term:
- A critical point is a point where the derivative is either zero or undefined.
- The critical point is the temperature at which the two phases are in equilibrium.
- "Critical point" - Program of investigative journalism on TV channel Ukraine
Critical point (thermodynamics)
Critical phase transition temperature is the temperature value at the critical point. Above the critical temperature, the gas cannot be condensed at any pressure.
Critical point (mathematics)
critical point differentiable function $f:D\to\R$, where D- the area in $\R^n$, is the point at which all its partial derivatives vanish. This condition is equivalent to the vanishing of the differential of the function at a given point, and is also equivalent to the horizontality of the tangent hyperplane to the graph of the function. This condition is necessary for an interior point of the region to be a point of local minimum or maximum of a differentiable function.
The value of the function at the critical point is called critical. According to Sard's lemma, the set of critical values of any C-smooth function $f: \to\R$ has zero Lebesgue measure (although there can be as many critical points in this case, for example, for the function f = const any point is critical).
The notion of a critical point can be generalized to the case of differentiable mappings $f:\R^n\to\R^m$, and to the case of differentiable mappings of arbitrary manifolds f : N → M. In this case, the definition of a critical point is that the rank of the Jacobian matrix of the mapping f in it is less than the maximum (equal to the number min( n, m}).
Critical points of functions and mappings play an important role in areas of mathematics such as differential equations, calculus of variations, stability theory, as well as in mechanics and physics. The study of critical points of smooth mappings is one of the main questions of catastrophe theory.
The notion of a critical point is also generalized to the case of functionals defined on infinite-dimensional function spaces. The search for critical points of such functionals is an important part of the calculus of variations. Critical points of functionals are called extremals.
The phase equilibrium curve (in the plane P, T) may end at some point (Fig. 16); such a point is called critical, and the temperature and pressure corresponding to it are called critical temperature and critical pressure. At higher temperatures and higher pressures, there are no different phases, and the body is always homogeneous.
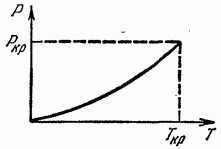
It can be said that at the critical point the difference between the two phases disappears. The concept of a critical point was first introduced by D. I. Mendeleev (1860).
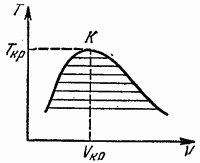
In the coordinates T, V, the equilibrium diagram in the presence of a critical point looks like it is shown in Fig. 17. As the temperature approaches its critical value, the specific volumes of the phases that are in equilibrium with each other approach each other and at the critical point (K in Fig. 17) coincide. A diagram in the coordinates Р, V has a similar form.
In the presence of a critical point between any two states of matter, a continuous transition can be made, in which at no time does separation into two phases occur - for this, the state must change along some curve that envelops the critical point and does not intersect the equilibrium curve anywhere. In this sense, in the presence of a critical point, the very concept of different phases becomes conditional, and it is impossible in all cases to indicate which states are one phase and which are another. Strictly speaking, one can speak of two phases only when they both exist simultaneously, touching each other, i.e., at points lying on the equilibrium curve.
It is clear that a critical point can exist only for such phases, the difference between which has only a purely quantitative character. Such are liquid and gas, differing from each other only in a greater or lesser role of interaction between molecules.
The same phases as liquid and solid(crystal) or various crystalline modifications of a substance are qualitatively different from each other, as they differ in their internal symmetry. It is clear that any property (element) of symmetry can only be said either that it exists or that it does not exist; it can appear or disappear only at once, abruptly, and not gradually. In each state, the body will have either one or the other symmetry, and therefore it is always possible to indicate which of the two phases it belongs to. A critical point, therefore, cannot exist for such phases, and the equilibrium curve must either go to infinity or end, intersecting with the equilibrium curves of other phases.
The usual phase transition point does not mathematically represent a singularity for the thermodynamic quantities of a substance. Indeed, each of the phases can exist (at least as a metastable one) on the other side of the transition point; thermodynamic inequalities are not violated at this point. At the transition point chemical potentials both phases are equal to each other: ; for each of the functions, this point is unremarkable.
Let us depict in the plane P, V any isotherm of liquid and gas, i.e., the curve of the dependence of P on V during isothermal expansion of a homogeneous body in fig. eighteen). According to the thermodynamic inequality, there is a decreasing function V. Such a slope of the isotherms must also persist for some distance beyond the points of their intersection with the equilibrium curve of liquid and gas (points b and sections of the isotherms correspond to metastable superheated liquid and supercooled steam, in which thermodynamic inequalities are still observed (the completely equilibrium isothermal change of state between points b does not, of course, correspond to a horizontal segment on which separation into two phases occurs).
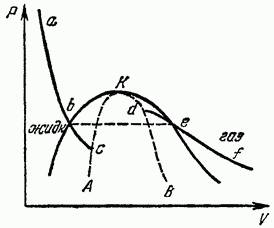
If we take into account that the points have the same ordinate P, then it is clear that both parts of the isotherm cannot go into each other in a continuous way, and there must be a gap between them. The isotherms end at points (c and d) at which the thermodynamic inequality is violated, i.e.
Having constructed the locus of endpoints of the liquid and gas isotherms, we obtain the AKB curve, on which thermodynamic inequalities are violated (for a homogeneous body); it limits the region in which the body under no circumstances can exist as homogeneous. The regions between this curve and the phase equilibrium curve correspond to superheated liquid and supercooled vapor. Obviously, at the critical point, both curves must touch each other. Of the points lying on the AKB curve itself, only the critical point K corresponds to the actually existing states of a homogeneous body - the only one at which this curve comes into contact with the region of stable homogeneous states.
In contrast to the usual points of phase equilibrium, the critical point is mathematically a singular point for the thermodynamic functions of matter (the same applies to the entire AQW curve, which limits the region of existence of homogeneous states of the body). The nature of this singularity and the behavior of matter near the critical point will be considered in § 153.






