Compression and rarefaction of gases
Ideal Machines in real life does not exist, it is just a mental construct. Each of these hypothetical machines, among which carnot engine occupies an important place, illustrates some important theoretical conclusion. (Even a castle in the air called a perpetual motion machine serves, in fact, only to show that you cannot get energy from nothing.) The Carnot engine, which underlies the operation of an ideal heat engine, was invented by the French engineer Sadi Carnot twenty years before how the foundations of thermodynamics were formulated, however, it illustrates an important consequence of the second law of thermodynamics.
The working part of a Carnot engine can be thought of as a piston in a cylinder filled with gas. Since the Carnot engine is a purely theoretical, that is, ideal machine, the friction forces between the piston and the cylinder and the heat losses are considered to be zero. The piston can move freely between two thermal reservoirs- With high temperature and with low temperature. (For convenience, imagine that a hot heat reservoir is heated by burning a mixture of gasoline with air, and a cold one is cooled by water or air at room temperature.) In this heat engine, the following ideal four-phase cycle occurs:
1. First, the cylinder comes into contact with the hot reservoir, and the ideal gas expands at constant temperature. During this phase, the gas receives some heat from the hot reservoir.
2. The cylinder is then surrounded by perfect thermal insulation, whereby the amount of heat available to the gas is conserved and the gas continues to expand until its temperature drops to that of the cold thermal reservoir.
3. In the third phase, the thermal insulation is removed, and the gas in the cylinder, being in contact with the cold reservoir, is compressed, while giving off part of the heat to the cold reservoir.
4. When the compression reaches a certain point, the cylinder is again surrounded by thermal insulation, and the gas is compressed by raising the piston until its temperature equals that of the hot reservoir. After that, the thermal insulation is removed and the cycle repeats again from the first phase.
The Carnot engine has much in common with real engines: it works in a closed loop (called, respectively, Carnot cycle); it receives energy from the outside due to a high-temperature process (for example, when burning fuel); part of the energy is dissipated in environment. In this case, a certain work is performed (in the case of a Carnot engine, due to forward movement piston). efficiency, or efficiency The Carnot engine is defined as the ratio of the work it produces to the energy (in the form of heat) taken from the hot reservoir. It is easy to prove that the efficiency ( E) is expressed by the formula:
E = 1 - (T c/ T h),
where T c and T h - respectively, the temperature of the cold and hot reservoirs (in kelvins). Obviously, the efficiency of the Carnot engine is less than 1 (or 100%).
Carnot's great insight is that he showed that no heat engine operating at two given temperatures can be more efficient than an ideal Carnot engine (this statement is called Carnot's theorem). Otherwise, we would encounter a violation of the second law of thermodynamics, since such an engine would take heat from a less heated reservoir and transfer it to a hotter one. (In fact, the second law of thermodynamics is a consequence of Carnot's theorem.) Thus, the relation Carnot obtained establishes efficiency limit real engines running in the real world. It can be approached, but engineers will not be able to achieve and, moreover, surpass it. So, a purely hypothetical Carnot engine plays an important role in the world of real, noisy and smelling of heated engine oil technology, and this is another example of the applied value of purely theoretical, at first glance, research.
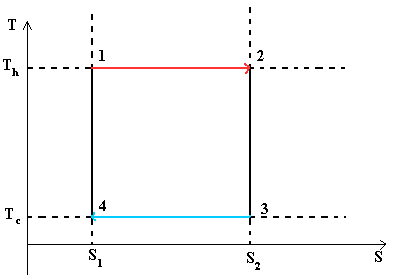
Figure 1 - The Carnot cycle is an ideal thermodynamic cycle.
This diagram shows a closed loop. The system sequentially passes from point 1 to 2 then 3, 4 and again to 1. The graph shows that process 1® 2 is isothermal (occurs at T 1) and process 3® 4 is also isothermal (occurs at T 2)
Processes 2® 3 and 4® 1 are adiabatic. Since there is no entropy change in them, then dS = 0, hence dQ = 0 or Q = const.
