The structure of the atom. Rutherford's experience. History of the development of natural sciences in the Middle Ages
The planetary model of the atom was proposed by E. Rutherford in 1910. The first studies of the structure of the atom were made by him with the help of alpha particles. Based on the results obtained in experiments on their scattering, Rutherford suggested that the entire positive charge an atom is concentrated in a tiny nucleus at its center. On the other hand, negatively charged electrons are distributed throughout the rest of its volume.
A little background
The first brilliant guess about the existence of atoms was made by the ancient Greek scientist Democritus. Since then, the idea of the existence of atoms, the combinations of which give all the substances around us, has not left the imagination of people of science. From time to time it was approached by its various representatives, but before early XIX centuries of their construction were just hypotheses, not supported by experimental data.
Finally, in 1804, more than a hundred years before the planetary model of the atom appeared, the English scientist John Dalton provided evidence for its existence and introduced the concept of atomic weight, which was his first quantitative characteristic. Like his predecessors, he imagined atoms to be the smallest pieces of matter, like solid balls, which could not be divided into even smaller particles.
Discovery of the electron and the first model of the atom
Almost a century passed when, finally, in late XIX century, the Englishman J. J. Thomson also discovered the first subatomic particle, the negatively charged electron. Since atoms are electrically neutral, Thomson thought they must be composed of a positively charged nucleus with electrons scattered throughout its volume. Based on various experimental results, in 1898 he proposed his model of the atom, sometimes called "plums in a pudding", because the atom in it was represented as a sphere filled with some positively charged liquid, into which electrons were embedded, as "plums into the pudding. The radius of such a spherical model was about 10 -8 cm. The total positive charge of the liquid is symmetrically and uniformly balanced by the negative charges of the electrons, as shown in the figure below.
This model satisfactorily explained the fact that when a substance is heated, it begins to emit light. Although this was the first attempt to understand what an atom was, it failed to satisfy the results of the experiments carried out later by Rutherford and others. Thomson agreed in 1911 that his model simply could not answer how and why the scattering of α-rays observed in experiments occurs. Therefore, it was abandoned, and it was replaced by a more perfect planetary model of the atom.
How is the atom arranged anyway?
Ernest Rutherford gave an explanation of the phenomenon of radioactivity, which brought him Nobel Prize, but his most significant contribution to science came later, when he established that the atom consists of a dense nucleus surrounded by orbits of electrons, just as the Sun is surrounded by the orbits of planets. 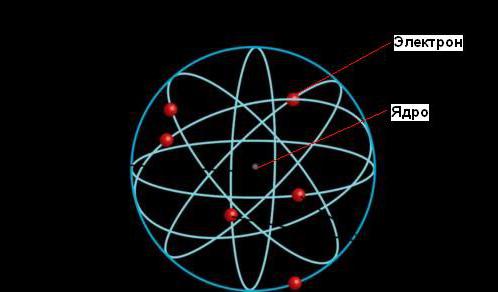
According to the planetary model of an atom, most of its mass is concentrated in a tiny (compared to the size of the entire atom) nucleus. Electrons move around the nucleus, traveling at incredible speeds, but most of the volume of atoms is empty space.
The size of the nucleus is so small that its diameter is 100,000 times smaller than that of an atom. The diameter of the nucleus was estimated by Rutherford as 10 -13 cm, in contrast to the size of the atom - 10-8 cm. Outside the nucleus, electrons revolve around it at high speeds, resulting in centrifugal forces that balance the electrostatic forces of attraction between protons and electrons.
