Heat engine efficiency
cycles. Thermal and refrigeration machines. The efficiency of the Carnot cycle for ideal gas
A cyclic (circular) process or cycle is a process in which the initial and final states of the system coincide. In the cycle shown in Fig. 8.1, in the section 1-a-2, the system does positive work, and returning to its original state along the path 2-b-1 - negative, but smaller in absolute value. Wherein full work completed per cycle is positive. It is equal to the area of the figure 1-a-2-b-1 covered by the cycle on P-V diagram.
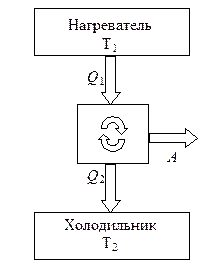 |
|
| Rice. 8.1 | Rice. 8.2 |
Since the internal energy U is a state function, its change in the cyclic process is zero (D U= 0). Then it follows from the first law of thermodynamics that the total work BUT, performed by the system per cycle, is equal to the total amount of heat Q received by the system in a cycle. If the cycle work BUT is positive, we say that the cycle is traversed in forward direction(clockwise). Such cyclical processes can be used to create heat engines - devices that perform mechanical work due to heat received from thermal reservoirs.
The heat engine includes a working body, i.e. a system that performs a cycle and performs work, and at least two heat reservoirs with which the working fluid exchanges heat.
Protozoa heat engine shown schematically in Fig. 8.2. Thermal reservoirs from which the working fluid in a direct cycle (at which BUT> 0) receive a positive amount of heat are called heaters. Tanks from which a negative amount of heat is received are called refrigerators. The sum of positive amounts of heat received by the system from heaters at all stages of the cycle is usually denoted Q + = Q 1 , and the sum of negative heat received from refrigerators Q - = - Q 2. Wherein Q 2 is called the amount of heat given off by the system to the refrigerator ( Q 2 > 0).
The work of the cycle is equal to the algebraic sum of the amounts of heat received by the system at all stages of the cycle
A = Q + + Q - = Q 1 – Q 2 .
Coefficient useful action(efficiency) of a cycle is the ratio of work A performed by the system during the passage of the cycle to the amount of heat Q 1 º Q + received by the system from the heater
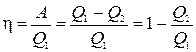 . (8.1)
. (8.1)
The efficiency h calculated in this way is sometimes called the thermodynamic efficiency to emphasize its difference from the technical efficiency, which is always lower due to various losses that accompany the operation of real machines.
If the direction of the cycle bypass is reversed, then the work and the amount of heat at all its stages will change sign. Such a cycle is called reverse. When going through the reverse cycle, complete work BUT arr, perfect by the working body, is negative BUT arr = - BUT(external forces acting on the system do positive work BUT). The system receives a positive amount of heat from the refrigerator and releases heat to the heater.
Refrigerating machines operate on the reverse cycle. They consume mechanical energy, take away heat from a relatively cold body and transfer heat to a warmer body. If the purpose of the machine is to heat a warmer body (for example, raising the temperature of the air in the room due to the heat taken from the outside air), it is called a heat pump. Its efficiency is determined by the performance of the heat pump x T.N, which is equal to the ratio of the amount of heat received by the heated body to the work expended on this
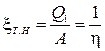 .
.
The useful effect exceeds the work expended, x T..N> 1, but there is, of course, no violation of the law of conservation of energy here. The work of external forces does not turn into heat, but provides a “pumping” of heat from a less heated body to a hotter one.
If the task is to take heat from a colder body, the machine is called a refrigeration unit. Its efficiency is characterized by the refrigeration coefficient x X, equal to the ratio of the extracted heat to the work expended
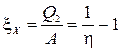 .
.
x coefficients T.N and x X are mainly used in technical applications of thermodynamics.
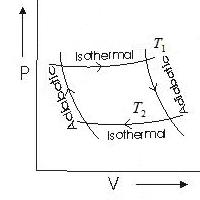
Of the various cyclic processes, the Carnot cycle is of particular importance in thermodynamics. It consists of two isotherms (a-b and c-d) and two adiabats (b-c and d-a) (Figure 8.3).
