What is the saturation vapor pressure. Dependence of pressure of saturated vapor on temperature. Boiling
Topic: Fundamentals of molecular kinetic theory
Lesson: Pressure dependency saturated steam from temperature. Boiling
In the previous lessons, we introduced the concept of an ideal gas as a model in which all gas laws that we have studied. However, this does not mean that molecular physics and, in particular, molecular-kinetic theory is limited to the study of only ideal gases. For real gases, our calculations on the topic "fundamentals of molecular-kinetic theory", of course, are valid. However, the relationship between the parameters of real gases is expected to have a slightly different form than this relationship for ideal gases.
Consider such a real gas as saturated steam. Recall that just the default ferry is called gaseous state a certain substance (most often, when they say “steam”, they mean exactly water vapor). Saturated steam means the following:
Definition. Saturated steam A vapor in dynamic equilibrium with its liquid. That is, the number of liquid molecules leaving the liquid over a certain period of time is, on average, equal to the number of vapor molecules returning back to the liquid (see Fig. 1). There is always a region of saturated vapor above any liquid surface. In order to create a wider area, the vapor molecules must be prevented from escaping into the environment (hermetically close the vessel).
To understand the differences between saturated steam and an ideal gas, you need to imagine two experiments.
First, let's take a hermetically sealed vessel with water and start heating it. As the temperature increases, the liquid molecules will have an increasing kinetic energy, and an increasing number of molecules will be able to escape from the liquid (see Fig. 2), therefore, the vapor concentration will increase and, consequently, its pressure. So the first position:

Rice. 2. T 2 > T 1
However, this provision is quite expected and not as interesting as the following. If you place a liquid with saturated steam under the movable piston and start lowering this piston, then, undoubtedly, the concentration of saturated steam will increase due to a decrease in volume. However, after some time, the vapor will move with the liquid to a new dynamic equilibrium by condensing an excess amount of vapor, and the pressure will not change in the end. The second position of the theory of saturated steam:
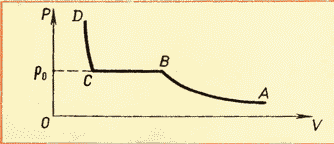
Now, it should be noted that the saturated vapor pressure, although it depends on temperature, like an ideal gas, but the nature of this dependence is somewhat different. The fact is that, as we know from the basic equation of the MKT, the gas pressure depends on both temperature and gas concentration. And therefore, the pressure of saturated vapor depends on temperature non-linearly until the vapor concentration increases, that is, until all the liquid has evaporated. The graph below (Fig. 3) shows the nature of the dependence of saturated vapor pressure on temperature,

Rice. 3
moreover, the transition from a non-linear section to a linear one just means the point of evaporation of the entire liquid. Since the pressure of a saturated gas depends only on temperature, it is possible to absolutely unambiguously determine what the saturated vapor pressure will be at a given temperature. These ratios (as well as the values of the density of saturated steam) are listed in a special table.
Let us now turn our attention to the important physical process like boiling. In the eighth grade, boiling was already defined as a process of vaporization more intense than evaporation. Now we will expand this concept somewhat.
Definition. Boiling- the process of vaporization occurring throughout the volume of the liquid. What is the boiling mechanism? The fact is that there is always dissolved air in water, and as a result of an increase in temperature, its solubility decreases, and microbubbles form. Since the bottom and walls of the vessel are not perfectly smooth, these bubbles cling to the irregularities on the inside of the vessel. Now the water-air section exists not only at the surface of the water, but also inside the volume of water, and water molecules begin to pass into the bubbles. Thus, saturated steam appears inside the bubbles. Further, these bubbles begin to float, increasing in volume and taking more water molecules into themselves, and burst near the surface, releasing saturated steam into the environment (Fig. 4).
Rice. 4. Boiling process ()
The condition for the formation and ascent of these bubbles is the following inequality: the saturated vapor pressure must be greater than or equal to atmospheric pressure.