Rutherford's experiments
The planetary model of the atom arose in 1911, after the famous experiment with gold foil, which made it possible to obtain some fundamental information about its structure. Rutherford's path to the discovery of the atomic nucleus is good example the role of creativity in science. His search began as early as 1899 when he discovered that certain elements emit positively charged particles that can penetrate anything. He called these particles alpha (α) particles (now we know they were helium nuclei). Like all good scientists, Rutherford was curious. He wondered if alpha particles could be used to find out the structure of an atom. Rutherford decided to aim a beam of alpha particles at a sheet of very thin gold foil. He chose gold because it can produce sheets as thin as 0.00004 cm. Behind a sheet of gold foil, he placed a screen that glowed when alpha particles hit it. It was used to detect alpha particles after they had passed through the foil. A small slit in the screen allowed the alpha particle beam to reach the foil after exiting the source. Some of them must pass through the foil and continue to move in the same direction, the other part must bounce off the foil and be reflected under sharp corners. You can see the scheme of the experiment in the figure below. 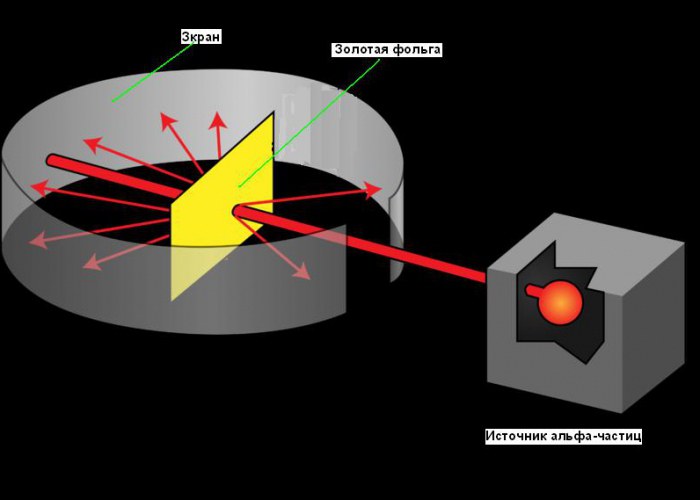
What happened in Rutherford's experiment?
Based on J. J. Thomson's model of the atom, Rutherford assumed that the solid regions of positive charge filling the entire volume of gold atoms would deviate or bend the trajectories of all alpha particles as they passed through the foil.
However, the vast majority of the alpha particles passed right through the gold foil as if it wasn't there. They seemed to be passing through empty space. Only a few of them deviate from the straight path, as it was supposed at the beginning. Below is a plot of the number of particles scattered in the respective direction versus the scattering angle. 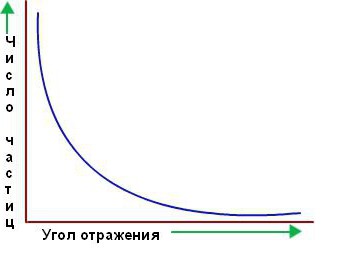
Surprisingly, a tiny percentage of the particles bounced back from the foil, like a basketball bouncing off a backboard. Rutherford realized that these deviations were the result of a direct collision between alpha particles and the positively charged components of the atom.
The nucleus takes center stage
Based on the negligible percentage of alpha particles reflected from the foil, we can conclude that all the positive charge and almost all the mass of the atom are concentrated in one small area, and the rest of the atom is mostly empty space. Rutherford called the area of concentrated positive charge the nucleus. He predicted and soon discovered that it contained positively charged particles, which he named protons. Rutherford predicted the existence of neutral atomic particles, called neutrons, but he couldn't detect them. However, his student James Chadwick discovered them a few years later. The figure below shows the structure of the nucleus of a uranium atom. 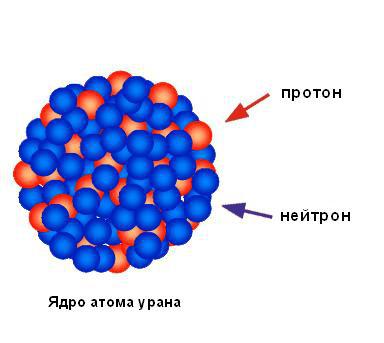
Atoms consist of positively charged heavy nuclei surrounded by negatively charged extremely light particles-electrons rotating around them, and at such speeds that mechanical centrifugal forces simply balance their electrostatic attraction to the nucleus, and in this connection the stability of the atom is allegedly ensured.