 Rice. 8.3 Rice. 8.3 |
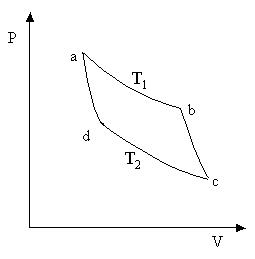
Let us find the efficiency of the Carnot cycle, the working substance of which is one mole of an ideal gas. On the section a-b the working fluid is in thermal contact with a heater having a temperature T one . Produced quasi-static isothermal expansion of the volume V a to volume V b. In this case, the gas receives from the heater the amount of heat Q ab. Since the internal energy of an ideal gas depends only on temperature and does not change during an isothermal process, then, according to the first law of thermodynamics,
On the stage b-c an adiabatic ( Q bc = 0) gas expansion. As a result, its temperature drops. When it reaches refrigerator temperature T 2 , the gas is brought into thermal contact with the refrigerator, and the process of isothermal c-d compression. The gas does negative work. BUT cd and gets a negative amount of heat Q cd (gives a positive amount of heat to the refrigerator)
From the consideration of the stages of the cycle, it can be seen that the gas receives a positive amount of heat only at section a-b, i.e. Q 1 = Q ab. The gas receives a negative amount of heat in the section c-d, which means that the heat given off to the refrigerator Q 2 =- Q cd . Then the cycle efficiency
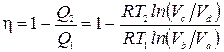 .
.
We write the adiabatic equations b-c and d-a, given that T a = T b= T 1 , T c= T d= T 2 ,
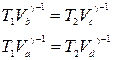 .
.
Dividing one equation by another, we get ![]() . Then the efficiency of the Carnot cycle for an ideal gas
. Then the efficiency of the Carnot cycle for an ideal gas
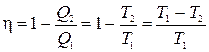 . (8.2)
. (8.2)
Note that from the expression for the efficiency, written as , it follows
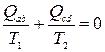 . (8.3)
. (8.3)
Relation (8.3) is special case the Clausius equalities (for details, see § 13).
We, as ordinary people, rarely think about how heat engines work, and even more so - we do not try to understand the essence of what is happening inside these same engines from the point of view of thermodynamics. The average knowledge of mechanics and technicians is limited to the fact that, it seems, something inside burns out, and thanks to this, the pistons begin to move (in the common people - "piston"), rotating other parts and, as they say, "the process has begun."
But, as always, among the human race-tribe there are the most meticulous representatives who just need to know how everything really happens and what everything depends on. Probably, on these "meticulous" and "ubiquitous", as on the branches of trees, the fruits that feed science grow.
So, let's try to figure it out - how does a heat engine work, and what determines its efficiency?
A bit of theory.
A heat engine is a machine that converts heat energy into mechanical energy. That is, inside these mechanisms, which are a system, something begins to rotate, move and somersault, if the temperature inside this system is somehow changed (as a rule, heat is supplied to the working fluid, which is most often a gas for a number of "good" reasons).
Well, a little more - all engines are divided, by and large, into internal combustion engines and external combustion engines.
For the former, heat is supplied to the element of the system inside the engine, for the latter, somewhere outside. Looking ahead, let's give an example: external combustion engines include, in particular, steam engines, in which heat to the working fluid (ice, water or steam or any liquid) supplied outside the engine, by burning some kind of fuel (coal, fuel oil, firewood, etc.) in a separately located furnace under the tank (boiler) with the working fluid. Then the heated working fluid is introduced into the heat engine (enters the cylinder), and does useful work, while giving off heat.
Internal combustion engines (ICE) include (for example) diesel engines and carburetor engines familiar to everyone since childhood, in which the working fluid is burned and generates heat inside the system (in the cylinder).
In both cases we are talking about about thermodynamic processes, i.e. processes that cause temperature fluctuations (or caused by temperature fluctuations) inside the system.
In the general case, the essence of what is happening from the point of view of modern thermodynamics is described.