Thus, since the saturation vapor pressure depends on the temperature, the boiling point is determined by the pressure environment: the smaller it is, the lower temperature the liquid boils, and vice versa.
In the next lesson, we will begin to consider the properties of rigid bodies.
Bibliography
- Myakishev G.Ya., Sinyakov A.Z. Molecular physics. Thermodynamics. - M.: Bustard, 2010.
- Gendenstein L.E., Dick Yu.I. Physics grade 10. - M.: Ileksa, 2005.
- Kasyanov V.A. Physics grade 10. - M.: Bustard, 2010.
- Physics.ru ().
- Chemport.ru ().
- Narod.ru ().
Homework
- Page 74: No. 546-550. Physics. Task book. 10-11 grades. Rymkevich A.P. - M.: Bustard, 2013. ()
- Why can't climbers boil eggs at altitude?
- What are some ways you can cool hot tea? Justify them in terms of physics.
- Why should the gas pressure on the burner be reduced after boiling water?
- * How can water be heated above one hundred degrees Celsius?
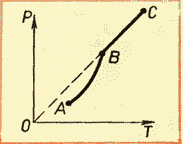
Familiarity with the isotherms of a real gas at various temperatures (see Fig. 6.4) allows us to conclude that that the saturation vapor pressure increases with increasing temperature. Since the saturation vapor pressure does not depend on volume, it therefore depends only on temperature. However, dependence p(T), found experimentally, is not proportional, as in an ideal gas at constant volume(Charles law). With increasing temperature, the pressure of saturated vapor increases faster than the pressure of an ideal gas (Fig. 6.5, section of the curve AB). This becomes especially obvious if we draw an isochore through the point BUT(dashed line). Why is this happening?
When a liquid is heated with steam in a closed vessel, part of the liquid turns into steam. As the temperature rises, the evaporation rate increases and the equilibrium between liquid and vapor is disturbed. The concentration of molecules and, consequently, the vapor density increase. This continues until the vapor density increases so much that the condensation process balances the evaporation process. As a result, according to the formula p =nkT the pressure of saturated steam increases not only due to an increase in temperature, but also due to an increase in the concentration of molecules (density) of the steam. In this case, the main role in increasing the pressure of saturated vapor is played by an increase in the concentration of vapor molecules, and not an increase in its temperature.
The main difference in the behavior of an ideal gas and saturated steam is that when the temperature of the vapor in a closed vessel changes (or when the volume of the vapor changes at a constant temperature), the mass of the vapor changes. The liquid partially turns into vapor or, conversely, the vapor partially condenses. FROM ideal gas nothing like that happens.
When all the liquid evaporates, the vapor, upon further heating, will cease to be saturated and its pressure at constant volume will increase in direct proportion to the absolute temperature in accordance with Charles's law (see Fig. 6.5, section BC).
Isotherms of a real gas, obtained experimentally, describe the state of the gas, the equilibrium between gas and liquid, and the liquid state. They can be used to trace the dependence of saturated vapor pressure on temperature.
§ 6.4. critical temperature. Critical situation
The substance may be in liquid state not at any temperature. There is a limit.
Critical temperature
At sufficiently high temperatures, the horizontal section of the isotherm of a real gas (see Fig. 6.4) becomes very short and at a certain temperature turns into a point (in Fig. 6.4 - point TO). This temperature is called critical. The critical temperature is the temperature at which differences in physical properties between a liquid and a vapor in dynamic equilibrium with it. Each substance has its own critical temperature. For example, the critical temperature for carbon dioxide CO 2 is t to = 31 °С, and for water - t to = 374 °С.
Critical situation
State corresponding to a point TO, into which the horizontal section of the isotherm turns at a temperature T = T to , called the critical state (critical point). The pressure and volume in this state are called critical. The critical pressure for carbon dioxide is 7.4 10 6 Pa (73 atm), and for water 2.2 10 7 Pa (218 atm). In the critical state, the liquid has a maximum volume, and the saturated vapor- maximum pressure.
