natural level of radiation. The concept of radiation background
Under background radiation commonly understood as ionizing radiation from natural sources of cosmic and terrestrial origin, as well as from artificial radionuclides dispersed in the biosphere as a result of human activities.
Radiation background affects the population the globe, with a relatively constant level. There are natural (natural) background radiation, technologically modified natural radiation background, artificial radiation background.
Natural radiation background is an ionizing radiation that affects a person on the surface of the Earth from natural sources of cosmic and terrestrial origin.
Technologically modified natural radiation background
is ionizing radiation from natural sources that have undergone certain changes as a result of human activity, for example, radiation from natural radionuclides entering the biosphere together with minerals extracted from the Earth's interior from its bowels, as a result of the combustion products of fossil fuels entering the environment, radiation in rooms built from materials containing natural radionuclides.
Artificial radiation background due to the radioactivity of the products of nuclear explosions, waste nuclear power and accidents.
The measure of the radiation background is the exposure dose rate, while in geophysics we mean the dose rate absorbed in the air on the ground due to external radiation sources.
For the convenience of comparing biological efficacy and assessing the risk of long-term effects when various types exposure, including cases of uneven exposure, doses due to the radiation background are often expressed in terms of the so-called effective dose - a conventional concept that characterizes the calculated dose of uniform external exposure to the whole body, adequate in terms of the risk of long-term stochastic consequences of the real absorbed dose in one or another organ.
9.1. Natural radiation background
Natural sources of ionizing radiation that form the natural radiation background are divided into external sources of extraterrestrial origin (cosmic radiation); external sources of earthly origin, i.e. radionuclides present in the earth's crust, water, air; internal sources, i.e. radionuclides of natural origin contained in the human body.
Cosmic rays are a stream of nuclear particles coming to the earth's surface from various areas world space is the so-called primary cosmic radiation. The average energy of cosmic particles is 10 10 eV. In the general flow of particles, there are particles with a much lower energy level, and particles with energies up to 10 19 eV. Primary cosmic radiation consists of protons (92%), α-particles (helium nuclei 7%), nuclei of atoms of lithium, beryllium, carbon, nitrogen and oxygen (0.78%) and nuclei of atoms, the charge of which
more than 10 (0.22%).
When cosmic particles fall on the Earth's surface, they interact with the atoms and molecules of the atmosphere. There is a secondary cosmic radiation; in this case, electron-photon and electron-nuclear interaction processes are most significant. In the electron-photon process, charged particles, interacting with the field of the atomic nucleus, give rise to photons, which form pairs of electrons and positrons. These particles, in turn, give rise to new photons. The cascade process of an avalanche-like increase in the number of particles and photons continues until their energy becomes sufficient.
exactly small and is not lost on the ionization and excitation of air atoms and molecules.
The electron-nuclear process is due to the interaction of primary cosmic particles, whose energy is not less than 3×10 9 eV, with the nuclei of atoms in the air. In this case, a number of new particles appear simultaneously - protons and neutrons (fragments of the nucleus) and π-mesons of three types: negatively charged, carrying positive charge and uncharged. Charged π-mesons (the mass of π-mesons in relation to the mass of an electron is 273) decay (average lifetime - 2.5?10 -8 s) into more stable μ-mesons (mass - 207 units) and neutrinos; neutral π-mesons in turn (τ = 2.5?10 -16 s) decay into 2 photons, and μ-mesons - into electrons, positrons and neutrinos. Thus, secondary cosmic radiation consists of electrons, neutrons, mesons and photons. As we approach the Earth's surface, the intensity of the primary cosmic radiation decreases, and the intensity of the secondary radiation reaches a maximum at an altitude of 20-30 km; at a lower altitude, the processes of absorption of this type of radiation prevail over the processes of its generation. At sea level, the intensity of the primary radiation is approximately 0.05% of the original value. The secondary radiation consists of mesons (80%) and electrons (20%). It should be noted that the level of cosmic radiation to a certain extent depends on the geomagnetic latitude, increasing from the equator to the poles (up to 14% at sea level). In table. 29 shows the intensity of cosmic radiation as a function of latitude and height above sea level.