The disadvantages of this model
Rutherford's main idea was related to the idea of a small atomic nucleus. The assumption about the orbits of the electrons was pure conjecture. He did not know exactly where and how electrons revolve around the nucleus. Therefore, Rutherford's planetary model does not explain the distribution of electrons in orbits.
In addition, the stability of the Rutherford atom was possible only with the continuous movement of electrons in orbits without loss of kinetic energy. But electrodynamic calculations have shown that the movement of electrons along any curvilinear trajectories, accompanied by a change in the direction of the velocity vector and the appearance of a corresponding acceleration, is inevitably accompanied by the emission of electromagnetic energy. In this case, according to the law of conservation of energy, the kinetic energy of the electron must be very quickly spent on radiation, and it must fall on the nucleus, as shown schematically in the figure below. 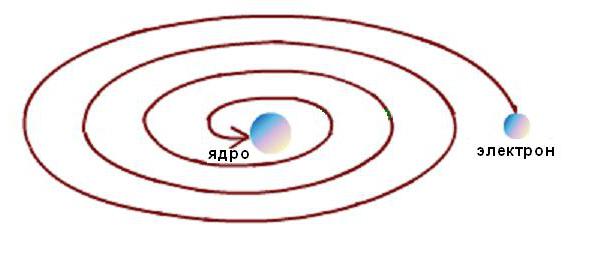 But this does not happen, since atoms are stable formations. A typical scientific contradiction arose between the model of the phenomenon and the experimental data.
But this does not happen, since atoms are stable formations. A typical scientific contradiction arose between the model of the phenomenon and the experimental data.
From Rutherford to Niels Bohr
The next major step forward in atomic history came in 1913, when the Danish scientist Niels Bohr published a description of a more detailed model of the atom. She determined more clearly the places where electrons could be. Although later scientists would develop more sophisticated atomic designs, Bohr's planetary model of the atom was basically correct, and much of it is still accepted today. It had many useful applications, for example, it is used to explain the properties of various chemical elements, the nature of their radiation spectrum and the structure of the atom. The planetary model and the Bohr model were the most important milestones that marked the emergence of a new direction in physics - the physics of the microworld. Bohr received the 1922 Nobel Prize in Physics for his contributions to our understanding of the structure of the atom.
What new did Bohr bring to the model of the atom?
While still a young man, Bohr worked in Rutherford's laboratory in England. Since the concept of electrons was poorly developed in Rutherford's model, Bohr focused on them. As a result, the planetary model of the atom was significantly improved. Bohr's postulates, which he formulated in his article "On the Structure of Atoms and Molecules", published in 1913, read:
1. Electrons can move around the nucleus only at fixed distances from it, determined by the amount of energy they have. He called these fixed levels energy levels or electron shells. Bohr envisioned them as concentric spheres, with a nucleus at the center of each. In this case, electrons with lower energy will be found at lower levels, closer to the nucleus. Those who have more energy will be found on more high levels, away from the core.
2. If an electron absorbs some (quite certain for a given level) amount of energy, then it will jump to the next, higher energy level. Conversely, if he loses the same amount of energy, he will return back to his original level. However, an electron cannot exist on two energy levels.
This idea is illustrated by a figure. 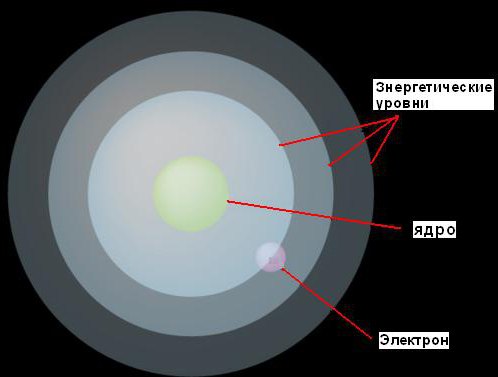
Energy portions for electrons
The Bohr model of the atom is actually a combination of two different ideas: Rutherford's atomic model with electrons revolving around the nucleus (essentially the Bohr-Rutherford planetary model of the atom), and Max Planck's idea of quantizing the energy of matter, published in 1901. A quantum (plural - quanta) is the minimum amount of energy that can be absorbed or emitted by a substance. It is a kind of discretization step for the amount of energy.