AT early XIX centuries, the talented French engineer Sadi Carnot (1796-1832) studied the thermodynamic processes that take place in heat engines using an ideal gas as a working fluid. At the same time, all processes in machines were considered by him as equilibrium (reversible).
Reversible process- this is a process that proceeds so slowly that it can be considered as a successive transition from one equilibrium state to another, etc., and the whole process can be carried out in reverse direction without changing the work done and the amount of heat transferred. (It should be noted that all real processes are irreversible).
The purpose of Carnot's research was to determine the conditions under which it is possible to obtain the maximum work from the heat supplied to the heat engine, i.e., to convert thermal energy into mechanical energy most efficiently.
At the end of the 18th - beginning of the 19th century, the only type of heat engines used by mankind for practical purposes were external combustion engines - that is, steam engines. The efficiency of these machines was extremely low - no more 2 %
, while there was no convincing theory indicating ways to improve their efficiency.
Carnot conducted a thorough analysis of the various ways of converting heat into work on the example of an idealized model of a reciprocating steam engine, while the results and conclusions drawn by him turned out to be valid for any type of machine that uses thermal energy to perform mechanical work.
As a result of theoretical conclusions, Carnot came to the conclusion that the maximum effect from the conversion of heat into mechanical energy can be achieved using circular cycle, consisting of four successive processes - isothermal, adiabatic, isothermal and again adiabatic, which completed the cycle, returning the system to its original state.
This sequence thermodynamic processes in a heat engine called ideal carnot cycle.
It is impossible to manufacture a real engine that converts heat energy into mechanical energy strictly according to the cycle proposed by Carnot for technological reasons, therefore the Carnot cycle is considered unfeasible and ideal.
Nicolas Leonard Sadie Carnot considered one of the founders of thermodynamics. At the age of 28, he wrote the only work that has come down to posterity - “Reflections on the driving force of fire and on machines capable of developing this force”, in which he outlined fundamentally new views for that time on processes in heat engines, which were reflected in the second law of thermodynamics.
Sadi Carnot introduced the basic concepts of thermodynamics into scientific terminology - an ideal heat engine, an ideal cycle, reversibility and irreversibility of thermodynamic processes.
At the beginning of the 19th century, only primitive steam engines were used, the efficiency of which did not exceed a few percent, since there was no theory that could explain how to increase the efficiency of using thermal energy in engines. Carnot's work served as the first guide for engineers to find the efficient use of heat in engines.
Carnot died very young, at the age of 36 from cholera.
Since in those years cholera was considered a terrible and incurable disease, the bodies and belongings of the dead were supposed to be burned. Surely many valuable works of this most talented engineer perished in the fire. Miraculously, only the famous “Reflections on driving forces fire…”, which this very fire, which destroyed all other works of Carnot and his lifeless body, regretted…
Sequence of processes in the Carnot cycle
Consider the sequence of thermodynamic processes proposed by Carnot, called the ideal Carnot cycle.
As is known, mechanical work can be carried out by a thermodynamic system only in the case when a process occurs that is accompanied by a change in the volume of the working fluid, i.e. isothermal, isobaric or adiabatic. At the same time, all thermal energy can only be converted into work in an isothermal process (with an isobaric and adiabatic process, part of the heat is spent on changing internal energy working body).
In an isochoric process (occurring at a constant volume of the working fluid), the transformation of heat into mechanical work is excluded.
In the initial state of the ideal Carnot cycle, the working fluid (ideal gas) has some parameters p 1 , V 1 , T 1 .
Heat is supplied to the working fluid from an external source called heater, which the system (heat engine) starts to use by isothermal process.
As noted above, in an isothermal process, the variables are two main parameters of the working fluid - pressure and volume, the ratio between which is inversely proportional (Boyle-Mariotte pattern). In this case, all the heat supplied to the working fluid is spent exclusively on the performance of mechanical work; the internal energy of the working fluid remains unchanged and does not require the heat received from the external heater. Therefore, the choice of the first thermodynamic process in the Carnot cycle according to the isotherm is quite logical - this allows the maximum use of the heat received from the heater to perform mechanical work.