The amount of heat supplied to the system:
Q 1 \u003d T 1 ´ (S 2 -S 1) or the area of \u200b\u200ba rectangle 1-2-S 2 -S 1 -1
The amount of heat given off by the system:
Q 2 \u003d T 2 ´ (S 2 -S 1) or the area of \u200b\u200ba rectangle 3-S 2 -S 1 -4-3
Cycle work L = Q 1 - Q 2
Cycle efficiency h \u003d (Q 1 - Q 2) / Q1.
An important consequence of the formula for cycle efficiency Carnot is that to increase the efficiency it is necessary to increase the heat supply temperature T 1 and reduce the heat removal temperature T 2 .
AT heat engine, the gas is (reversibly) heated (reversibly heated) and then cooled. The cycle model is as follows: Position 1 --(isothermal expansion) --> Position 2 --(adiabatic expansion) --> Position 3 --(isothermal compression) --> Position 4 --(adiabatic compression) --> Position 1
Position 1 - Position 2: Isothermal expansion
Isothermal expansion. At the beginning of the process, the working fluid has a temperature T h , that is, the temperature of the heater. Then the body is brought into contact with the heater, which isothermally (at a constant temperature) transfers to it the amount of heat Q H . At the same time, the volume of the working fluid increases. Q H \u003d∫Tds \u003d T h (S 2 -S 1) \u003d T h ΔS
Position 2 - Position 3: Adiabatic expansion
Adiabatic (isentropic) expansion. The working fluid is detached from the heater and continues to expand without heat exchange with the environment. At the same time, its temperature decreases to the temperature of the refrigerator.
Position 3 - Position 4: Isothermal compression
Isothermal compression. The working fluid, which by that time has a temperature T c , is brought into contact with the cooler and begins to contract isothermally, giving the cooler the amount of heat Q c . Q c \u003d T c (S 2 -S 1) \u003d T c ΔS
Position 4 - Position 1: Adiabatic compression
Adiabatic (isentropic) compression. The working fluid is detached from the refrigerator and compressed without heat exchange with the environment. At the same time, its temperature increases to the temperature of the heater.
In isothermal processes, the temperature remains constant, in adiabatic processes there is no heat transfer, which means that entropy is conserved.
Therefore, it is convenient to represent the Carnot cycle in the coordinates T and S (temperature and entropy).
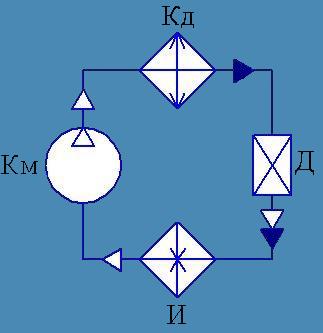
3. Work cycle refrigeration machine(to lesson No. 16)
Refrigeration machine This is a reverse carnot cycle machine.. That is, if you cycle through reverse direction, heat will be taken from the refrigerator and transferred to the heater (due to the work of external forces).
In refrigeration systems, the transfer of heat from an environment with a lower temperature to an environment with a higher temperature is carried out using a working fluid called coolant.
Getting cold occurs by circular process, or a cycle in which the process of removing heat from the cooled medium is accompanied by a compensating process - the supply of energy (for example, when compressing refrigerant vapor in a compressor).
The process of transferring heat from a less heated body to a more heated one at a cost mechanical work is called the reverse Carnot cycle. The cycle consists of the following processes:
1-2 - adiabatic compression of the vaporous refrigerant (final compression temperature T);
2-3 - isothermal condensation of refrigerant vapor at temperature T with return to the environment (for example, water) of condensation heat Q;
3-4 - adiabatic expansion of the liquid refrigerant (final expansion temperature T o);
4-1 - isothermal evaporation of a liquid refrigerant at a temperature T o with the removal of the cooled medium of the heat of evaporation Q o.
![]()
Such a cycle is feasible only if the entropy of the system is constant. Therefore, if during the evaporation of the refrigerant, the entropy of the cooled medium decreases by Q o /T o , then the entropy of a more heated medium (water) should increase by the same value, to which heat Q o is transferred, taken from the cooled medium, and heat equivalent to the work L k expended on compressing the refrigerant. As a result, the increase in the entropy of a more heated medium is (Q o + L c)/T.
