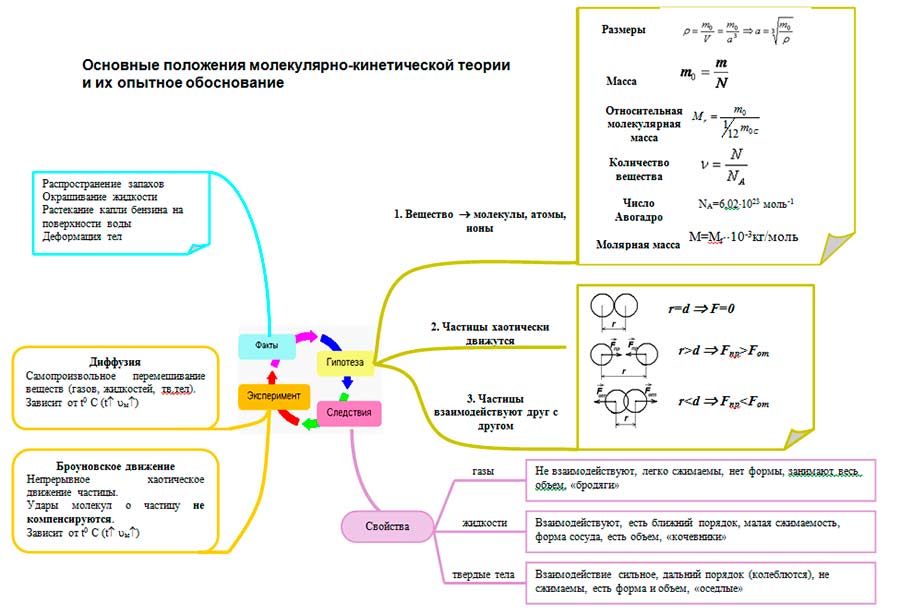
Formulate the main provisions of molecular kinetics. All bodies are made up of particles - atoms, molecules, ions. Basic Provisions of Molecular Kinetic Theory
Basic Provisions of Molecular Kinetic Theory
What is the main task of molecular physics? What is Molecular Kinetic Theory? Formulate the main provisions of the molecular-kinetic theory. What observations and experiments confirm the main provisions of the molecular kinetic theory? What is a molecule? atom?
What is the main task of molecular physics? Explain the properties of macroscopic bodies and the thermal processes occurring in them, based on the idea that all bodies consist of separate, randomly moving particles.
What is Molecular Kinetic Theory? Molecular-kinetic theory (MKT) is a theory that considers the structure of a substance from the point of view of three main approximately correct provisions.
Formulate the main provisions of the molecular-kinetic theory. all bodies are made up of particles, between which there are gaps particles are in continuous chaotic motion particles interact with each other
What observations and experiments confirm the main provisions of the molecular kinetic theory? Diffusion Brownian motion Experiments by Dunoyer and Stern
What is a molecule? atom? A molecule is an independent particle, a mandatory component of any substance that has all the chemical and physical properties this substance. Any molecule consists of the simplest independent particles - atoms. Atom - the smallest possible particle of any of the simplest chemical substances called elements.
The main provisions of the molecular kinetic theory have been subjected to comprehensive experimental verification. The most famous experiments demonstrating the molecular structure of a substance and confirming the molecular kinetic theory are the Dunoyer experiment and the Otto Stern experiment (1888 - 1969), performed in 1911 and 1920, respectively. In these experiments, molecular beams were created by evaporation of various metals, and therefore the molecules of the studied gases were atoms of these metals. Such experiments made it possible to verify the predictions of the molecular-kinetic theory, which it gives for the case of gases, the molecules of which can be considered as material points, that is, for monatomic gases.
Dunoyer's experiment The scheme of Dunoyer's experiment with molecular beams is shown in fig. 1. A glass vessel, the material of which was chosen in such a way as to provide a high vacuum, was divided into three compartments 1, 2 and 3 by two partitions with diaphragms 4. In compartment 1 there was a gas, which in this experiment was used sodium vapor obtained by heating it. Molecules of this gas could freely fly through the holes in the diaphragms, collimating the molecular beam 5, that is, allowing it to pass only within a small solid angle. In compartments 2 and 3, an ultra-high vacuum was created, such that sodium atoms could fly through them without collisions with air molecules. The unscattered molecular beam left a trace 6 on the end wall of the vessel. But even in the case of ultrahigh vacuum, the molecular beam was scattered at the edges of diaphragms 4. Therefore, there was a “penumbra” region 7 on the end wall of the vessel, in which particles that underwent scattering left traces. As the vacuum in compartment 3 worsened, region 7 increased. From the magnitude of the smearing of the trail of scattered sodium atoms, it was possible to estimate the length of their free path. Such estimates were made by Max Born (1882 - 1970) based on the results of experiments similar to Dunoyer's.