At the end of the isothermal process, the working fluid has parameters p 2 , V 2 , T 1 .
This process of the Carnot cycle in the diagram (Fig. 1) is indicated by numbers 1-2
.
Since the Carnot cycle is reversible and circular, i.e., all thermodynamic processes occurring in it must return the working fluid to its original parameters, it becomes obvious that at least one more isothermal process must be present in the cycle. At the same time, its flow must be accompanied by cooling of the working fluid, i.e., the transfer of heat from the system to external environment, otherwise you cannot return to the point with the initial parameters. If immediately after the first process, the second isothermal process is started, then the total work of the cycle will be minimal, since the area of the graph characterizing the mechanical work performed by the system (shaded in Fig. 1) will be small or even zero. (if the forward and reverse isotherms are the same).

For this reason, S. Carnot used an adiabatic process as the second thermodynamic process for his cycle, which proceeds without heat exchange between the system and the environment. In this case, the work is performed due to a change in the internal energy of the working fluid, which continues to expand and cool down to a temperature T 2 . On the Carnot cycle diagram, this section is enclosed between the numbers 2-3
.
The use of an adiabatic process after an isothermal process allows one to obtain some mechanical work from the system already without heat supply from the heater, due to the use of the internal energy of the working fluid.
The parameters of the working fluid at the end of this process are p 3 , V 3 , T 2 .
The next link in the Carnot cycle is the second isothermal process, which, as already discussed above, must be negative, that is, accompanied by the transfer of heat from the working fluid to the external environment to another body, called in this case refrigerator.
On the cycle diagram, this process is indicated by numbers. 3-4
.
The course of the process is accompanied by a decrease in volume and an increase in pressure of the working fluid (compression), while its temperature remains constant due to heat transfer to the refrigerator.
The parameters of the working fluid at the end of this process - p 4 , V 4 , T 2 .
The final process of the Carnot cycle, which returns the system to its original state with initial parameters p 1 , V 1 , T 1 is adiabatic.
The heat transfer to the refrigerator is stopped. At the same time, the working fluid continues to decrease in volume (compress), due to the performance of some external work on it, which is negative for the process.
In this case, the internal energy of the working fluid increases, since part of the external work is spent on heating it.
This process is indicated by numbers in the diagram. 4-1
.
An analysis of the circular p-V cycle diagram obtained by Carnot shows that the system has performed mechanical work, the value of which is characterized by the area enclosed between the curve bounded by points 1-2-3 and a curve bounded by points 3-4-1 . In this case, all the work performed by the system will be equal to the sum of the work performed during each of the four successive thermodynamic processes listed above.
It is obvious that the work performed by the working body during the direct and reverse adiabatic processes is equal in magnitude, but has a different sign (positive in the first process, and negative in the second), i.e., the sum of these works is equal to zero. And the work done during the direct isothermal process is greater than the work done during the reverse isothermal process.
This is illustrated graphically different area diagram enclosed between the abscissa and, respectively, the first and second isotherms. The higher the first isotherm is located on the diagram of the relative second (inverse) isotherm, the more work the working fluid will do.
If we consider the T-V process diagram, then it will represent a flat figure (for example, a rhombus), in which two isotherms (direct and reverse) parallel to one of the axes (temperature), and the adiabats will be parallel to each other.
It follows from this that the useful work performed by the system will be the greater, the greater the difference between the temperature of the heater and the temperature of the refrigerator, i.e., the greater the temperature difference between T 1 and T 2 (distance between upper and lower isotherm on T-V diagram).
Mathematical analysis of the ideal cycle model proposed by Sadi Carnot shows that the maximum thermal efficiency thermal machine can be determined from the relation:
η t \u003d 1 - T 2 /T 1;
where: T 1 and T 2 - the temperature of the working fluid (gas), respectively, at the beginning and end of the cycle.