If energy is compared to water and you want to add it to matter in the form of a glass, you cannot just pour water in a continuous stream. Instead, you can add it in small amounts, like a teaspoonful. Bohr believed that if electrons can only absorb or lose fixed amounts of energy, then they should only vary their energy by these fixed amounts. Thus, they can only occupy fixed energy levels around the nucleus, which correspond to quantized increments of their energy.
So from the Bohr model grows a quantum approach to explaining what the structure of the atom is. The planetary model and the Bohr model were a kind of steps from classical physics to quantum physics, which is the main tool in the physics of the microworld, including atomic physics.
In 1903, the English scientist Thomson proposed a model of the atom, which was jokingly called the "bun with raisins." According to him, an atom is a sphere with a uniform positive charge, in which negatively charged electrons are interspersed like raisins.
However, further studies of the atom showed that this theory is untenable. And a few years later, another English physicist, Rutherford, conducted a series of experiments. Based on the results, he built a hypothesis about the structure of the atom, which is still recognized worldwide.
Rutherford's experience: the proposal of his model of the atom
In his experiments, Rutherford passed a beam of alpha particles through thin gold foil. Gold was chosen for its plasticity, which made it possible to create a very thin foil, almost one layer of molecules thick. Behind the foil was a special screen that was illuminated when bombarded by alpha particles falling on it. According to Thomson's theory, alpha particles should have passed through the foil unhindered, deviating quite a bit to the sides. However, it turned out that some of the particles behaved in this way, and a very small part bounced back, as if hitting something.
That is, it was found that inside the atom there is something solid and small, from which alpha particles bounced off. It was then that Rutherford proposed a planetary model of the structure of the atom. Rutherford's planetary model of the atom explained the results of both his experiments and those of his colleagues. To this day, no better model has been proposed, although some aspects of this theory still do not agree with practice in some very narrow areas of science. But basically, the planetary model of the atom is the most useful of all. What is this model?
Planetary model of the structure of the atom
As the name implies, an atom is compared to a planet. In this case, the planet is the nucleus of an atom. And electrons revolve around the nucleus at a fairly large distance, just like satellites revolve around the planet. Only the speed of rotation of electrons is hundreds of thousands of times greater than the speed of rotation of the fastest satellite. Therefore, during its rotation, the electron creates, as it were, a cloud above the surface of the nucleus. And the existing charges of electrons repel the same charges formed by other electrons around other nuclei. Therefore, the atoms do not "stick together", but are located at a certain distance from each other.
And when we talk about the collision of particles, we mean that they come close enough to each other long distance and are repelled by the fields of their charges. There is no direct contact. Particles in matter are generally very far apart. If by any means it were possible to implode together the particles of any body, it would be reduced by a billion times. The earth would become smaller than an apple. So the main volume of any substance, strange as it may sound, is occupied by a void in which charged particles are located, held at a distance by electronic forces of interaction.
Need help with your studies?
Previous topic: Radioactivity: alpha, beta, gamma radiationNext topic: Atomic Nucleus: Nuclear Charge
2.5. Rutherford's experiments. Rutherford model of the atom
A. Rutherford's experiments
In 1911, Rutherford conducted experiments of exceptional significance that proved the existence of the atomic nucleus. To study the atom, Rutherford used its probing (bombardment) with the help of α-particles, which arise during the decay of radium, polonium and some other elements. Rutherford and his collaborators, even in earlier experiments in 1909, found that α-particles have a positive charge equal in modulus to twice the electron charge q =+2e, and a mass coinciding with the mass of a helium atom, i.e.
m a\u003d 6.62 10 -27 kg,
which is about 7300 times the mass of an electron. Later it was found that α-particles are the nuclei of helium atoms. With these particles, Rutherford bombarded the atoms of heavy elements. Electrons due to their small mass cannot change the trajectory of the α-particle. Their scattering (changing the direction of movement) can only be caused by the positively charged part of the atom. Thus, from the scattering of α-particles, one can determine the nature of the distribution of the positive charge, and hence the mass inside the atom.