Otto Stern's Experiment One of the most famous experiments with molecular beams was Stern's experiments, in which for the first time it was possible to carry out direct measurements of molecular velocities. The most famous scheme of Stern's experiment is shown in fig. 2. Platinum thread 1, on which a drop of silver was applied, was located on the axis of two coaxial cylinders 2 and 3, and in cylinder 2 there was a slot parallel to its axis. The cylinders could rotate around their axis. In Stern's experiments, the angular velocity of their rotation was 2...3 thousand revolutions per minute. When passed through a platinum thread electric current she warmed up maximum temperature about 1200 oС. As a result, silver began to evaporate, its atoms flew through slot 4 of cylinder 2 and settled on the surface of cylinder 3, leaving trace 5 on it. sector corresponding to their rectilinear distribution. The rotation of the cylinders led to a curvature of the trajectory of molecules in the reference frame associated with the cylinders and, as a result, to a change in the position of the silver atoms that settled on the outer cylinder. Analyzing the density of settled molecules, it was possible to estimate the characteristics of the distribution of molecules by velocity, in particular, the maximum and minimum speeds corresponding to the edges of the track, and also to find the most probable velocity corresponding to the maximum density of settled molecules.
Rice. 1 - Scheme of the Dunoyer experiment 1 - compartment filled with gas 2 and 3 - compartments with ultrahigh vacuum 4 - partitions with diaphragms 5 - molecular beam 6 - trace of an unscattered beam 7 - trace of scattered molecules
Fig.2 - Schematic of the Stern experiment 1 - source of molecules 2 and 3 - rotating cylinders 4 - slit limiting the molecular beam 5 - trace of the molecular beam
Diffusion Diffusion is the phenomenon of mutual penetration of a molecule of one substance between the molecules of another. Diffusion can occur in gases (very fast), in liquids (fast), in metals (very slow).
Brownian motion Brownian motion is the thermal motion of particles suspended in a liquid or gas. The causes of Brownian motion are: 1. Random movement of molecules 2. Deviation from the average value of the pressure produced by them. The following have been established: 1. Brownian motion is carried out at any t and lasts indefinitely. 2. Brownian particles make random trajectories. 3. The nature of the motion of a Brownian particle does not depend on its nature Brownian motion is a direct proof of the random motion of molecules.
We are surrounded by various things. We can see that they are either solids or liquids or gases. There are a lot of questions about everything that surrounds us. Gives answers to many questions molecular kinetic theory.
Molecular-kinetic theory is a set of views used to describe the observed and measured properties of a substance based on the study of the properties of atoms and molecules of a given substance, their interaction and movement.
Basic Provisions of Molecular Kinetic Theory
All bodies are made up of particles - atoms, molecules, ions.
All particles are in continuous chaotic thermal motion.
Between the particles of any body there are forces of interaction - attraction and repulsion.
Thus, in the molecular-kinetic theory, the object of study is a system consisting of a large number of particles - macrosystem. To explain the behavior of such a system, the laws of mechanics are not applicable. Therefore, the main research method is statistical method studying the properties of matter.
To explain and predict phenomena, it is important to know main characteristics of molecules:
- Dimensions

An estimate of the size of a molecule can be made as the size of a cube a containing one molecule, based on the density of solid or liquid substances and the mass of one molecule:

- Mass of molecules
The ratio of the mass of a substance m to the number of molecules N in this substance:
- Relative molecular weight
The ratio of the mass of a molecule (or atom) of a given substance to 1/12 of the mass of a carbon atom:
- Amount of substance
The amount of substance is equal to the ratio of the number of particles N in the body (atoms - in the atomic substance, molecules - in the molecular) to the number of molecules in one mole of the substance NBUT:

- Avogadro constant
The number of molecules contained in 1 mol of a substance.
- Molar mass
The molar mass of a substance is the mass of a substance taken in an amount of 1 mole.
AT international system units molar mass of a substance is expressed in kg/mol.