This simple formula allows us to draw two main conclusions - about the way to increase the efficiency of heat engines and that it is impossible to create a heat engine, the efficiency of which will be equal to unity, i.e. 100%. Indeed, the fraction T 2 /T 1 can be equal to zero only if its numerator is equal to zero, or the denominator is equal to infinity. Both are unrealistic, since it is impossible to cool a material body to a temperature absolute zero, and impossible starting temperature to make the working body infinite, since the very concept of the body in this case will lose its meaning; in addition, it is impossible to manufacture a real engine, the parts and components of which are able to withstand such a temperature.
The Carnot cycle is the benchmark that engineers who design heat engines aspire to. Under conditions of real temperatures, the upper limit of which is determined by the strength of the materials, and the lower one corresponds to the temperature environment, the thermal efficiency of the Carnot cycle can reach 0.7…0.8.
Any real heat engine will be the more perfect, the closer its efficiency is to the calculated efficiency of the Carnot cycle, which proceeds within the same temperature limits.
Modern realities involve the widespread operation of heat engines. Numerous attempts to replace them with electric motors have so far failed. The problems associated with the accumulation of electricity in autonomous systems are solved with great difficulty.
Still relevant are the problems of technology for the manufacture of electric power accumulators, taking into account their long-term use. The speed characteristics of electric vehicles are far from those of cars on internal combustion engines.
The first steps towards the creation of hybrid engines can significantly reduce harmful emissions in megacities, solving environmental problems.
A bit of history
The possibility of converting steam energy into motion energy was known in antiquity. 130 BC: Philosopher Heron of Alexandria presented to the audience a steam toy - aeolipil. A sphere filled with steam began to rotate under the action of jets emanating from it. This prototype of modern steam turbines did not find application in those days.
For many years and centuries, the development of the philosopher was considered only a fun toy. In 1629, the Italian D. Branchi created an active turbine. Steam set in motion a disk equipped with blades.
From that moment began the rapid development of steam engines.
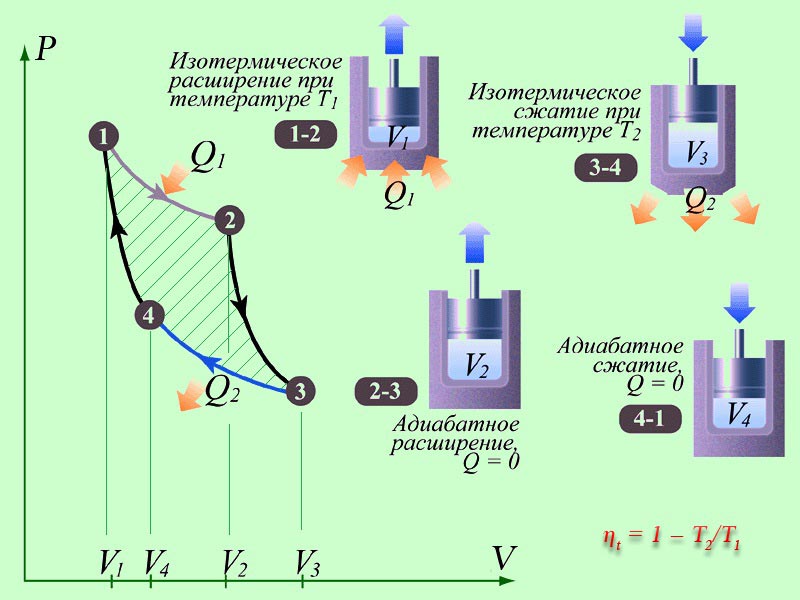
heat engine
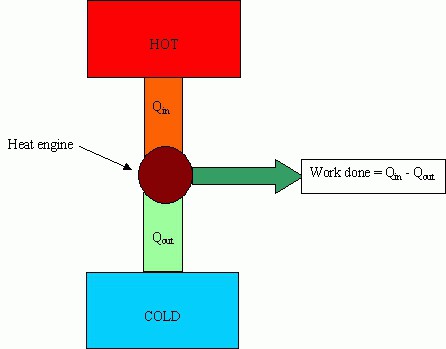
The main parts of machines: a heater (a system for obtaining energy from outside), a working fluid (performs a useful action), a refrigerator.
The heater is designed to ensure that the working fluid has accumulated a sufficient supply of internal energy to perform useful work. The refrigerator removes excess energy.
