What is the work of a gas cycle. Heat engines, carnot cycle
LECTURE 13
HEAT MACHINES
13.1. Reversible and irreversible processes
circular process- the process in which the system, after passing through a series of states, returns to its original state. On the state diagram, the cycle is represented by a closed curve.
direct cycle is the cycle in which positive work is done:  . The cycle proceeds clockwise.
. The cycle proceeds clockwise.
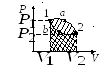
The cycle carried out ideal gas can be broken down into expansion and contraction processes. Extension work ( 
 - positive (
- positive (  ).
).
Compression work (  ) is determined by the area of the figure
) is determined by the area of the figure 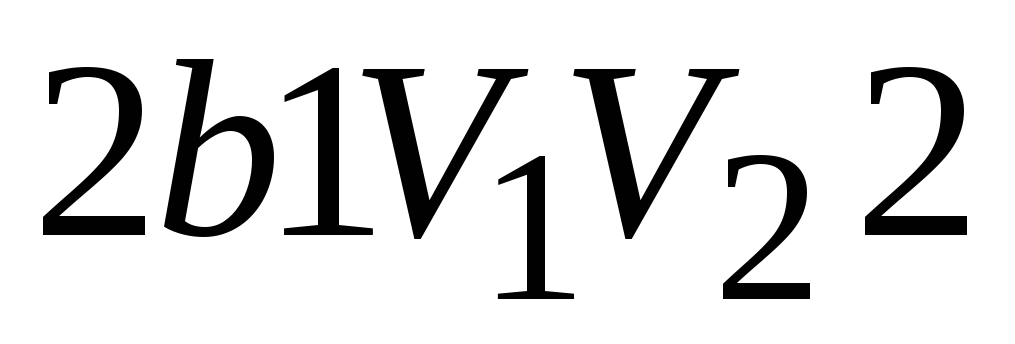 - negative (
- negative (  ).
).
The work done by the gas per cycle is determined by the area covered by the closed curve.
reverse cycle is the cycle in which negative work is done 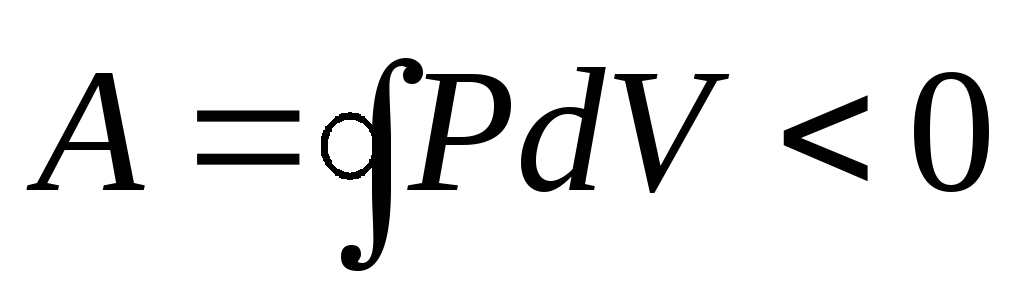 . The cycle runs counterclockwise.
. The cycle runs counterclockwise.
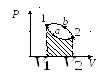
Extension work 1 a 2 positive  . Compression work 2 b 1 negative
. Compression work 2 b 1 negative  . The work done by the gas per cycle is determined by the area covered by the closed curve.
. The work done by the gas per cycle is determined by the area covered by the closed curve.
Coefficient of performance (COP) for a circular process. As a result of the circular process, the system returns to its original state, i.e. the change in the internal energy of the gas is zero. We write the first law of thermodynamics:  . Because
. Because 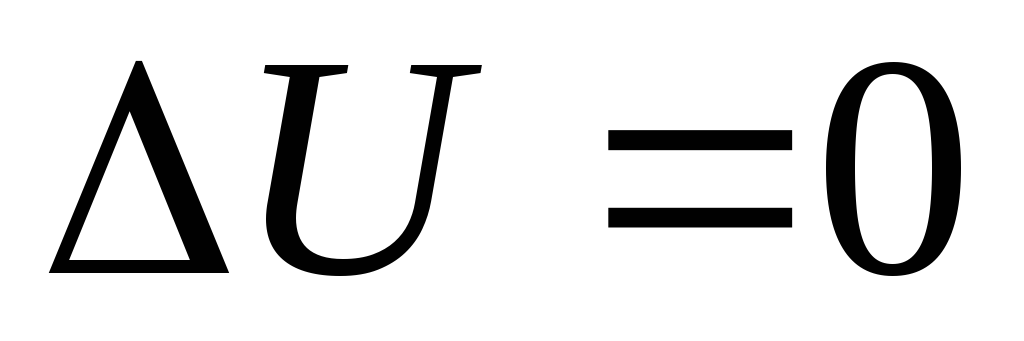 , Consequently
, Consequently 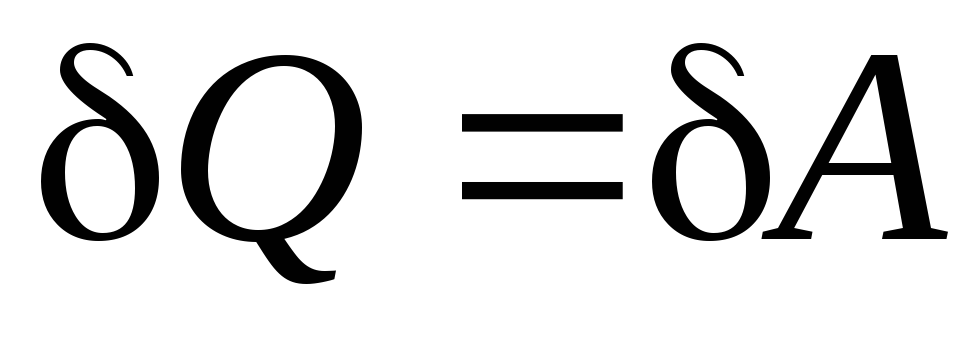 , i.e. the work done per cycle is equal to the amount of heat received from outside. But as a result of a circular process, the system can both receive heat and give it away, then
, i.e. the work done per cycle is equal to the amount of heat received from outside. But as a result of a circular process, the system can both receive heat and give it away, then
 ,
,
where 
quantity of heat obtained about the heater, and
quantity of heat obtained about the heater, and  - the amount of heat transferred by the system to the cooler.
- the amount of heat transferred by the system to the cooler.