Dependence of pressure of saturated vapor on temperature. The state of saturated vapor is approximately described by the equation of state of an ideal gas (3.4), and its pressure is approximately determined by the formula
As the temperature rises, the pressure rises. Since the saturation vapor pressure does not depend on volume, it therefore depends only on temperature.
However, this dependence found experimentally is not directly proportional, as in an ideal gas at constant volume. With increasing temperature, the pressure of saturated vapor increases faster than the pressure of an ideal gas (Fig. 52, section of the curve AB).
This happens for the following reason. When a liquid is heated with steam in a closed vessel, part of the liquid turns into steam. As a result, according to formula (5.1), the vapor pressure increases not only due to an increase in temperature, but also due to an increase in the concentration of molecules (density) of the vapor. The main difference in the behavior of an ideal gas and saturated steam is that when the temperature of the vapor in a closed vessel changes (or when the volume changes at constant temperature) the mass of the vapor changes. The liquid partially turns into vapor or, conversely, the vapor partially condenses. Nothing like this happens with an ideal gas.
When all the liquid evaporates, the vapor, upon further heating, will cease to be saturated and its pressure at constant volume will increase in direct proportion to absolute temperature(Section BC in Figure 52).
Boiling. The dependence of saturation vapor pressure on temperature explains why the boiling point of a liquid depends on pressure. When boiling, rapidly growing vapor bubbles form throughout the volume of the liquid, which float to the surface. Obviously, a vapor bubble can grow when the pressure of the saturated vapor inside it slightly exceeds the pressure in the liquid, which is the sum of the air pressure on the surface of the liquid (external pressure) and the hydrostatic pressure of the liquid column.
Boiling begins at a temperature at which the saturation vapor pressure in the bubbles is equal to the pressure in the liquid.
The greater the external pressure, the higher the boiling point. Thus, at a pressure in a steam boiler reaching Pa, water does not boil even at a temperature of 200°C. In medical institutions, boiling water in hermetically sealed vessels - autoclaves (Fig. 53) - also occurs at elevated pressure. Therefore, the boiling point is much higher than 100°C. Autoclaves are used to sterilize surgical instruments, dressings, etc.
Conversely, by reducing the pressure, we thereby lower the boiling point. By pumping out air and water vapor from the flask, you can make the water boil at room temperature (Fig. 54). When climbing mountains Atmosphere pressure decreases. Therefore, the boiling point decreases. On high


7134 m (Lenin Peak in the Pamirs) the pressure is approximately equal to Pa (300 mm Hg). The boiling point of water there is about 70 °C. It is impossible to cook, for example, meat under these conditions.
The difference in the boiling points of liquids is determined by the difference in their pressure saturated vapors. The higher the saturated vapor pressure, the lower the boiling point of the corresponding liquid, since at lower temperatures the saturated vapor pressure becomes equal to atmospheric pressure. For example, at 100 ° C, the pressure of saturated water vapor is (760 mm Hg), and mercury vapor is only 117 Pa (0.88 mm Hg). Mercury boils at 357°C at normal pressure.
critical temperature. With an increase in temperature, simultaneously with an increase in the pressure of saturated vapor, its density also increases. The density of a liquid in equilibrium with its vapor, on the contrary, decreases due to the expansion of the liquid when heated. If in one figure we draw curves for the dependence of the density of a liquid and its vapor on temperature, then for the liquid the curve will go down, and for steam it will go up (Fig. 55).
At a certain temperature, called the critical temperature, both curves merge, i.e., the density of the liquid becomes equal to the density of the vapor.

The critical temperature is the temperature at which the differences in physical properties between the liquid and its saturated vapor disappear.
At the critical temperature, the density (and pressure) of saturated vapor becomes maximum, and the density of the liquid in equilibrium with vapor becomes minimum. Specific heat vaporization decreases with increasing temperature and becomes zero at the critical temperature.
Each substance has its own critical temperature. For example, the critical temperature of water, while liquid carbon monoxide (IV)