The main characteristic of efficiency is called the efficiency of heat engines. This value shows what part of the energy spent on heating is spent on doing useful work. The higher the efficiency, the more profitable the operation of the machine, but this value cannot exceed 100%.
Efficiency calculation
Let the heater acquire from outside the energy equal to Q 1 . The working fluid did work A, while the energy given to the refrigerator was Q 2 .
Based on the definition, we calculate the efficiency:
η= A / Q 1 . We take into account that A \u003d Q 1 - Q 2.
From here, the efficiency of the heat engine, the formula of which has the form η = (Q 1 - Q 2) / Q 1 = 1 - Q 2 / Q 1, allows us to draw the following conclusions:
- Efficiency cannot exceed 1 (or 100%);
- to maximize this value, either an increase in the energy received from the heater or a decrease in the energy given to the refrigerator is necessary;
- an increase in the energy of the heater is achieved by changing the quality of the fuel;
- reducing the energy given to the refrigerator, make it possible to achieve the design features of the engines.

Ideal heat engine
Is it possible to create such an engine, the efficiency of which would be maximum (ideally, equal to 100%)? The French theoretical physicist and talented engineer Sadi Carnot tried to find the answer to this question. In 1824, his theoretical calculations about the processes occurring in gases were made public.
The main idea behind an ideal machine is to carry out reversible processes with an ideal gas. We start with the expansion of the gas isothermally at a temperature T 1 . The amount of heat required for this is Q 1. After the gas expands without heat exchange. Having reached the temperature T 2, the gas is compressed isothermally, transferring the energy Q 2 to the refrigerator. The return of the gas to its original state is adiabatic.
The efficiency of an ideal Carnot heat engine, when accurately calculated, is equal to the ratio of the temperature difference between the heating and cooling devices to the temperature that the heater has. It looks like this: η=(T 1 - T 2)/ T 1.
The possible efficiency of a heat engine, the formula of which is: η= 1 - T 2 / T 1 , depends only on the temperature of the heater and cooler and cannot be more than 100%.
Moreover, this ratio allows us to prove that the efficiency of heat engines can be equal to unity only when the refrigerator reaches temperatures. As you know, this value is unattainable.
Carnot's theoretical calculations make it possible to determine the maximum efficiency of a heat engine of any design.
The theorem proved by Carnot is as follows. An arbitrary heat engine under no circumstances is capable of having a coefficient of efficiency greater than the similar value of the efficiency of an ideal heat engine.
Example of problem solving
Example 1 What is the efficiency of an ideal heat engine if the heater temperature is 800°C and the refrigerator temperature is 500°C lower?
T 1 \u003d 800 o C \u003d 1073 K, ∆T \u003d 500 o C \u003d 500 K, η -?
By definition: η=(T 1 - T 2)/ T 1.
We are not given the temperature of the refrigerator, but ∆T = (T 1 - T 2), from here:
η \u003d ∆T / T 1 \u003d 500 K / 1073 K \u003d 0.46.
Answer: efficiency = 46%.
Example 2 Determine the efficiency of an ideal heat engine if 650 J of useful work is performed due to the acquired one kilojoule of heater energy. What is the temperature of the heat engine heater if the coolant temperature is 400 K?
Q 1 \u003d 1 kJ \u003d 1000 J, A \u003d 650 J, T 2 \u003d 400 K, η -?, T 1 \u003d?
In this problem, we are talking about a thermal installation, the efficiency of which can be calculated by the formula:
To determine the temperature of the heater, we use the formula for the efficiency of an ideal heat engine:
η \u003d (T 1 - T 2) / T 1 \u003d 1 - T 2 / T 1.
After performing mathematical transformations, we get:
T 1 \u003d T 2 / (1- η).
T 1 \u003d T 2 / (1- A / Q 1).
Let's calculate:
η= 650 J / 1000 J = 0.65.
T 1 \u003d 400 K / (1- 650 J / 1000 J) \u003d 1142.8 K.
Answer: η \u003d 65%, T 1 \u003d 1142.8 K.