It was known that α-particles emitted by polonium fly at a speed of 1.6-107 m/s. The polonium was placed inside a lead case, along which a narrow channel was drilled. The α-particle beam, having passed through the channel and the aperture, was incident on the foil. Gold foil can be made extremely thin - 4-10 -7 m thick (400 gold atoms; this number can be estimated by knowing the mass, density and molar mass of gold). After the foil, the α-particles hit a semitransparent screen coated with zinc sulfide. The collision of each particle with the screen was accompanied by a flash of light (scintillation) due to fluorescence, which was observed under a microscope.
With a good vacuum inside the device (so that there was no scattering of particles from air molecules), in the absence of foil, a bright circle appeared on the screen from scintillations caused by a thin beam of α-particles. When a foil was placed in the path of the beam, the vast majority of α-particles still did not deviate from their original direction, that is, they passed through the foil as if it were empty space. However, there were alpha particles that changed their path and even bounced back.
Marsden and Geiger, Rutherford's students and collaborators, counted more than a million scintillations and determined that about one in 2,000 alpha particles deflected through angles greater than 90°, and one in 8,000 through 180°. It was impossible to explain this result on the basis of other models of the atom, in particular Thomson.
Calculations show that when distributed throughout the atom, a positive charge (even without taking into account electrons) cannot create a sufficiently intense electric field capable of throwing an α-particle back. The electric field strength of a uniformly charged ball is maximum on the surface of the ball and decreases to zero as it approaches the center. Scattering of α-particles at large angles occurs as if the entire positive charge of the atom was concentrated in its nucleus - a region that occupies a very small volume compared to the entire volume of the atom.
The probability of α-particles hitting the nucleus and deflecting them through large angles is very small, so for the majority of α-particles the foil did not seem to exist.
Rutherford theoretically considered the problem of the scattering of α-particles in the Coulomb electric field of a nucleus and obtained a formula that makes it possible to determine the number N elementary positive charges +e contained in the nucleus of atoms of a given scattering foil. Experiments have shown that the number N equal to the ordinal number of the element in the periodic system of D. I. Mendeleev, that is N=Z(for gold Z= 79).
Thus, Rutherford's hypothesis about the concentration of a positive charge in the nucleus of an atom made it possible to establish the physical meaning of the ordinal number of an element in the periodic system of elements. The neutral atom must also contain Z electrons. It is essential that the number of electrons in an atom, determined by various methods, coincided with the number of elementary positive charges in the nucleus. This served as a test of the validity of the nuclear model of the atom.
B. Rutherford's nuclear model of the atom
Summarizing the results of experiments on the scattering of α-particles by gold foil, Rutherford established:
♦ atoms by their nature are largely transparent to α-particles;
♦ deviations of α-particles at large angles are possible only if there is a very strong electric field inside the atom, created by a positive charge associated with a large mass concentrated in a very small volume.
To explain these experiments, Rutherford proposed a nuclear model of the atom: in the atomic nucleus (regions with linear dimensions of 10 -15 -10 -14 m) all of its positive charge and almost the entire mass of the atom (99.9%) are concentrated. Around the nucleus in a region with linear dimensions of ~10 -10 m (the dimensions of the atom are estimated in the molecular-kinetic theory), negatively charged electrons move in closed orbits, the mass of which is only 0.1% of the mass of the nucleus. Consequently, the electrons are located at a distance from the nucleus from 10,000 to 100,000 diameters of the nucleus, that is, the main part of the atom is empty space.