- Interaction (quantitative based on experiences)

The interaction of molecules is characterized by both attraction and repulsion at the same time: at distances r
Molecular-kinetic theory makes it possible to understand why a substance can be in gaseous, liquid and solid states. From the point of view of the MKT, the states of aggregation differ in terms of the value of the average distance between molecules and the nature of the movement of molecules relative to each other.
The main provisions of the molecular kinetic theory have been repeatedly confirmed by various physical experiments. For example, research:
A) diffusion
B) Brownian motion
Brief summary
Molecular-kinetic theory explains the structure and properties of bodies on the basis of the movement and interaction of atoms, molecules and ions. MKT is based on three positions, which are fully confirmed experimentally and theoretically:
1) all bodies consist of particles - molecules, atoms, ions;
2) the particles are in continuous chaotic thermal motion;
3) between the particles of any body there are forces of interaction - attraction and repulsion.
The molecular structure of a substance is confirmed by the direct observation of molecules in electron microscopes, as well as the dissolution of solids in liquids, the compressibility and permeability of a substance. Thermal motion - Brownian motion and diffusion. The presence of intermolecular interaction strength and elasticity solids, surface tension liquids.
Reference outline for the lesson:
Questions for self-control in the block "Basic provisions of the molecular kinetic theory and their experimental substantiation"
- Formulate the main provisions of the molecular-kinetic theory.
- What observations and experiments confirm the main provisions of the molecular kinetic theory?
- What is a molecule? atom?
- What is called relative molecular weight? What formula expresses this concept?
- What is the quantity of a substance? What formula expresses this concept? What is the unit of quantity of a substance?
- What is called the Avogadro constant?
- What is the molar mass of a substance? What formula expresses the meaning of this concept? What is the unit molar mass?
- What is the nature of intermolecular forces?
- What are the properties of molecular forces?
- How do the forces of interaction depend on the distance between them?
- Describe the nature of the movement of molecules in gases, liquids and solids.
- What is the nature of particle packing in gases, liquids and solids?
- What is the average distance between molecules in gases, liquids and solids?
- List the main properties of gases, liquids, solids.
- What is called Brownian motion?
- What does Brownian motion indicate?
- What is called diffusion? Give examples of diffusion in gases, liquids and solids.
- 18. How does the diffusion rate depend on the temperature of bodies?
DEFINITION
Atom - the smallest particle of a given chemical element. All atoms that exist in nature are represented in Mendeleev's periodic system of elements.
Atoms are combined into a molecule by chemical bonds based on electrical interaction. The number of atoms in a molecule can be different. A molecule can consist of one, two, three, or even several hundred atoms.
DEFINITION
Molecule- the smallest particle of a given substance that has its chemical properties.
Molecular Kinetic Theory- the doctrine of the structure and properties of matter based on the concept of the existence of atoms and molecules.
The founder of the molecular kinetic theory is M.V. Lomonosov (1711-1765), who formulated its main provisions and applied them to explain various thermal phenomena.
Basic Provisions of Molecular Kinetic Theory
The main provisions of the ICB:
- all bodies in nature consist of the smallest particles (atoms and molecules);
- particles are in continuous chaotic motion, which is called thermal;
- particles interact with each other: forces of attraction and repulsion act between the particles, which depend on the distance between the particles.
The molecular kinetic theory is confirmed by many phenomena.
The mixing of various liquids, the dissolution of solids in liquids, is explained by the mixing of molecules of various kinds. In this case, the volume of the mixture may differ from the total volume of its constituent components. which indicates different sizes of molecular compounds.
DEFINITION
Diffusion- the phenomenon of penetration of two or more adjoining substances into each other.
Diffusion proceeds most intensively in gases. The spread of odors is due to diffusion. Diffusion indicates that the molecules are in constant chaotic motion. Also, the phenomenon of diffusion indicates that there are gaps between the molecules, i.e. matter is discrete.
DEFINITION
Brownian motion- thermal motion of the smallest microscopic particles suspended in a liquid or gas.
This phenomenon was first observed by the English botanist R. Brown in 1827. Observing flower pollen suspended in water through a microscope, he saw that each pollen particle makes fast random movements, moving over a certain distance. As a result of individual movements, each pollen particle moved along a zigzag trajectory (Fig. 1a).
Fig.1. Brownian motion: a) trajectories of motion of individual particles suspended in a liquid; b) transfer of momentum by liquid molecules to a suspended particle.
Further studies of Brownian motion in various liquids and with various solid particles showed that this motion becomes more intense, the smaller the particle size and the higher the temperature of the experiment. This movement never stops and does not depend on any external causes.