Table 2 9.Cosmic ray intensity for mid-latitudes and the equator, as well as various heights above sea level
Natural radioactivity is due to radionuclides of natural origin, present in all shells of the Earth: the lithosphere, hydrosphere, atmosphere and biosphere. Radioactive elements can be conditionally divided into three groups:
Radionuclides that are part of the radioactive families, the ancestors of which are uranium (238 U), thorium (232 Th) and actinouranium (235 As) (the decay of the families of uranium, thorium and actinouranium is shown in Scheme 1);
Radioactive elements not included in the families 40 K, 48 Ca, 87 Rb, etc.;
Radioactive isotopes that continuously arise on Earth as a result of nuclear reactions under the influence of cosmic rays. The most important of these are carbon (14 C) and tritium (3 H).
Scheme 1.The decay of families of uranium (a), thorium (b), actinouranium (c)
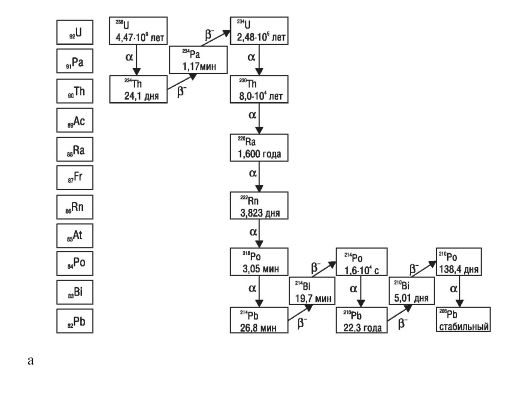
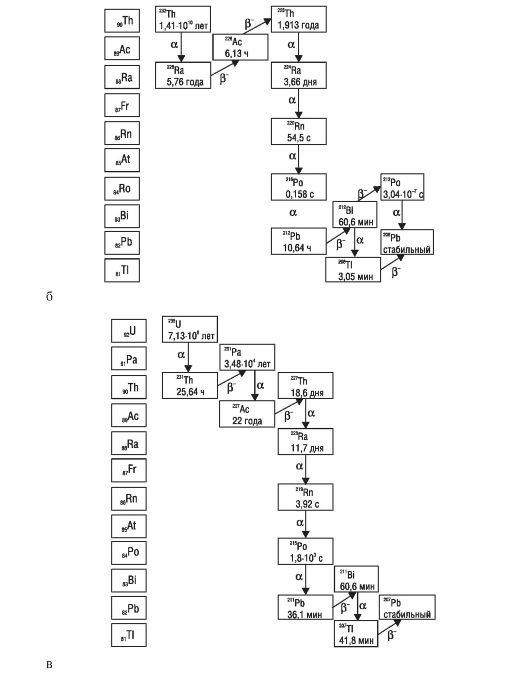
In table. 30 shows data characterizing the magnitude of the specific activity of the main radioactive isotopes and elements containing these isotopes in their composition.
Table 30Characterization of the main natural radioactive isotopes

In addition to those listed in Table. 30 radioactive isotopes, decay products of radioactive families, primarily radon, thoron and actinon, participate in the formation of the natural background.
The main source of natural radionuclides entering the environment, which are now widely distributed in all shells of the Earth, are rocks, the origin of which is inextricably linked with the inclusion in their composition of all radioactive elements that arose during the formation and development of the planet. Due to the continuous destructive processes of meteorological, hydrological, geochemical and volcanic nature, radionuclides were widely dispersed.
No matter how much terrestrial matter we take, we can always find several tens of chemical elements. Many elements can be found in the form of traces - in negligible amounts. For example, in the air there is the rarest gas -
xenon, which is only four hundred thousandths of a percent (by mass). However, each cubic centimeter of air contains about a billion xenon atoms. In the water of the oceans in dissolved form, there are up to 50 different elements. The atoms of each of them can be found in a drop of water.
Despite the negligible content of individual elements in seawater, they can have a significant impact by entering into biogeochemical processes that occur continuously here. For example, manganese contained in sea water in the amount of one ten millionth of a percent as a result of biogeochemical processes contributed to the deposition of many millions, as, for example, in Chiatura (Georgia). We observe the same phenomenon (scattering) in rocks. Even the purest mineral rock crystal contains millions of atoms of other elements in 1 g.
It is important that for a number of elements, being in nature in a diffuse form is a characteristic state. These elements include all natural radionuclides.