Real conditions
The ideal heat engine is designed with ideal processes. Work is done only in isothermal processes, its value is defined as the area bounded by the Carnot cycle graph.

In fact, it is impossible to create conditions for the process of changing the state of a gas without accompanying changes in temperature. There are no materials that would exclude heat exchange with surrounding objects. adiabatic process becomes impossible to implement. In the case of heat transfer, the temperature of the gas must necessarily change.
The efficiency of heat engines created in real conditions differ significantly from the efficiency of ideal engines. Note that the processes in real engines are so fast that the variation in the internal thermal energy of the working substance in the process of changing its volume cannot be compensated by the inflow of heat from the heater and return to the cooler.
Other heat engines
Real engines operate on different cycles:
- Otto cycle: the process at a constant volume changes adiabatically, creating a closed cycle;
- Diesel cycle: isobar, adiabat, isochor, adiabat;
- the process occurring at constant pressure is replaced by an adiabatic one, closing the cycle.
Create equilibrium processes in real engines (to bring them closer to ideal ones) under conditions modern technology does not seem possible. The efficiency of thermal engines is much lower, even taking into account the same temperature regimes as in an ideal thermal installation.
But you should not reduce the role of the efficiency calculation formula, since it is it that becomes the starting point in the process of working to increase the efficiency of real engines.
Ways to change efficiency
When comparing ideal and real heat engines, it is worth noting that the temperature of the refrigerator of the latter cannot be any. Usually the atmosphere is considered to be a refrigerator. The temperature of the atmosphere can be taken only in approximate calculations. Experience shows that the temperature of the coolant is equal to the temperature of the exhaust gases in the engines, as is the case in internal combustion engines (abbreviated internal combustion engines).
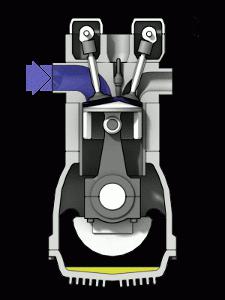
ICE is the most common heat engine in our world. The efficiency of a heat engine in this case depends on the temperature created by the burning fuel. An essential difference between an internal combustion engine and steam engines is the merging of the functions of the heater and the working fluid of the device in the air-fuel mixture. Burning, the mixture creates pressure on the moving parts of the engine.
An increase in the temperature of the working gases is achieved by significantly changing the properties of the fuel. Unfortunately, it is not possible to do this indefinitely. Any material from which the combustion chamber of an engine is made has its own melting point. The heat resistance of such materials is the main characteristic of the engine, as well as the ability to significantly affect the efficiency.
Motor efficiency values
If we consider the temperature of the working steam at the inlet of which is 800 K, and the exhaust gas is 300 K, then the efficiency of this machine is 62%. In reality, this value does not exceed 40%. Such a decrease occurs due to heat losses during heating of the turbine housing.
The highest value of the efficiency of internal combustion engines does not exceed 44%. Increasing this value is a matter of the near future. Changing the properties of materials, fuels is a problem that the best minds of mankind are working on.
Consider a reversible cycle Carnot performed by an ideal heat engine.
The Carnot cycle consists of four reversible processes: two isotherms and two adiabats. On fig. 3.10 shows the direct Carnot cycle.
![]()
Rice. 3.10
1. Plot 1-2. The ideal gas, located in the cylinder under the piston, in the process of isothermal expansion (T 1 = const) is brought into thermal contact with the heater, which transfers heat Q 1 to the ideal gas.
2. Plot 2-3. In state 2, the gas is completely thermally isolated from the heater. There is an adiabatic expansion of it, and the temperature drops to T 2 .
3. Plot 3-4. In state 3, the ideal gas is brought into contact with the cooler. Isothermal compression occurs (T 2 = const), in which the ideal gas transfers heat Q 2 to the refrigerator.
4. Plot 4-1. In state 4, the gas is thermally insulated from the refrigerator. Then there is adiabatic compression. The gas temperature rises to T 1 .
The dynamics of thermodynamic processes can be observed in the computer model "Carnot cycle".