Efficiency for circular process:
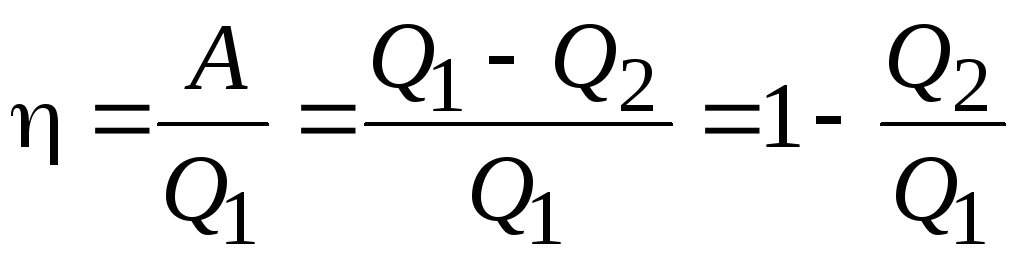 .
.
The process is called reversible, if it can occur both in direct and in reverse direction, and the system returns to its original state, i.e. in environment and there is no change in the system. Any other process is called irreversible.
All real processes are accompanied by energy dissipation (due to friction, heat conduction). Thus, reverse processes are idealized real processes. They are more economical and have maximum efficiency. We consider them for two reasons: 1) many processes in nature and technology are practically reversible; 2) you can see ways to improve the efficiency of real engines.
Circular processes underlie the operation of heat engines and refrigeration machines.
13.2. Heat engines and refrigeration machines
heat engine- This is a periodically operating engine that performs work due to heat received from outside. Heat engines use a direct cycle.
The principle of operation of a heat engine. From a thermostat with a higher temperature 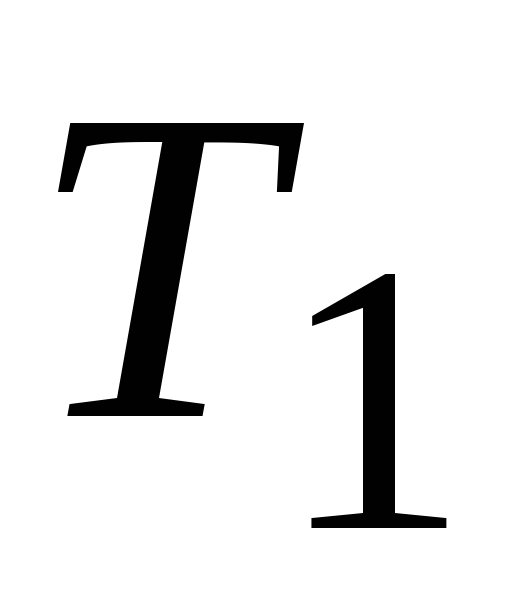 called heater, the amount of heat is taken away per cycle
called heater, the amount of heat is taken away per cycle  , and a thermostat with a lower temperature
, and a thermostat with a lower temperature 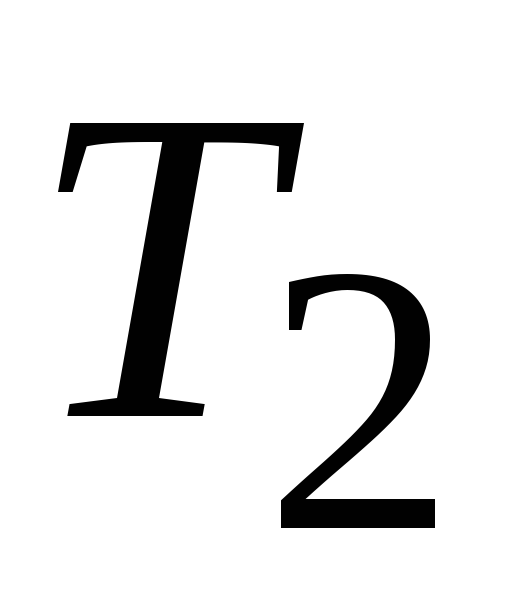 , called a refrigerator, the amount of heat transferred per cycle
, called a refrigerator, the amount of heat transferred per cycle  , while doing work
, while doing work 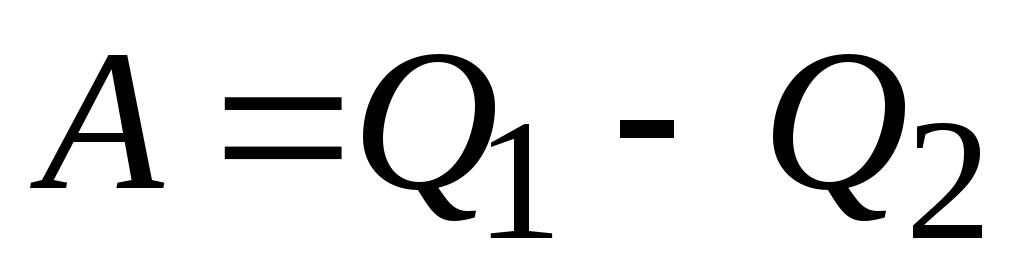 (Fig. 13.1).
(Fig. 13.1).
Rice. 13.1 Heat engine
The efficiency of a heat engine is the ratio of work BUT performed by the engine to the expended energy, i.e. to the amount of heat taken from the heater:
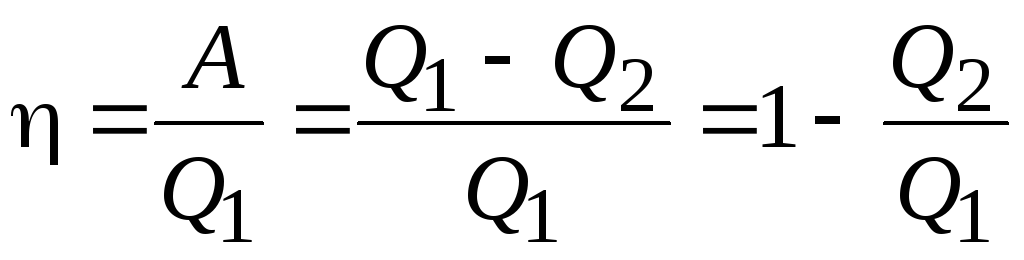 .
.
It can be seen from this expression that even for an ideal heat engine, the efficiency is less than 1, i.e. less than 100%. Carnot showed that for the operation of a heat engine, at least two sources of heat with different temperatures are needed, because in the process of compression, it is necessary to cool the working fluid. Otherwise, no work will be done.