Rutherford's nuclear model of atoms resembles the solar system: in the center of the system is the "sun" - the nucleus, and around it "planets" - electrons move in orbits, therefore this model is called planetary. The electrons do not fall on the nucleus because the electrical forces of attraction between the nucleus and the electrons are balanced by the centrifugal forces due to the rotation of the electrons around the nucleus.
In 1914, three years after the creation of the planetary model of the atom, Rutherford investigated the positive charges in the nucleus. By bombarding hydrogen atoms with electrons, he found that neutral atoms turned into positively charged particles. Since the hydrogen atom has one electron, Rutherford decided that the nucleus of an atom is a particle carrying an elementary positive charge +e. He called this particle proton.
The planetary model is in good agreement with experiments on the scattering of α-particles, but it cannot explain the stability of the atom. Consider, for example, a model of a hydrogen atom containing a proton nucleus and one electron that moves at a speed v around the nucleus in a circular orbit of radius r. The electron must spiral into the nucleus, and the frequency of its revolution around the nucleus (hence, the frequency of electromagnetic waves emitted by it) must continuously change, that is, the atom is unstable, and its electromagnetic radiation must have a continuous spectrum.
In fact, it turns out that:
a) the atom is stable;
b) an atom radiates energy only under certain conditions;
c) the radiation of an atom has a line spectrum determined by its structure.
Thus, the application of classical electrodynamics to the planetary model of the atom led to a complete contradiction with the experimental facts. Overcoming the difficulties that arose required the creation of a qualitatively new quantum- the theory of the atom. However, despite its inconsistency, the planetary model is still accepted as an approximate and simplified picture of the atom.
Planetary model of the atom
Planetary model of an atom: nucleus (red) and electrons (green)
Planetary model of the atom, or Rutherford model, - historical model of the structure of the atom, which was proposed by Ernest Rutherford as a result of an experiment with alpha particle scattering. According to this model, the atom consists of a small positively charged nucleus, in which almost all the mass of the atom is concentrated, around which electrons move, just as the planets move around the sun. The planetary model of the atom corresponds to modern ideas about the structure of the atom, taking into account the fact that the movement of electrons is of a quantum nature and is not described by the laws of classical mechanics. Historically, Rutherford's planetary model succeeded Joseph John Thomson's "plum pudding model", which postulates that negatively charged electrons are placed inside a positively charged atom.
Rutherford proposed a new model for the structure of the atom in 1911 as a conclusion from an experiment on the scattering of alpha particles on gold foil, carried out under his leadership. In this scattering, an unexpectedly large number of alpha particles were scattered at large angles, which indicated that the scattering center had small dimensions and a significant amount of energy was concentrated in it. electric charge. Rutherford's calculations showed that a scattering center, positively or negatively charged, must be at least 3000 times smaller than the size of an atom, which at that time was already known and estimated to be about 10 -10 m. Since electrons were already known at that time, and their mass and charge are determined, then the scattering center, which was later called the nucleus, must have had the opposite charge to the electrons. Rutherford did not link the amount of charge to atomic number. This conclusion was made later. And Rutherford himself suggested that the charge is proportional to the atomic mass.
The disadvantage of the planetary model was its incompatibility with the laws of classical physics. If electrons move around the nucleus like a planet around the Sun, then their movement is accelerated, and, therefore, according to the laws of classical electrodynamics, they should radiate electromagnetic waves, lose energy and fall on the nucleus. The next step in the development of the planetary model was the Bohr model, postulating other, different from the classical, laws of electron motion. Completely the contradictions of electrodynamics were able to solve quantum mechanics.
Wikimedia Foundation. 2010 .