R. Brown could not explain the observed phenomenon. The theory of Brownian motion was built by A. Einstein in 1905 and received experimental confirmation in the experiments of the French physicist J. Perrin (1900-1911).
Liquid molecules that are in constant chaotic motion, when colliding with a suspended particle, transfer some impulse to it (Fig. 1, b). In the case of a particle large sizes the number of molecules attacking it from all sides is large, their impacts are compensated at each moment of time, and the particle remains practically motionless. If the particle size is very small, then the impacts of the molecules are not compensated - on the one hand, it can hit more molecules than with the other, as a result of which the particle will begin to move. It is precisely such a movement under the influence of random impacts of molecules that Brownian particles perform. Although Brownian particles are billions of times larger than the mass of individual molecules and move at very low speeds (compared to the speeds of molecules), their movement can still be observed under a microscope.
Examples of problem solving
EXAMPLE 1
EXAMPLE 2
Molecular Kinetic Theory called the doctrine of the structure and properties of matter based on the idea of the existence of atoms and molecules as the smallest particles of chemical substances.
The molecular kinetic theory is based on three main points:
1.
All substances - liquid, solid and gaseous - are formed from the smallest particles - molecules, which themselves consist of atoms.
Molecules and atoms are electrically neutral particles. Under certain conditions, molecules and atoms can acquire additional electric charge and turn into positive or negative ions.
2. Atoms and molecules are in continuous chaotic motion.
3. Particles interact with each other by forces that are electrical in nature. Gravitational interaction between particles
negligible.
The most striking experimental confirmation of the ideas of the molecular kinetic theory about the random motion of atoms and molecules is Brownian motion.
Brownian motion - e that is the thermal movement of the smallest microscopic particles suspended in a liquid or gas. It was discovered by the English botanist R. Brown.
Brownian particles move under the influence of random collisions of molecules. Due to the chaotic thermal motion of the molecules, these impacts never balance each other. As a result, the speed of a Brownian particle randomly changes in magnitude and direction, and its trajectory is a complex zigzag curve.
The constant chaotic movement of the molecules of a substance is also manifested in another easily observed phenomenon - diffusion.
By diffusion The phenomenon of penetration of two or more adjoining substances into each other is called.
The process proceeds most rapidly in a gas if it is heterogeneous in composition. Diffusion leads to the formation of a homogeneous mixture, regardless of the density of the components. So, if in two parts of the vessel, separated by a partition, there are oxygen O 2 and hydrogen H 2, then after the removal of the partition, the process of interpenetration of gases into each other begins, leading to the formation of an explosive mixture - explosive gas.
Diffusion and Brownian motion are related phenomena. The interpenetration of contacting substances into each other and the random movement of the smallest particles suspended in a liquid or gas occur due to the chaotic thermal motion of molecules.
Define the term "heat capacity". What types of heat capacities are used in thermal engineering calculations. Explain what determines the heat capacity of gases? Write down the Mayer equation.
Heat capacity- a value equal to the ratio of the heat supplied to the body or removed from it to the corresponding change in its temperature: C= , [J/K]
where C is the heat capacity; Q is the supplied (or removed) heat; ∆T is the change in body temperature.
In thermal calculations apply different kinds reduced heat capacities (referred to a unit of mass, quantity and volume)
Mass (or specific) heat capacity is the ratio of the heat capacity of the body to its mass: c \u003d,
where c is the mass heat capacity; m - body weight.
molar heat capacity is the ratio of heat capacity to the amount of substance: µ c = ,
where μ c is the molar heat capacity; n is the amount of substance.
Volumetric heat capacity is the ratio of heat capacity to 1 m 3 of gas under normal conditions: c`=,
where c` is the volumetric heat capacity; v 0 is the volume of gas under normal conditions.
In SI, mass heat capacity c is measured in J / kg * K, molar heat capacity µ s - J / mol * K, volumetric heat capacity c "- J / m 3 * K.
The heat capacities of gases and vapors are variable; for ideal gases they depend on their temperature, and for real gases and vapors also on their pressure.
The heat capacity of gases depends to a large extent on the conditions under which they are heated or cooled. Among these processes in technology, the most important are the processes occurring during constant volume gas (isochoric process) and at constant pressure gas (isobaric process).
In this regard, distinguish heat capacity at constant volume(with v) and heat capacity at constant pressure(with p).