Computer model "Carnot cycle"
The model is designed to study a reversible cyclic process in an ideal gas, consisting of two isotherms and two adiabats (Carnot cycle). When bypassing the Carnot cycle, the working substance is sequentially brought into thermal contact with two thermal reservoirs - a heater and a refrigerator. In the model, it is possible to change the temperatures T 1 and T 2 of the heater and refrigerator. An energy diagram is presented, which shows the amount of heat Q received by the gas, the work done A and the change ΔU in internal energy.
Remember that a heat engine running on Carnot cycle, has a maximum efficiency at given temperatures of the heater and refrigerator.
The work done by the working body per cycle,
where T 1 - temperature of the heater; T 2 - refrigerator temperature.
Formula (3.50) expresses the first theorem Carnot:
Efficiency an ideal heat engine operating according to the Carnot cycle depends only on the temperatures of the heater and refrigerator; does not depend on the device of the machine and the type of working fluid.
For a real heat engine (according to the second Carnot theorem):
The efficiency of any heat engine operating according to the Carnot cycle with the same heater and cooler temperatures as an ideal heat engine cannot exceed the efficiency of an ideal engine,
those. The efficiency is found by the formula
|
. |
(3.51) |
According to the direct Carnot cycle, internal combustion engines, diesel engines, etc. work. Consider the reverse Carnot cycle. Gas work per cycle A \u003d (Q 1 -Q2)< 0, где Q 1 < 0 - heat removed from the refrigerator; Q2 > 0 - heat supplied to the gas at T 2< T 1 .
The transfer of heat from a cold body to a hot one occurs due to the work of external forces.
The efficiency of an ideal heat engine operating on the reverse Carnot cycle is
All refrigeration machines operate according to the reverse Carnot cycle.
We assume that all molecules, except for the one under consideration, are motionless. The molecules will be considered as balls with diameter d. Collisions will occur whenever the center of the immobile molecule is at a distance less than or equal to d from the straight line along which the center of the considered molecule moves. During collisions, the molecule changes its direction of motion and then moves in a straight line until the next collision. Therefore, the center of a moving molecule moves along a broken line due to collisions (Fig. 1).
| rice. one |
The molecule will collide with all immobile molecules whose centers are within a broken cylinder with a diameter of 2d. In a second, a molecule travels a path equal to . Therefore, the number of collisions occurring during this time is equal to the number of molecules whose centers fall inside a broken cylinder with a total length and radius d. We will take its volume equal to the volume of the corresponding straightened cylinder, i.e., equal to If there are n molecules in the unit volume of the gas, then the number of collisions of the molecule under consideration in one second will be equal to
For an ideal gas. That's why
| | (3.1.10) |
This shows that during isothermal expansion (compression), the mean free path increases (decreases). As noted in the introduction, the effective diameter of molecules decreases with increasing temperature. Therefore, at a given concentration of molecules, the mean free path increases with increasing temperature. Calculation of the mean free path for nitrogen (d \u003d 3 10 -10 m) under normal conditions (p \u003d 1.01 10 5 Pa, T \u003d 273.15 K) gives: ![]() , and for the number of collisions per second:
, and for the number of collisions per second: ![]() . Thus, the mean free path of molecules under normal conditions is fractions of microns, and the number of collisions is several billion per second. Therefore, the processes of equalization of temperatures (thermal conductivity), velocities of gas layers (viscous friction) and concentrations (diffusion) are quite slow, which is confirmed by experience.
. Thus, the mean free path of molecules under normal conditions is fractions of microns, and the number of collisions is several billion per second. Therefore, the processes of equalization of temperatures (thermal conductivity), velocities of gas layers (viscous friction) and concentrations (diffusion) are quite slow, which is confirmed by experience.
Molecule mean free path is the average distance (denoted by λ) that the particle travels during its free path from one collision to the next.
The mean free path of each molecule is different, so in kinetic theory the concept of mean free path is introduced (<λ>). Value<λ>is a characteristic of the entire set of gas molecules at given values of pressure and temperature.
Where σ is the effective cross section of the molecule, n is the concentration of molecules.