See what the "Planetary Model of the Atom" is in other dictionaries:
planetary model of the atom- planetinis atomo modelis statusas T sritis fizika atitikmenys: angl. planetary atom model vok. Planetenmodell des Atoms, n rus. planetary model of the atom, f pranc. modele planétaire de l'atome, m … Fizikos terminų žodynas
The Bohr model of a hydrogen-like atom (Z is the charge of the nucleus), where the negatively charged electron is enclosed in atomic shell, surrounding a small, positively charged atomic nucleus... Wikipedia
Model (French modèle, Italian modello, from Latin modulus measure, measure, sample, norm), 1) a sample that serves as a standard (standard) for serial or mass reproduction (M. of a car, M. of clothes, etc.). ), as well as the type, brand of any ... ...
I Model (Model) Walter (January 24, 1891, Gentin, East Prussia, April 21, 1945, near Duisburg), Nazi German General Field Marshal (1944). In the army since 1909, participated in the 1st World War of 1914 18. From November 1940 he commanded the 3rd tank ... ... Great Soviet Encyclopedia
An atom consists of a positively charged nucleus and negatively charged particles rotating around it - electrons that make up its electron shell.
The sum of the electron charges is equal in absolute value to the positive charge of the nucleus, so the atom as a whole is an electrically neutral system. The size of an atom is determined by its size electron shell and make up a value of the order of 10-8 cm.
The electrons in the shell of an atom are arranged in layers. The number of electron layers is equal to the serial number chemical element in the periodic system of elements D.I. Mendeleev.
In the first layer K, which is closest to the nucleus, no more than two electrons rotate. In the next layer L - no more than 8, in the M layer - no more than 18, and in the fourth layer N - no more than 32 electrons. In this way, largest number electrons of these layers is equal to twice the square of the layer number Z = 2n2. In subsequent layers, this rule is violated, and the number of electrons can be: in the fifth layer O - from 1 to 29, in the sixth layer P - from 1 to 9 and in the additional (last) layer Q - no more than 2 electrons.
Each atom exists only in certain discrete energy states corresponding to a strictly defined value of its energy.
The transition of an atom from one energy state to another is accompanied by the absorption or emission of energy. In its normal state, an atom does not radiate.
If one of the electrons, when colliding with some particle from the outside, receives some additional energy, then it will go to a more distant orbit of the layer to which its new energy corresponds. In this case, the atom enters an excited state, and then one of the electrons of the outer layer jumps to the vacant place. After a short time (of the order of 10-8 s), the atom returns to its normal state, while emitting visible light, ultraviolet or x-ray radiation.
If an electron of an atom receives a large energy, then it will be completely knocked out (removed) from the atom. This process is called ionization.
The nucleus of an atom consists of positively charged particles (protons) and neutral particles devoid of charge (neutrons). Both of these particles are usually called nucleons.
A proton is a material particle that has a mass mр = 1.6726. 10-24 years = 1.007275 amu The positive charge is equal to 1e+. Since the mass of the neutron (mn = 1.008665 amu) is only 0.14% greater than the mass of the proton, this difference is usually not taken into account in calculations and the mass of the neutron is practically considered equal to the mass of the proton.
The dimensions of the nucleus are very small: 10-12-10-13 cm (the nucleus is 100,000 times smaller than an atom). Despite the small size of the nucleus, 99.95% of the mass of the atom is concentrated in it. In view of this, the density of nuclear matter is very high and amounts to about 1017 kg/m3.
The charge of the nucleus, expressed in elementary units, is numerically equal to the ordinal number of the element in the periodic system of D.I. Mendeleev. This makes it possible to determine the number of protons in the nucleus of a given atom by the ordinal number of the element Z.
The total number of nucleons in the nucleus of an atom can be determined by the so-called mass number A. The mass number is the atomic weight of the element rounded to whole units. Since the number of protons in the nucleus is numerically equal to the ordinal number of the element Z, the number of neutrons is equal to the difference mass number A and serial number Z, i.e. N \u003d A - Z. For example, helium has Z \u003d 2 and A \u003d 4, which means that there are two protons and two neutrons in the nucleus of a helium atom.