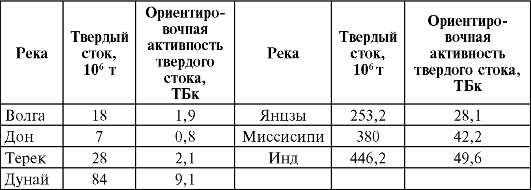
At present, there is a relative balance between the entry of radionuclides into the circulation sphere and their amount that is eliminated from this dynamic process due to the formation of sedimentary rocks and radioactive decay. In these processes, water plays the main role as a universal solvent. In contact with rock material during filtration through cracks and pores, water dissolves and carries out from the depths of the earth's crust to its surface a number of both stable and radioactive elements. In addition, water carries rock particles with it and deposits them in the form of sediments at a considerable distance from the site of primary erosion. The mass of suspended matter carried, including radionuclides, for example, only by river water is quite significant. In table. 31 gives a description of this phenomenon for individual rivers.
With the water of the rivers, a significant amount of dissolved substances is carried out. So, only one r. The Mississippi annually releases about 136 million tons of various dissolved salts into the world's oceans. As a result of these processes, which have been taking place on our planet for many millions of years, it turned out that the waters of the world ocean contain a truly huge amount of natural radioactive elements in a dissolved state. So, in the water of the Pacific Ocean
contains about 2.95 billion tons 40 K, which corresponds to an activity of approximately 7.4 × 10 20 Bq.
Table 31Annual removal to the sea of suspended materials (solid runoff) and their total activity(according to M.A. Velikanov and L.A. Pertsov)
A significant place in the processes of migration and circulation of radionuclides in nature is occupied by flora and fauna.
Most of the natural radioactive elements are contained in the rocks that form the thickness of the earth's crust. The average concentrations of potassium, thorium, uranium, and radium in them are given in Table 1. 32.
Table 32Average content of potassium, thorium, uranium and radium in earth rocks, %

The amount of radioactive elements contained in the soil is largely determined by the concentration of radionuclides in the parent rock. Soils derived from the destruction products of acidic igneous rocks contain relatively more uranium, radium, thorium, and potassium than soils formed from ultramafic and basic rocks. Clay soils due to the high content of colloidal fractions, well sorbing and retaining
living radioactive isotopes, is always richer in radioactive elements than sandy ones. Thus, the content of uranium in the upper soil horizon of the Central Russian Upland ranges from 1 × 10 -5 to 1.8 × 10 -4%, thorium - from 2.3 × 10 -4 to 14 × 10 -4%, potassium - from 0.3 to 2.6%.
As a rule, there is no equilibrium in the soil between the precursor and the daughter nuclide due to their unequal chemical properties. At the same time, an excess (relative to 226 Ra) amount of 210 Pb in the upper soil horizon (0–5 cm) is noted everywhere, and the excess 210 Pb in the upper soil horizons varies widely. It is believed that the main reason for the accumulation of 210 Pb in the upper layers of the soil is atmospheric fallout caused by atmospheric precipitation and "dry" fallout.
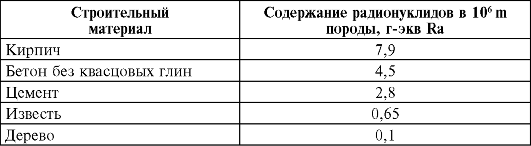
In certain regions of the globe there are zones with a high content of radioactive elements in rocks and soils, for example, the regions of the Pamirs and Tibet, the territories of Brazil, India, France, and Russia. Thus, in India, in the state of Kerala, on whose territory about 100 thousand people live, the content of thorium and its daughter products is high (up to 0.1%); in the state of Rio de Janeiro (Brazil), in the area of monazite sands, where up to 50 thousand people live, the content of ThO 2 in the sands reaches 6.15%; in the regions of France, the Pamirs and Tibet, the content of uranium and radium in volcanic rocks is high. Of great interest is the level of radionuclides in building materials made from various rocks; they are very diverse in terms of the content of natural radioactive elements. The specific activity of building materials is presented below.
Natural radioactivity in the air
It is due to the presence of radionuclides arising in the atmosphere as a result of exposure to cosmic radiation, radioactive gases coming from the upper layers of the earth's crust, and their daughter products, radionuclides, as a result of human activity, etc.