The quantitative relationship between c p and c v is set using Mayer's equations: with p − with v = R, where R is the universal gas constant.
Thus, the difference between the isobaric and isochoric heat capacities for all gases is a constant value and is equal to the universal gas constant.
Define the term " disperse systems". Name two main features of dispersed systems. How are dispersed systems classified? Using each attribute, describe the disperse systems.
dispersed called systems consisting of many small particles distributed in a liquid, solid or gaseous medium.
All disperse systems are characterized by two main features: high fragmentation (dispersion) and heterogeneity.
Heterogeneity disperse systems is manifested in the fact that these systems consist of two (or more) phases. All dispersed systems consist of a continuous phase - dispersion medium and discontinuous phase (particles) - dispersed phase.
High dispersion gives substances new qualitative features: increased reactivity and solubility, color intensity, light scattering, etc.
Classification of dispersed systems carried out on the basis of various features, namely: 1) by particle size, 2) by the state of aggregation of the dispersed phase and the dispersion medium, 3) by the nature of the interaction of the particles of the dispersed phase with each other and with the medium.
Depending on particle size disperse systems are divided into groups:
1) coarse systems - systems in which particles have a size of 1000 nm or more;
2) colloidal systems - particles have a size from 1 to 500 nm.
3) true solutions - particles have a size of up to 1 nm.
According to the state of aggregation of the dispersed phase and the dispersion medium systems are classified as follows:
With respect to the ratio of colloidal particles to the dispersion medium distinguish lyophilic and lyophobic systems ( from the Greek "philia" - love, "phobia" - hate).
Lyophilic systems- those in which colloidal particles are associated with the molecules of the dispersion medium and have a shell of them (if the dispersion medium is water, the systems are called hydrophilic). For example, lyophilic colloidal systems include solutions formed by dissolving natural or synthetic IUDs. These are solutions of proteins, starch, cellulose ethers and various resins, both natural and synthetic.
Lyophobic systems(hydrophobic) - those in which colloidal particles are weakly bound to the molecules of the dispersion medium or solvent. For example, lyophobic systems include precious metal sols, sulfur sols, sols of iron and aluminum hydroxides, etc. These systems are characterized by aggregative instability and require stabilization.
7. Define the concept of "chemical equilibrium". Formulate Le Chatelier's principle. Justify how changes in pressure affect the equilibrium position of the reaction: N 2 +3H 2 ⇄2NH 3 . For this process, write an expression for the equilibrium constant and predict its value if the equilibrium concentrations of the reactants nitrogen, hydrogen, and ammonia are 3 mol/l, 9 mol/l, 4 mol/l, respectively.
Chemical equilibrium
For a system in chemical equilibrium, the concentrations of reagents, temperature, and other parameters of the system do not change with time.
Le Chatelier's principle: if a system in a state of equilibrium is acted upon from the outside, changing any of the equilibrium conditions (temperature, pressure, concentration), then the processes in the system are intensified, aimed at compensating (or weakening) the external influence.
Reaction N 2 + 3H 2 ⇄ 2NH 3 proceeds with the participation of gaseous substances and is reversible. Pressure significantly affects the equilibrium position in such reactions, since they are accompanied by a change in volume due to a change in the amount of substance in the transition from the starting substances to the products of the reaction: with an increase in pressure, the equilibrium shifts in the direction in which the total number of moles of gases decreases and vice versa.
According to the reaction equation N 2 + 3H 2 ⇄ 2NH 3, it can be seen that the amount of initial gaseous substances is 4 mol (1 mol of nitrogen and 3 mol of hydrogen), and the amount of gaseous products is 2 mol (2 mol of ammonia). Consequently, with an increase in pressure, the chemical equilibrium will shift to the right, towards a smaller amount of gaseous substances, and with a decrease in pressure, to the left, towards the starting substances.
Equilibrium constant- a value that determines for a given chemical reaction the ratio between thermodynamic activities (or, depending on the conditions of the reaction, partial pressures, concentrations) of starting substances and products in a state of chemical equilibrium (in accordance with the law of mass action). Knowing the equilibrium constant of the reaction, it is possible to calculate the equilibrium composition of the reacting mixture, the limiting yield of products, and determine the direction of the reaction.
Given:\u003d 3 mol / l, \u003d 9 mol / l, \u003d 4 mol / l
Find: K with -?