Carnot's theorem. Of all periodic operating heat engines with the same heater temperatures ( 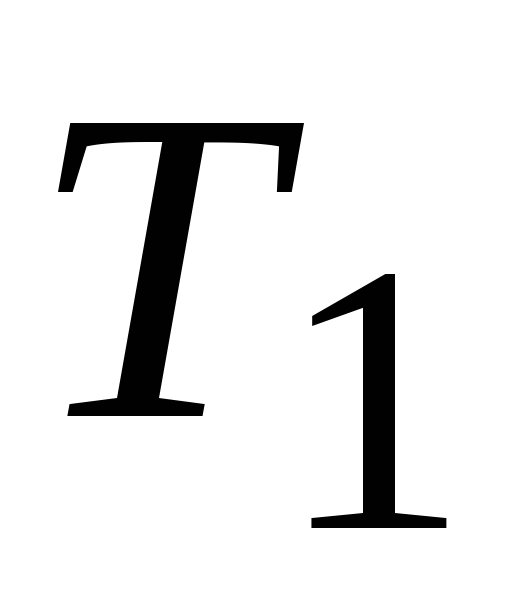 ) and refrigerators (
) and refrigerators (  ), reversible machines have the highest efficiency. At the same time, the efficiency of reversible machines operating at the same heater temperatures (
), reversible machines have the highest efficiency. At the same time, the efficiency of reversible machines operating at the same heater temperatures ( 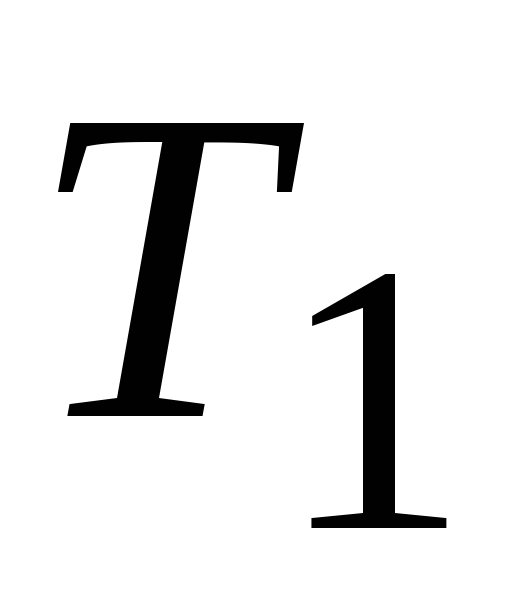 ) and refrigerators (
) and refrigerators (  ), are equal to each other and do not depend on the nature of the working fluid (the body that performs circular process and exchanging energy with other bodies), but is determined only by the temperatures of the heater and refrigerator.
), are equal to each other and do not depend on the nature of the working fluid (the body that performs circular process and exchanging energy with other bodies), but is determined only by the temperatures of the heater and refrigerator.
 .
.
Refrigeration machines- intermittent installations in which, due to the work of external forces, heat is transferred to a body with a higher temperature.
The principle of operation of refrigeration machines. System per cycle from a thermostat with a lower temperature  the amount of heat is taken
the amount of heat is taken  and is given to the thermostat with a higher temperature
and is given to the thermostat with a higher temperature 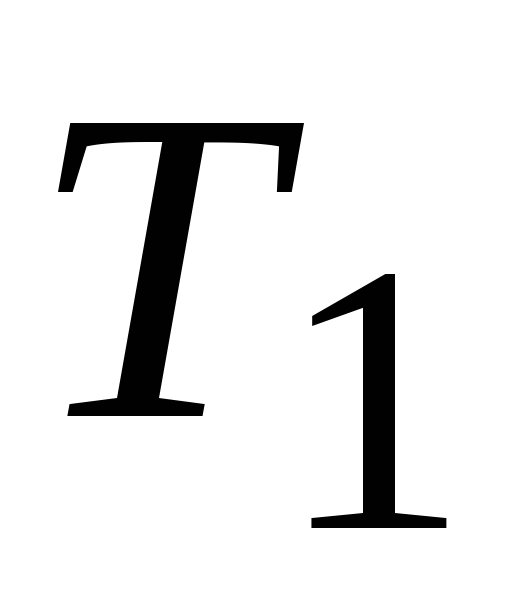 quantity of heat
quantity of heat  (Fig. 13.2).
(Fig. 13.2).
Rice. 13.2. Refrigeration machine
Since this process is unnatural (heat cannot spontaneously transfer from a cold body to a hot one), work must be done on the system.
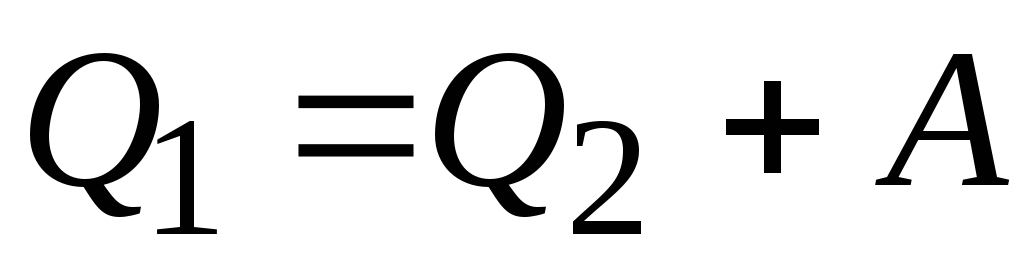 .
.
So the amount of heat Q 1 given by the system to the thermostat with a higher temperature T 1 , more warmth Q 2 obtained from a thermostat with a lower temperature T 2, on the amount of work BUT perfect over the system.
Chiller efficiency (coefficient of performance):
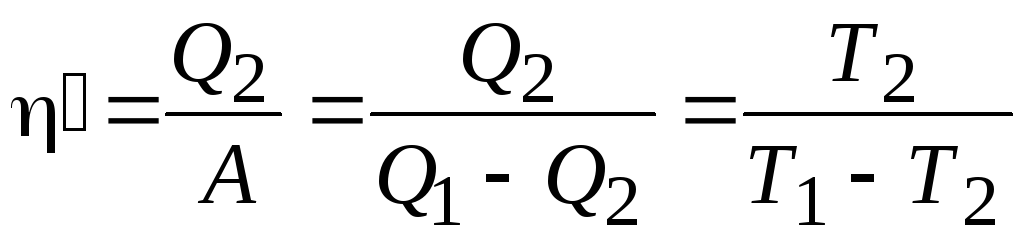 .