Radionuclides under the influence of cosmic radiation owe their origin to secondary cosmic radiation, which has in its composition neutrons of various energies. Most of the neutrons, interacting with the nitrogen nuclei of the air, give rise to radioactive carbon - 14 C. It should be noted that such processes are observed only at an altitude of over 9000 m above sea level. As a result of the impact of cosmic radiation on atmospheric nitrogen, about 10 kg of 14 C is produced on our planet annually, and its total amount in the planet’s atmosphere is approximately 80 tons. Radioactive carbon formed in the upper layers of the atmosphere, combining with oxygen, gives carbon dioxide, which is included in the usual carbon exchange cycle between the atmosphere, hydrosphere, soil and organic world. Over a centuries-long period, radioactive carbon has been evenly distributed in stable isotopes, and the equilibrium concentration in the mixture of isotopes is approximately 0.3 Bq per 1 g. This corresponds to the concentration of radioactive carbon in the atmospheric air, equal to 4.8 × 10 -5 Bq/l.
Another radioactive isotope that occurs under the influence of cosmic radiation is tritium (3 H), which is formed mainly by the reactions 14 N (n, 3 H) 12 C and 16 O (p, 3 H) 14 O. Due to the same reasons that led to the widespread distribution of 14 C, the content of tritium in the environment as a whole is constant and very small and reaches 10 -14 in relation to stable hydrogen.
Beryllium-7, beryllium-Yu, phosphorus-32, sulfur-35, and other radioactive elements also appear under the influence of cosmic radiation. The latter make an even smaller contribution to the dose of background exposure to a person compared to tritium, so they have no hygienic significance.
To the radioactive gases that come from the upper layers earth's surface, include emanations arising from dis-
a drop of daughter products of uranium (222 Rn), thorium (220 Rn) and actinium (219 Rn). The rate of formation of emanation in rocks depends on the content of the ancestors of radioactive series in them. Each of the resulting gaseous isotopes diffuses to some extent into the atmospheric air. At the same time, of course, radon, all other things being equal, has a greater possibility of escaping into the atmosphere than thoron and actinon, since its half-life is 3.8 days, while the half-life of thoron is 54 s, and that of actinon is 3.9 s . The content of emanations in the soil increases with depth and reaches constant values at a depth of 5 m. The rate of entry of radioactive emanations into the atmospheric air depends on a number of reasons: diffusion of soil gases towards a decreasing concentration, convection flows of air masses as a result of heating the earth's surface due to solar radiation, changes in barometric pressure, soil freezing depth, snow cover thickness, etc.
The flow of emanation into the air increases with a decrease atmospheric pressure and drops to almost 0 during snowmelt and ice formation. Are celebrated seasonal fluctuations during radon intake with a minimum in winter and a maximum in summer.
As a result of the continuous flow of radioactive gases from the soil into the atmosphere, their highest concentrations are found in the surface layer, and their content decreases with height.
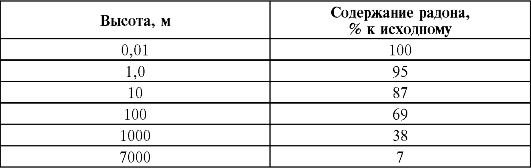
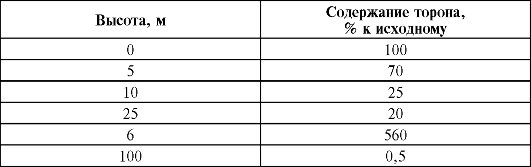
In the air of regions, the rocks of which contain an increased amount of radionuclides, the concentrations of emanation are increased, and, conversely, they decrease over surfaces composed of materials depleted in radioactive isotopes. Yes, activity atmospheric air over land for radon - an average of 4.8?10 -3 Bq/l, over the ocean near the coast - 1.4?10 -3 Bq/l, and over the ocean away from the coast - 3.5?10 -5 Bq/l l. In atmospheric air, the content of thoron is much less (10-100 times) than that of radon. An even smaller contribution to the total air activity is made by actinon, as a result of its short lifespan and the relatively low abundance of the parent element, actinouranium.
During decay, radioactive emanations give rise to short- and long-lived active aerosols (isotopes of polonium, bismuth and lead). The data of a number of authors indicate that the α-activity of air for short-lived emanation products averages (1.8-2.5) × 10 -3 Bq / l, β-activity - 22.2 × 10 -3 Bq / l . The specific activity of long-lived decay products of radon is less: over land in 210 Bi it ranges from 1.1 × 10 -7 to 14.8 × 10 -7 Bq / l, and in 210 Ro - (2.5-5.5)? 10 -8 Bq/l.