Solution: 1) Let's write the reaction equation N 2 +3H 2 ⇄2NH 3
2) The equilibrium constant for this reaction has the expression: K c \u003d 2 / * 3; 3) calculate the equilibrium constant K c \u003d 4 2 / 3 * 9 3 \u003d 0.0073
Answer: K c \u003d 0.0073
8. Define the term "disperse system". Give a classification of dispersed systems according to the state of aggregation of the dispersed phase and the dispersed medium. Systematize this knowledge and determine the state of aggregation of the dispersed phase and dispersion medium for the proposed systems: oil; dusty air. Predict and explain the stability of these colloidal systems.
Disperse system- these are formations of two or more phases (bodies) that do not mix at all or practically and do not chemically react with each other.
Most general classification of dispersed systems is based on the difference in the state of aggregation of the dispersion medium and the dispersed phase. Combinations of three types state of aggregation allow distinguishing nine types of dispersed systems. For brevity, they are usually denoted by a fraction, the numerator of which indicates the dispersed phase, and the denominator indicates the dispersion medium, for example, for the “gas in liquid” system, the designation G/L is adopted.
| Designation | dispersed phase | Dispersion medium | Name and example |
| Y/Y | gaseous | gaseous | The dispersed system is not formed |
| F/G | Liquid | gaseous | Aerosols: fogs, clouds |
| T/Y | solid | gaseous | Aerosols (dust, fumes), powders |
| G/F | gaseous | Liquid | Gas emulsions and foams |
| F/F | Liquid | Liquid | Emulsions: oil, cream, milk |
| T/F | solid | Liquid | Suspensions and sols: pulp, silt, suspension, paste |
| G/T | gaseous | solid | porous bodies |
| F/T | Liquid | solid | Capillary systems: liquid in porous bodies, soil, soil |
| T/T | solid | solid | Solid heterogeneous systems: alloys, concrete, composite materials |
In turn, these systems are classified according to the degree of dispersion.
Systems with particles of the dispersed phase of the same size are called monodisperse, and systems with particles of different sizes are called polydisperse. As a rule, the real systems surrounding us are polydisperse.
Stability of dispersed systems is the possibility of their being in the initial state for an indefinitely long time.
The stability of dispersed systems can be:
1. To the precipitation
dispersed phase - characterizes the ability of a dispersed system to maintain an equilibrium distribution of the phase over the volume of the dispersion medium or its resistance to phase separation. This property is called sedimentation (kinetic) stability .
2. Towards aggregation
its particles. Aggregative stability
is the ability of a disperse system to keep the degree of dispersion unchanged over time, i.e. particle sizes and their individuality.
It is due to the ability of dispersed systems to form aggregates (i.e., grow larger). With respect to aggregation, dispersed systems can be stable kinetically
and thermodynamically
. Thermodynamically stable systems are formed as a result of spontaneous dispersion of one of the phases, i.e. spontaneous formation of a heterogeneous system.
Dispersion system: dusty air consists of a gaseous dispersion medium and a solid dispersion phase. This system is kinetically and aggregatively unstable.
Dispersion system: oil consists of a liquid dispersion medium and a liquid dispersion phase. And the system is kinetically and aggregatively stable.
9. Define the concept of "shift of chemical equilibrium." Comment on which factors influence and which do not affect the position of chemical equilibrium? Formulate Le Chatelier's principle. Predict in which direction the equilibrium shifts with an increase in pressure, temperature, concentration of the initial substances for the reaction:
+CaCO3 + O(steam)⇄Ca(HCO 3 +Q
Chemical equilibrium- the dynamic state of a chemical system in which a chemical reaction proceeds reversibly, and the rates of the forward and reverse reactions are equal to each other.
The position of chemical equilibrium depends on the following reaction parameters: temperature, pressure and concentration. Changing these parameters causes a change in the speeds of the flowing chemical reactions and shifts the chemical balance.
Factors Affecting Chemical Equilibrium: temperature, pressure, concentration.
1) temperature: when the temperature increases, the chemical equilibrium shifts towards an endothermic (absorption) reaction, and when it decreases, towards an exothermic (isolation) reaction.
2) pressure: when pressure increases, the chemical equilibrium shifts towards a smaller volume of substances, and when it decreases, towards a larger volume. This principle only applies to gases, i.e. if they participate in the reaction solids, they are not taken into account.
3) concentration of starting substances and reaction products
With an increase in the concentration of one of the starting substances, the chemical equilibrium shifts towards the reaction products, and with an increase in the concentration of the reaction products, towards the starting substances.