.
Conclusion: coefficient of performance characterizes the efficiency of the refrigeration machine and is defined as the ratio of the amount of heat taken from the thermostat with a lower temperature  to work BUT, which is spent on bringing the refrigeration machine into action. Without doing work, heat cannot be taken from a less heated body and transferred to a hotter body.
to work BUT, which is spent on bringing the refrigeration machine into action. Without doing work, heat cannot be taken from a less heated body and transferred to a hotter body.
13.3 Carnot cycle and work per cycle
The Carnot cycle is the most economical reversible circular cycle, consisting of two isotherms and two adiabats. Consider the direct Carnot cycle with an ideal gas as the working fluid (Fig. 13.3).
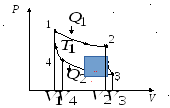
Rice. 13.3. Carnot cycle in diagram P, V
Process 1-2 isothermal expansion; process 2-3 adiabatic expansion; process 3-4 isothermal compression; process 4-1 adiabatic compression.
1-2 2-3 3-4 4-1
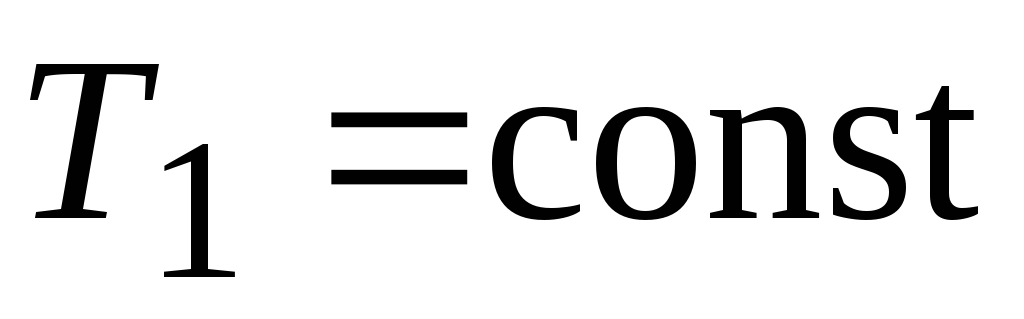

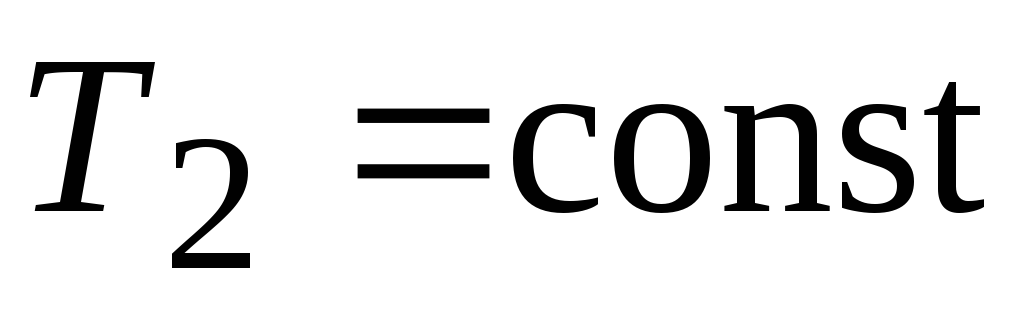

isothermal adiabatic isothermal adiabatic
expansion expansion compression compression
Consider thermodynamic processes and work in them.
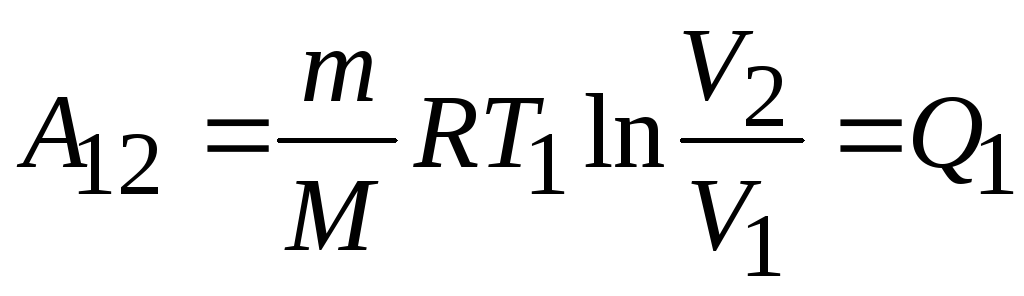 - isothermal expansion
- isothermal expansion  ,
,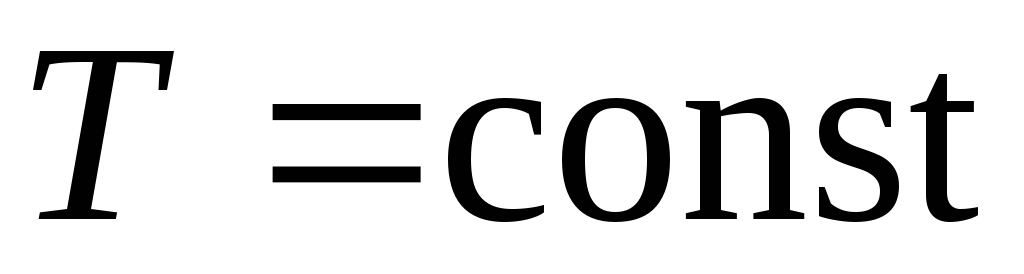 ,
,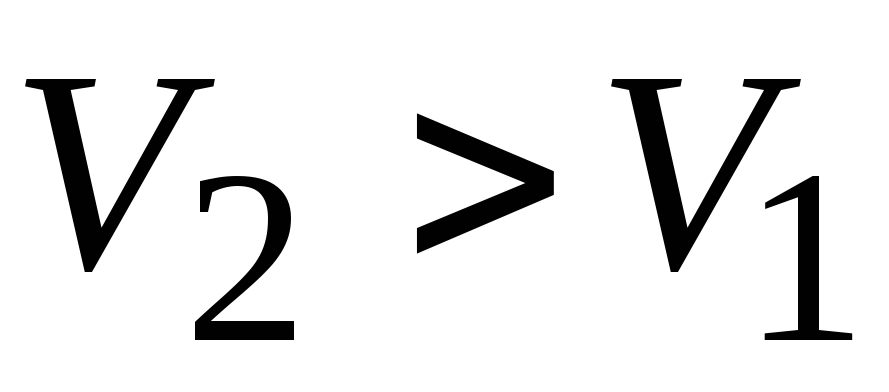 ;
;
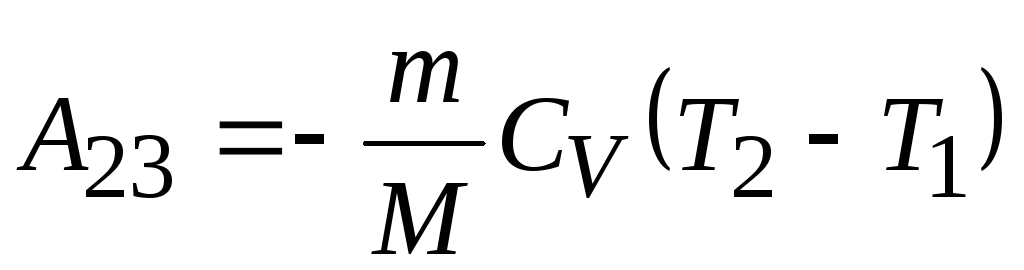 adiabatic expansion
adiabatic expansion  ,
, ,
,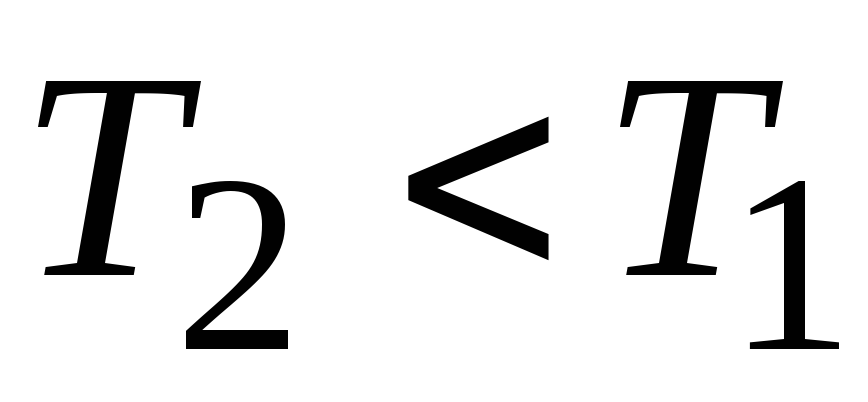 ;
; - isothermal compression
- isothermal compression  ,
,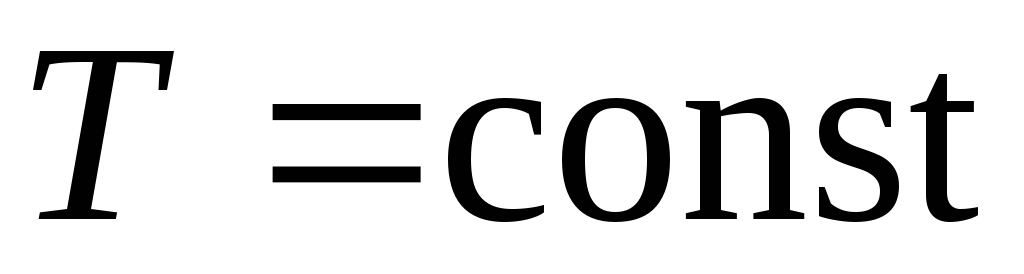 ,
, ;
;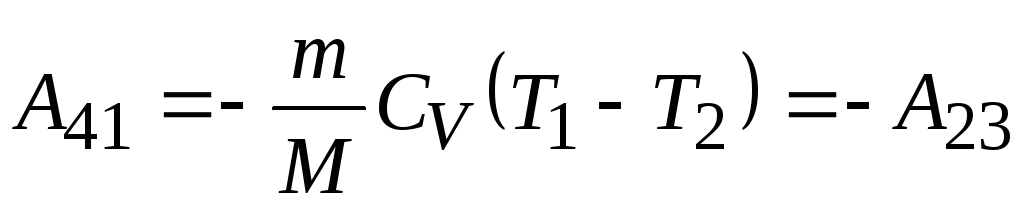 - adiabatic compression
- adiabatic compression 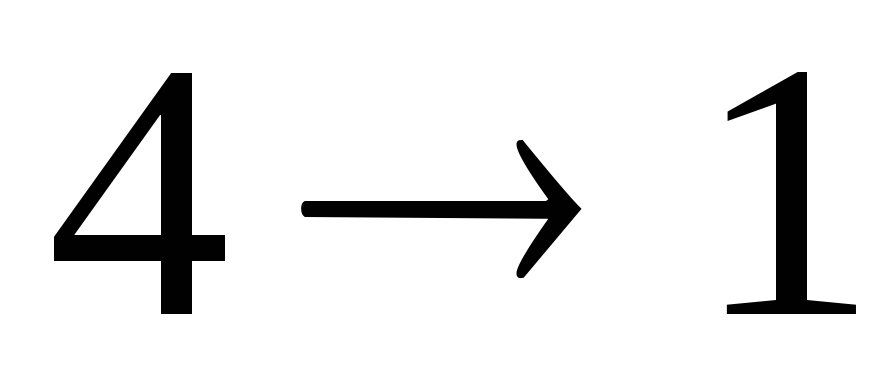 ,
, ,
,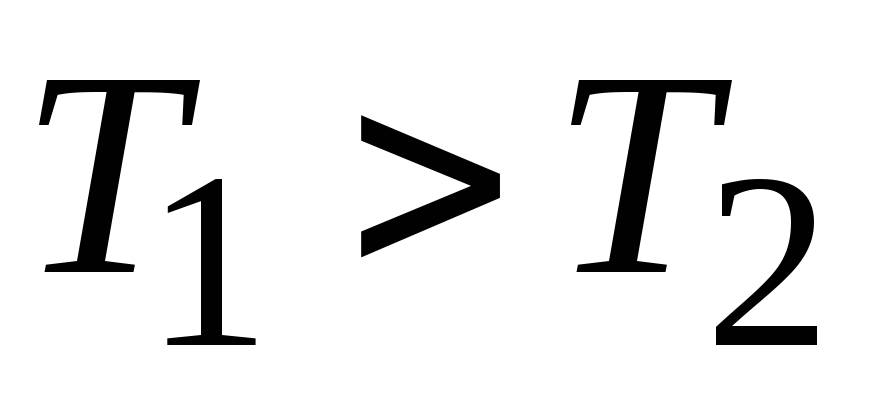 .