According to the energy balance
Q o /T o \u003d (Q o + L to) / T
Hence the work that must be expended in a refrigeration unit operating on the reverse Carnot cycle
L k \u003d Q o (T - T o) / T o
Heat Q o taken away by the refrigerant of the cooled medium at temperature T o< Т, определяет cooling capacity b cycle, or refrigeration plant.
Thus, using the example of the reverse Carnot cycle, the energy balance of any refrigeration machine:
where L is the work of the real cycle.
The thermodynamic efficiency of refrigeration cycles is expressed as the ratio of the cooling capacity Q o to the work expended, L and this relation is called coefficient of performance and is denoted by ε. The coefficient ε is expressed by the dependence
ε \u003d Q o / L \u003d Q / (Q - Q o) \u003d T o (S 1 - S 2) / [ T (S 1 - S 2) - T o (S 1 - S 2)] \u003d T o / T - T o
The coefficient of performance shows how much heat is perceived by the refrigerant from the cooled medium per unit of work expended.
The coefficient of performance, which characterizes the degree of use of mechanical work to obtain artificial cold, as can be seen from the expression, does not depend on the properties of the refrigerant or the scheme of operation of the refrigeration unit, but is only a function of temperatures T o and T. In this case, the degree of use of mechanical work will be the higher the smaller the difference between the temperatures of the refrigerant when giving off T and receiving T about heat.
Isothermal expansion process 1 - 2 superheated steam in coordinates is shown in fig. 12.8. The definition of the initial / and final 2 points of the process is clear from the previously stated.
An isothermal process of expansion or contraction can also be carried out reversibly under conditions of rapid heat exchange with external environment required to maintain a constant temperature.
AT isothermal process expansion of water, the amount of heat is reported, equal to heat vaporization / h and in the diagram s - T graphically defined area.
In an isothermal expansion process n, the heat input is completely converted into work, and the change in internal energy is zero.
The exception is the ideal isothermal expansion process, when all the heat goes into work, but the cooling capacity of such a process is zero.
So, in the isothermal process of expansion, all the supplied heat is spent on external work, and in the isothermal process of compression, the external work is completely converted into heat.
Thus, in the isothermal process of gas expansion internal energy system, converted into work against external pressure, is replenished due to the influx of heat. In the case of a reversible process considered here, the work done is identical to the maximum useful work, which, as shown below, is equal to the change in the state function. When the process is irreversible (friction loss, Ap0), part of the useful work is lost, turning into heat.
On fig. 4 - 3 shows an isothermal expansion process ideal gas in the ri system.
It should be noted that the measurement of the entropy AS Q / T obtained for the particular case of the isothermal expansion process is the same as that obtained earlier from the analysis of the Carnot cycle. In this way, statistical physics substantiates the existence of a state function - entropy, the increment of which in reversible processes is equal to the reduced heat, and the position that the entropy of a closed system tends to a maximum. This state function makes it possible to determine the direction of processes and equilibrium conditions by means of measurements of thermal quantities. With the principle of increasing entropy in closed systems related ideas about the thermal mixture of the Universe, put forward by Clausius, who argued: The energy of the world is constant, the entropy of the world tends to a maximum. Hence - the conclusion about the achievement as a result of one-sided processes occurring in nature, the final state of equilibrium, in which the entropy of the world is maximum and the Universe dies from heat death.
Comparing (15.16) and (15.4), we note that the specific work of a theoretical pneumatic motor during a complete isothermal expansion process is equal to the same value of the specific energy of air. This is also true for adiabatic and polytropic processes.
Comparing (288) and (272), we note that the specific work of a theoretical pneumatic motor during a complete isothermal expansion process is equal to the same value of the specific energy of air. This is also true for adiabatic and polytropic expansion processes.
From fig. 23 it can be seen that the SW segment equal to Asp - As0 simultaneously represents the entropy increment in the isothermal process of SW expansion.