Thus, the place of the element in the periodic system of elements of D.I. Mendeleev and its atomic weight reveal not only the structure of the atom, but also the structure of its nucleus.
The type of atoms with given numbers of protons and neutrons is called a nuclide.
The value of atomic weight in the table of elements is almost always expressed as a fractional number. This is because almost every element actually consists of several varieties of this element that have the same electrical charge, but different mass, i.e. the same number of protons in the nucleus, but a different number of neutrons. Varieties of a chemical element that have the same number of protons in the nucleus of an atom, but a different number of neutrons, are called isotopes.
All isotopes of a given element are placed in one cell of the table of elements of the periodic system. The fractional value of the atomic weight of an element reflects in this case the average value of the atomic weight of all isotopes of this element. Currently, more than 1500 isotopes are known, of which no more than 300 are stable (the nuclei of which do not undergo changes over a long period of time), the rest are radioactive (the nuclei of which decay over time).
The planetary model of the structure of the atom was first proposed by J. Perrin, trying to explain the observed properties by the orbital motion of electrons. But V. Vin considered it untenable. First, an electron during rotation, according to classical electrodynamics, must continuously radiate energy and, in the end, fall onto the nucleus. Secondly, due to the continuous loss of energy, the radiation of an atom must have a continuous spectrum, and a line spectrum is observed.
Experiments on the passage of a - particles through thin plates of gold and other metals were carried out by E. Rutherford's employees E. Marsden and H. Geiger (1908).
They found that almost all particles pass through the plate freely, and only 1/10,000 of them experience a strong deflection - up to 150 °. Thomson's model could not explain this, but Rutherford, his former assistant, made estimates of the fraction of deviations and came to a planetary model: the positive charge is concentrated in a volume of the order of 10 -15 with significant weight.
Considering the orbits of electrons in the atom to be fixed, Thomson in 1913 also arrived at a planetary model of the structure of the atom.
But, solving the problem of the stability of such an atom using Coulomb's law, he found a stable orbit for only one electron. Neither Thomson nor Rutherford could explain the emission of a - particles during radioactive decay - it turned out that electrons should also be in the center of the atom ?!
M. Sklodowska-Curie also spoke about this. Rutherford accepted this, but he had to assign the function of gluing the nuclei together to the electrons so that the Coulomb repulsion would not break the nucleus apart. These models did not allow obtaining quantitative results consistent with the experiments. In 1913, some experimental data on Rutherford's model were given weight. radioactive phenomena. His assistant G. Moseley measured the frequency spectral lines a number of atoms of the Periodic system and found that “a certain characteristic value is inherent in the atom, which regularly increases during the transition from atom to atom. This quantity cannot be anything other than the charge of the inner nucleus” [Cit. according to: 5, p. 194].
The construction of a theory of the structure of the atom on the basis of the planetary model ran into an abundance of contradictions.
At first, the Danish physicist N. Bohr tried to apply classical mechanics and electrodynamics to the problem of the deceleration of charged particles when moving through matter, but for a given value of the electron energy, it became possible to attribute arbitrary parameters of the orbit (or frequency) to it, which led to paradoxes.
Rutherford's planetary model of the structure of the atom turned out to be incompatible with Maxwell's electrodynamics.
In February 1913, articles appeared on the interpretation of the spectra of stars by J. Nicholson. He, extending Planck's idea to atoms, proposed to quantize the projections of the momentum of the electron. This is how an atom with discrete orbits appeared, along which groups of electrons rotated, emitting electromagnetic waves with a frequency equal to the frequency of circulation. Such a model was suitable for highly excited atoms, and Nicholson explained some of the features in the spectra of stars and nebulae based on the atom model - the idea of an electron ring rotating around a positively charged nucleus.
The atom was characterized, first of all, by its emission spectrum. He connected with the spectral frequencies the frequencies of specially postulated mechanical vibrations of electrons perpendicular to the plane of the ring.