.
Work per cycle:
Conclusion: work per cycle is determined by the area bounded by the isotherms and adiabats of the Carnot cycle.
Thermal efficiency of the Carnot cycle:
 .
.
We use rice. 13.3 and write the equation of the adiabatic process in the form 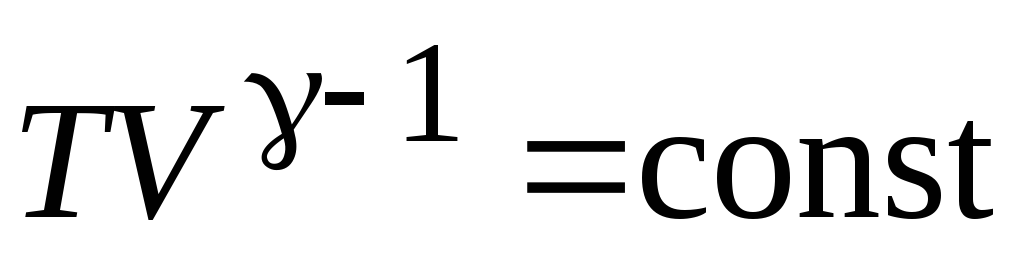 :
:
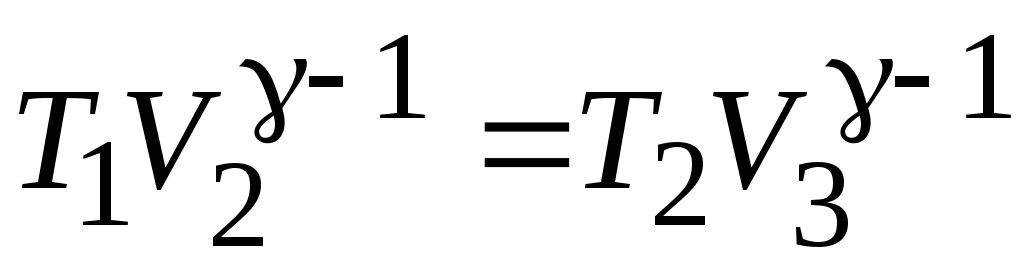 ;
;
 ;
;
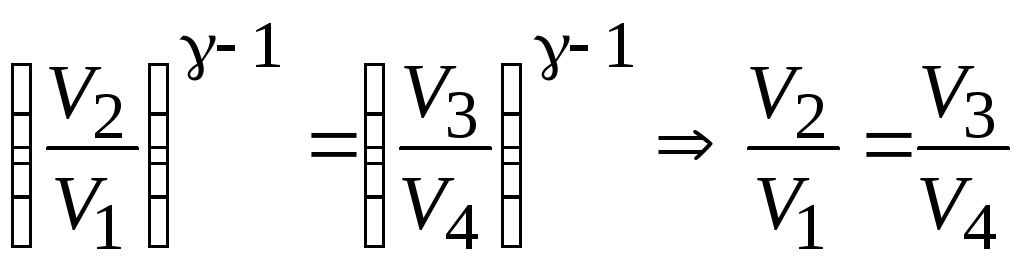 .
.
We substitute these expressions into the formula for the thermal efficiency of the circular process:
 .
.
Conclusion: for the Carnot cycle, the efficiency is really determined only by the temperatures of the heater and cooler.
test questions
What is a circular process?
What is forward and reverse cycle? Where are they applied?
What are heat engines and how do they work?
What are refrigerators and how do they work?
What is a Carnot cycle? What is the work per cycle?
Get an expression for the thermal efficiency of the Carnot cycle.
Tasks
An ideal heat engine operating according to the Carnot cycle does 2.94 kJ of work per cycle and gives the cooler an amount of heat of 13.4 kJ. Find cycle efficiency. [≈ 18 %].
An ideal heat engine operates on the Carnot cycle. At the same time, 80% of the amount of heat received from the heater is transferred to the refrigerator. The amount of heat received from the heater is 6.28 kJ. Find the cycle efficiency and the work done per cycle. .
A refrigerator operating on the reverse Carnot cycle does 37 kJ of work per cycle. At the same time, it takes heat from the calving body with a temperature of -10 С and transfers heat to the body with a temperature of 17 С. Find the coefficient of performance of the refrigerator and the amount of heat taken from the cold body per cycle. [≈ 9.74; 360 kJ].
LECTURE 14
ENTROPY. SECOND ORIGIN OF THERMODYNAMICS
14.1. Entropy and its change in some thermodynamic processes
The qualitative difference between the thermal motion of molecules and other forms of motion is its randomness, randomness. Therefore, to describe the thermal motion of molecules, a quantitative measure of the degree of molecular disorder is introduced - entropy.
thermal machines, Carnot cycle
Heat engines are an important application of thermodynamics. Under thermal machine understand a device that converts some part of the received amount of heat into mechanical work. Mechanical work in heat engines is performed in the process of expansion of a certain substance, which is called the working fluid. As a working fluid, gaseous substances (gasoline vapors, air, water vapor) are usually used.
Thermal engines are divided into two classes: disposable machines (rocket, gun, etc.) and cyclic machines (steam engines, internal combustion engines).
cycle A process is called, the beginning and end of which are the same. An example of a cyclic process is the process depicted in Figure 13.3. The work of the cycle consists of the work of the system itself ( plot abc) and work on the system ( plot cda).
Figure 13.3 - The circular process in the diagram ( p, V). abc is the expansion curve, cda is the compression curve. Work A in a circular process is equal to the area of the figure.
Cycle work numerically equal to the area of the figure abcd. Gas does work on the site abc from received from heater the amount of heat, and on the site cda Work is done on the gas by external forces. In order for the work of external forces to be less than the work of the gas, it is necessary to perform it at a lower temperature, and, consequently, a certain amount of heat must transfer from working body– gas - to a less heated body – refrigerator. The energy scheme of the heat engine is shown in Figure 13.4.
The statement that in order to perform useful work in a cyclic machine, the participation of two bodies with different temperatures, is called Carnot principle.

Figure 13.4 - Energy diagram of a heat engine: 1 - heater;
2 - refrigerator; 3 - working body. Q 1 > 0, A > 0, Q 2 < 0; T 1 > T 2 .
The cycle by which the amount of heat taken from a body can be best converted into mechanical work is called Carnot cycle. An ideal gas acts as a working fluid. The Carnot cycle consists of two isotherms and two adiabats (Figure 13.5).