The set of working processes in the expander, as well as in the compressor, is not a closed system. thermodynamic process-cycle. However, the isothermal expansion process is difficult to implement, and the processes in expanders are close to adiabatic.
On fig. 15.7 shows theoretical indicator diagrams for various air expansion processes in engines. To implement an isothermal expansion process (curve 2 - 3, polytropic index n 1) it is necessary to supply heat so that the air temperature is maintained constant, and for an adiabatic process (curve 2 - 3, n K), heat exchange with the environment should be excluded. The polytropic process will be in that case (curve 2 - 3, 1 p k), if the heat supply is less than in the isothermal process.
A reversible cycle can be carried out under such conditions as follows. First, in the isothermal expansion process, heat is reversibly supplied to the working fluid from a heat exchanger with a constant temperature.
The internal energy (see Table 1.1) consists of the Helmholtz energy F and the associated energy TS. The loss of energy F during the isothermal expansion process (S const) is equal to the work RT In (VVK2) - The absorption of heat by the system (at V const) leads to an increase in the associated energy by T (S2 - Sx), since dS 8Q / T. If there is no change in volume at a constant temperature, then the decrease in the Helmholtz energy allows us to obtain useful work.
The direct Carnot cycle consists of six processes (Fig. Curve A-B depicts the isothermal process of gas expansion at a constant temperature 7; curve 3-C depicts the adiabatic process of gas expansion in the absence of heat exchange with the external environment - 50H; C-D curve depicts an isothermal process of gas compression at a constant temperature Tz; Line D-A depicts the adiabatic process of gas compression in the absence of heat exchange with the environment. In pro-lesses A-B and B-C, the gas, expanding, does external work, 1 in C-D processes and D-A the gas is compressed and external work is applied to its compression. Applying the equation of the first law of thermodynamics to the entire cycle, we obtain Qt - Q2A (L. Therefore, in the direct Carnot cycle, the supplied heat Qi is partially spent on useful work, numerically equal to Square A-B-C-D-A(Fig. The efficiency of the dikla Carnot cannot, in principle, be equal to unity.
In general, the value of a depends on temperature and pressure. At the same time, in the isothermal process of gas expansion, the value a occurs as a product of ap.
Image reg - a process of expansion 4 - / - message to him. Despite this property of the Carnot cycle, other cycles are used as the basis for the operation of real engines, as will follow from what follows. This is mainly due to the impossibility of carrying out isothermal processes of expansion and contraction under real conditions.
The work done by compressed air during filling and expansion is positive, while the work done by compressed air is negative. As seen in fig. 93, a, the maximum work will be with an isothermal expansion process, and the minimum - with an adiabatic one.
Page 2
At first glance, it may seem that such a formulation contradicts, for example, the process of isothermal expansion of an ideal gas. Indeed, all the heat received by an ideal gas from some body is completely converted into work. However, obtaining heat and converting it into work is not the only end result of the process; in addition, as a result of the process, a change in the volume of gas occurs.
At first glance, it may seem that Thomson's formulation contradicts, for example, the process of isothermal expansion of an ideal gas. Indeed, during this process, all the amount of heat received by an ideal gas from a certain body is completely converted into work. However, obtaining heat and converting it into work is not the only end result process; in addition, there is a change in the volume of gas.
Knowing how isothermal and adiabatic processes in a Ts-diagram, one can construct a Carnot cycle in it. Here 1 - 2 is the process of isothermal expansion, during which the amount of heat qv is supplied, measured by the area 1 - 2 - 5 - 6 - 1; 2 - 3 - adiabatic expansion; 3 - 4 - isothermal compression, in which the amount of heat d% is removed, measured by the area 4 - 3 - 5 - 6 - 4; 4 - 1 - adiabatic compression. The amount of heat 70, converted into useful work w0, will be represented by square.
PRESSURE CRITICAL - see the state of matter is critical. SATURATION PRESSURE - the maximum pressure at which, in the process of isothermal expansion of oil or formation water, the release of the gas sorbed by them begins. However, the reliability of this method has not yet been substantiated. Oil in the presence of a gas cap is usually saturated.