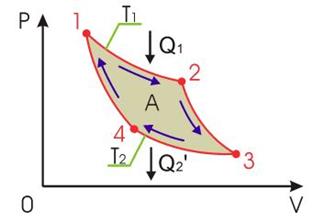
Figure 13.5 - Scheme of the Carnot cycle
In section 1-2, the working fluid contacts a heater (a body with a large heat capacity) and receives an amount of heat from it Q1. In this case, isothermal expansion of the gas is realized (due to the large heat capacity of the heater, its temperature does not change). This is the most profitable one-time process, in which all the received amount of heat is converted into mechanical work, according to the first beginning of thermodynamics:
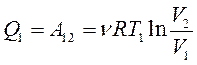 (13.25)
(13.25)
Section 2-3 corresponds to the adiabatic expansion of an ideal gas. At this stage, the contact with the heater is broken and the working fluid does not exchange the amount of heat with other bodies. This is also beneficial, since in this case the gas does work at the expense of its own internal energy, as a result of which it decreases, the gas temperature becomes equal to T 2. According to the first law of thermodynamics,
In section 3-4, the working fluid is brought into thermal contact with the refrigerator, which has a large heat capacity and temperature T 2 . Here, at a lower temperature, the gas is compressed isothermally, doing work on it that is numerically equal to the amount of heat given to the refrigerator, the work of the gas itself, as well as the given amount of heat, is negative:
 (13.27)
(13.27)
At lower temperatures, when internal energy less than the initial one, it is easier to compress the gas, so the work A 34 is less than the work A 12. Isothermal compression is again the most advantageous, since it is not necessary to change the internal energy of the gas, spending additional work on external forces. In the last section of the Carnot cycle, it is necessary to return the gas to its original state in the most advantageous way, that is, to compress it adiabatically. With adiabatic compression, there is no thermal contact between the working fluid and the cooler, and the work of external forces is completely spent on increasing the internal energy of the gas:
Useful work per cycle is equal to the algebraic sum of the work of each section of the Carnot cycle: . Comparison of formulas (13.25) and (13.28) allows us to conclude that the gas work in section 2-3 is equal in magnitude to the gas work in section 4-1, but is opposite in sign, therefore, the algebraic sum of the work in these sections is zero, and the work for the cycle will be determined by the sum of the works of sections 1-2 and 3-4:
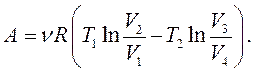 (13.29)
(13.29)
For further transformation of useful work, consider the adiabatic equations in sections 2-3 and 4-1, written in terms of volume and temperature: ![]() and
and ![]() . Divide the second equation by the first and get:
. Divide the second equation by the first and get: 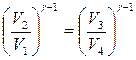 or . Given this equality, we can take out of brackets natural logarithm volume ratios in formula (13.29) and obtain an expression for useful work per Carnot cycle.
or . Given this equality, we can take out of brackets natural logarithm volume ratios in formula (13.29) and obtain an expression for useful work per Carnot cycle.
Is such an ideal heat engine possible, which receives 50 J from the heater per cycle and performs useful work of 100 J? What is the efficiency of such a heat engine?
1) possible,
2) possible,
3) possible,
4) impossible,
Solution.
Such a heat engine is impossible, since, according to the second law of thermodynamics, the useful work per cycle is always less than the amount of heat received from the heater. The efficiency of such a heat engine would be equal to
Correct answer: 4.
Answer: 4
An ideal gas performs a cyclic process 1→2→3→4→1, shown in the figure. As a result of this cyclic process
1) the total work done by the gas is zero.
2) the change in the internal energy of the gas is zero.
3) the total amount of heat received and released by the gas is zero.
4) all the heat received by the gas in the process 1→2→3 is completely converted into mechanical work.
Solution.
Internal energy is a function of the state of the gas, that is, it depends only on the state of the gas. Since, as a result of the cyclic process, the gas returns to its original state, the change in its internal energy ends up being zero.
Let's discuss why the other options are not correct.
In the diagram, the work done by an ideal gas per cycle corresponds to the cycle area. Since this area is non-zero, we conclude that the total work of the gas is not zero.
According to the first law of thermodynamics, the heat transferred to the gas goes to work against external forces and to change the internal energy: The change in energy in the process 1→2→3→4→1 is zero, the work is non-zero, therefore, the total amount of heat received and given away by the gas cannot be zero.
Finally, if we apply the first law separately for the process 1→2→3, then it is easy to see that, since the gas is heated, its internal energy in this section increases, which means that not all of the heat transferred to the gas goes to work.
Correct answer: 2.
Answer: 2
Source: Yandex: Training USE work in physics. Option 1.
Guest 19.12.2012 20:29
In the process 2-->3, the work was positive, because the volume increased and the gas itself did the work. In the process 4-->1, work is done on the gas, the work is negative, because the volume decreases. Why is the total work not equal to zero? Was it necessary to clarify in the problem what is meant by the sum of the modules of work?
Guest
Good afternoon!
That's right, the works have opposite signs, but they also differ in modulus. As a result, the total work is different from zero.
The efficiency of an ideal heat engine can be increased by
1) only by reducing the temperature of the heater
2) only by increasing the temperature of the refrigerator
3) using another gas as a working fluid
4) by decreasing the temperature of the refrigerator or increasing the temperature of the heater
Solution.
The efficiency of an ideal heat engine does not depend on the working fluid and is determined by the relation: Thus, this value can be increased by reducing the temperature of the refrigerator or increasing the temperature of the heater.
Correct answer: 4.
Answer: 4
Source: Yandex: USE training work in physics. Option 2.
The figure schematically shows the direction of heat transfer during the operation of two ideal heat engines. Which one is more efficient?
1) at the first
2) at the second
3) both machines have the same efficiency
4) it is impossible to answer unequivocally
Solution.
The efficiency of an ideal heat engine depends only on the temperatures of the heater and refrigerator and is given by the expression: For the first machine, the heater is schematically shown from above, and for the second - from below. Thus, the efficiency of the first machine is equal to
The efficiency of the second ideal heat engine:
Thus, the efficiency of the second machine is greater.
Correct answer: 2.
Answer: 2
Source: MIOO: Training work in physics 10/18/2013 option 1.
The figure shows two cyclic processes 1 → 2 → 3 → 4 → 1 and 5 → 6 → 7 → 8 → 5.