As can be seen from equation (VI, 5), entropy increases in the process of isothermal expansion. Just as in the previous example of the melting of crystalline mercury, in the process of isothermal expansion, a certain amount of heat (which is taken from a heat source - a thermostat) at a constant temperature is reversibly imparted to the expanding gas.
Consider the features phase transition from liquid state to gaseous - the transition of liquid to vapor. If, as a result of an accident, depressurization occurs, and the temperature retains its value by inertia, then the process of isothermal expansion dabe develops. Experience shows that when moving to isotherms with higher temperatures, the length of the ab section decreases, for lower temperatures the length of the ab section increases.
The reverse Carnot cycle is also depicted in the s - G - diagram as a rectangle / - 2 - 3 - 4 (Fig. 7.6, b), but all processes in it are directed counterclockwise. The cycle also consists of two isotherms and two adiabats: / - 2 - adiabatic expansion process, 2 - 3 - isothermal expansion process, 3 - 4 - adiabatic compression process, 4 - / - isothermal compression process.
Since this gas is isolated from the environment (Q O, A 0), its internal energy U, as follows from the first law of thermodynamics, must remain constant. This means that as a reversible process that transfers the gas to the same final state as the given process, we can consider the process of reversible isothermal expansion, during which the gas volume doubles.
In practice, the processes of isothermal expansion are usually quite difficult to implement due to insufficient thermal conductivity; nevertheless, it is interesting to compare the thermodynamic properties of such a flow with the more usual adiabatic flow. Under these conditions, isothermal expansion gives an upper limit to the coefficient useful action expansion process. The processes of isothermal expansion in rocket nozzles have been considered theoretically, but due to the short residence time of gas particles in the nozzle, the practical use of these processes, apparently, is not feasible.
From point / with parameters p1; uh, 7 the working fluid expands adiabatically to state 2 (process / - 2) and connects to a source with a low temperature. Further expansion (process 2 - 3) occurs with the supply of heat c / 2 to the working fluid. The process of isothermal expansion takes place.
Carnot can be imagined as follows. In the process of isothermal expansion 1 - Г, the gas is in thermal contact and equilibrium with a body having a temperature TV. Such a body. This body is called a heater. It is clear that the heat capacity of the heater must be, strictly speaking, infinitely large. Otherwise, the release of heat Q4 to the gas would cause a decrease in the temperature of the heater, and, consequently, a violation of the isothermal process of gas expansion. In the I - - 2 process, the gas is completely thermally insulated and its expansion continues to occur adiabatically.
The same result can be easily obtained directly from equation (3 - 177), Since we agreed that the source of work considered in this example has the properties of an ideal gas, and since the temperature of the source in states 1 and 2 is the same and equal to T0, then the internal energy of the source work in states 1 and 2 is also the same and the first term of equation (3 - 177) is equal to zero. The second term of the equation is the amount of heat supplied to the source of work in an isothermal process at a temperature T0, equal to work in this process (the internal energy remains unchanged. The entropy of the work source in the process of isothermal expansion increases (heat is supplied. Slt and therefore the second term of equation (3 - 177) will be positive. The last term of the equation will be negative (F2 Fj), and its numerical value is equivalent to area a-c-2 - b - a. Thus, L ts (area l - 2 - b - a - l) - (area a-c-2 - b - a) (area l - 2 - c - l ), which, as expected, coincides with the previously obtained result.
The main ways of transferring energy from one part of the system to another are heat and work. For example, isothermal expansion ideal gases is not accompanied by the release or absorption of heat, if the process proceeds without doing work by the gas. If the process of isothermal expansion of the gas is accompanied by work, then heat is absorbed.
The processes in the GCM proceed in the following sequence. The heat of compression in this process is removed to the environment. Then the compressed gas, while the pistons move to the left, is pushed through the cooled regenerator 2 (isochoric process), while the temperature and pressure of the gas are reduced. At the next moment, the process of isothermal expansion of the gas occurs, in which the right piston is stationary, and the expander piston goes to the left.