Which of the following statements is true?
A. The work of the gas in the case of a cyclic process 1 → 2 → 3 → 4 → 1 is greater than the work of the gas in the case of a cyclic process 5 → 6 → 7 → 8 → 5.
B. The change in the internal energy of the gas as a result of the cyclic process 1 → 2 → 3 → 4 → 1 is greater than the change in the internal energy of the gas as a result of the cyclic process 5 → 6 → 7 → 8 → 5.
1) only A
3) only B
4) neither A nor B
Solution.
In the diagram, the work of gas as a result of a cyclic process corresponds to the area "inside" the cycle. From the above figure, it can be seen that the area of the square 1-2-3-4-1 is greater than the area of the rhombus 5-6-7-8-5. Thus, the work of the gas in the case of a cyclic process 1 → 2 → 3 → 4 → 1 is greater than the work of the gas in the case of a cyclic process 5 → 6 → 7 → 8 → 5. Therefore, statement A is true.
After any cyclic process, the gas returns to its original state. Internal energy is a function of state, therefore, as a result of any cycle, the change in internal energy is always zero. So statement B is not true.
Correct answer: 1.
Answer: 1
Source: MIOO: Diagnostic work in physics 12/17/2012 option 1.
Which of the figures correctly shows the dependence of the efficiency of an ideal heat engine on the temperature of the heater at a constant temperature of the refrigerator?
Solution.
The efficiency of an ideal heat engine is given by the expression: Thus, at a fixed temperature of the refrigerator, the efficiency behaves as shown in Figure 3: at temperature it goes to zero, at an infinite temperature of the heater it tends to 1.
Correct answer: 3
Answer: 3
Source: MIOO: Training work in physics 04/11/2013 variant PHI1501.
Which of the figures correctly shows the dependence of the efficiency of an ideal heat engine on the refrigerator temperature at a constant heater temperature?
Solution.
The efficiency of an ideal heat engine is given by the expression: Thus, at a fixed temperature of the heater, the efficiency behaves as shown in Figure 1: it decreases linearly with an increase in the temperature of the refrigerator, and at a temperature it vanishes.
Correct answer: 1
Answer: 1
Source: MIOO: Training work in physics 04/11/2013 variant PHI1502.
The figure shows a graph of a cycle carried out with a monatomic ideal gas. In which of the sections did the internal energy of the gas decrease? The amount of gas substance is constant.
Solution.
The internal energy of a fixed amount of a monatomic ideal gas depends only on temperature, its change is determined by the expression: Thus, the internal energy decreases with decreasing temperature. From the above graph it can be seen that the temperature decreased in the area CD.
Correct answer: 3
Answer: 3
Source: Unified State Examination in Physics 06/06/2013. main wave. Far East. Option 1.
Solution.
It is clear from the diagram that this corresponds to the section DA.
Correct answer: 4
Answer: 4
Source: Unified State Examination in Physics 06/06/2013. main wave. Siberia. Option 1.
The figure shows a cycle carried out with an ideal gas. Work is not done on site
Solution.
BC.
Correct answer: 2
Answer: 2
Source: Unified State Examination in Physics 06/06/2013. main wave. Siberia. Option 2.
The figure shows a cycle carried out with an ideal gas. Work is not done on site
Solution.
The work of a gas is related to the change in its volume. If the gas expands, it does positive work on external forces. On the contrary, if its volume decreases, external forces do positive work on it. Thus, the work is zero if the volume of the gas does not change.
AB
Correct answer: 1
Answer: 1
Source: Unified State Examination in Physics 06/06/2013. main wave. Siberia. Option 3.
The figure shows a cycle carried out with an ideal gas. Work is not done on site
Solution.
The work of a gas is related to the change in its volume. If the gas expands, it does positive work on external forces. On the contrary, if its volume decreases, external forces do positive work on it. Thus, the work is zero if the volume of the gas does not change.
According to Charles's law, in an isochoric process, the ratio of pressure and temperature remains constant: It is clear from the diagram that the plot has a similar property DA. It is on him that no work is done.
Correct answer: 4
Answer: 4
Source: Unified State Examination in Physics 06/06/2013. main wave. Siberia. Option 5.
A. A positive amount of heat cannot spontaneously transfer from a colder body to a hotter one.
1) only A
2) only B
4) neither A nor B
Solution.
Both statements are true. They form the essence of the second law of thermodynamics.
Postulate of Clausius: “A process is impossible, the only result of which would be the transfer of heat from a colder body to a hotter one” (such a process is called the Clausius process). That is, heat can spontaneously transfer only from a hotter body to a colder one.
Correct answer: 3.
Answer: 3
Source: Unified State Examination in Physics 06/06/2013. main wave. Ural. Option 1.
Which of the following statements is true(s)?
A. A positive amount of heat spontaneously passes from a hotter body to a colder one.
B. It is impossible to create a cyclic heat engine, with the help of which it is possible to completely convert the energy received from the heater into mechanical work.
1) only A
2) only B
4) neither A nor B
Solution.
The second law of thermodynamics forbids the so-called perpetual motion machines of the second kind, showing that the efficiency cannot be equal to one.
There are several equivalent formulations of the second law of thermodynamics:
Postulate of Clausius: “A process is impossible, the only result of which would be the transfer of heat from a colder body to a hotter one” (such a process is called the Clausius process).
Thomson's (Kelvin's) postulate: "A circular process is impossible, the only result of which would be the production of work by cooling the thermal reservoir" (such a process is called the Thomson process).
This shows that both statements are true.
Correct answer: 3
Answer: 3
Source: Unified State Examination in Physics 06/06/2013. main wave. Ural. Option 2.
Which of the following statements is true(s)?
A. During thermal contact of two bodies having different temperatures, a positive amount of heat cannot spontaneously transfer from a body with a lower temperature to a body with a higher temperature.
B. It is impossible to create a cyclic heat engine, with the help of which it is possible to completely convert the energy received from the heater into mechanical work.
1) only A
2) only B
4) neither A nor B
Solution.
Both statements are true.
The second law of thermodynamics forbids the so-called perpetual motion machines of the second kind, showing that the efficiency cannot be equal to one.
There are several equivalent formulations of the second law of thermodynamics:
Postulate of Clausius: “A process is impossible, the only result of which would be the transfer of heat from a colder body to a hotter one” (such a process is called the Clausius process). That is, when two bodies come into contact with different temperatures heat can only move from a hotter body to a colder one.