In addition to radioactive aerosols resulting from the decay of emanation, the surface layer of the atmosphere contains other radioactive particles of natural origin: particles raised by the wind from the surface of the earth, and particles formed when droplets dry. sea water. So, according to L.A. Pertsov, the total mass of aerosols generated by the entire equator of the World Ocean is (5-7)?10 7 t/year, and their total activity at 40 K is approximately 17 PBq. These aerosols
also contain thorium and other radioactive isotopes, but in general, the specific activity of air due to this group of radioactive aerosols is insignificant. It should also be noted that in the air of cities with heavy traffic and developed industry, dust radioactivity is due to potassium, and smoke radioactivity is due to potassium and carbon isotopes. Finally, the observations recent years it was found that the relative content of carbon is somewhat lower in the atmosphere of industrial cities than in rural areas. The latter is explained by the fact that fossil fuels are burned in cities, in which the content of radioactive carbon due to its natural decay is less than in the biosphere.
meteoric watersare usually low-active and contain traces of 3 H, 14 C, 7 Be, resulting from the interaction of cosmic radiation with atoms and molecules of atmospheric air, as well as 40 K, 238 U, which are part of soluble salts that enter the atmosphere due to eolian (wind) erosion of the earth's surface.
Radioactivity groundwater depends on their conditions. According to the nature of occurrence, groundwater can be the waters of the first aquifer (they are sometimes called groundwaters), accumulating on the first water-resistant layer from the surface, and interlayer waters, located between water-resistant layers in the thickness of sedimentary rocks. The radiochemical composition of groundwater is affected by the amount of soluble radionuclides contained in the composition of the soil washed by this water. In addition, the concentration of radioactive isotopes in the water of the first aquifer is influenced by climatic and meteorological conditions. Thus, the radioactivity of this water at 40 K in the Hungry Steppe reaches 207 Bq/l, in the steppes of Fergana - 36 Bq/l, and in Karelia - 8.5 Bq/l. In deep waters and
more mineralized than surface ones, there is a proportional increase in specific activity with increasing total concentration salts. The radioactivity of groundwater is mainly due to the presence of 40 K, 226 Ra and 222 Rn. Groundwater in sedimentary rocks has the least activity, they are most often used for water supply to the population, since they contain uranium on average 5 × 10 -6 g / l, radium 7.4 × 10 -2 Bq / l and radon 1.85 Bq / l. The waters of acidic igneous rocks, for example, the waters of fractured granites, have a higher activity for these elements and may contain an increased amount of 226 Ra - up to 3.7 Bq/l - the waters of the resorts of Tskhaltubo, Istisu in Transcaucasia, 222 Rn 48 Bq/l - water resorts of Belokurikha, Zheleznovodsk, etc.
High concentrations of radium and uranium are found in the interstratal waters of oil-bearing regions.
Radioactivity waters of open reservoirs of land depends on chemical composition breeds and climatic conditions. The degree of radioactivity of river water is determined by the type of river feeding - surface or ground, and the type of feeding, in turn, is influenced by the change of seasons and meteorological factors. As a rule, surface waters (rain, glacier, snow) contain relatively less radionuclides, therefore, during the flood period, the radioactivity of river water is lower. In low water, during the feeding period of rivers, mainly due to groundwater, the specific activity of water increases. In winter, radon and thoron accumulate in the water of rivers covered with ice. The radioactivity of river water is mainly due to the presence of 40 K, 226 Ra, and the content of 40 K varies from 3.7?10 -2 to 0.6 Bq / l, uranium - from 2?10 -8 to 510 -5 g / l, radium - from 9.2?10 -3 to 7.4?10 -2 Bq/l.
Radioactivity lake water depends on the water activity of the tributaries and groundwater feeding the lakes. In the northern regions, the water activity of lakes is close to that of rivers. In the southern regions, where the evaporation of water from lakes exceeds the runoff from them, salts accumulate and, accordingly, water activity increases. Thus, the specific activity of water in the central regions of Kazakhstan at 40 K rises to 3.7 Bq/l and more, the radioactivity of water in salt lakes is especially high, where it reaches 370 Bq/l.
Waters of the seas and oceans depending on hydrological and climatic conditions, they differ in salt composition. Certain
fluctuations are also detected in the composition of radionuclides. The activity of sea and ocean water at 40 K is within 11-18 Bq / l, at 238 U - 2 × 10 -6 g / l, at 226 Ra - (2.2-3.7) × 10 -2 Bq / l.
Radioactivity of flora and fauna
The radioactivity of the plant and animal world is due to almost all those radioactive isotopes that occur in nature, and all of them can be conditionally divided into two groups.
The first group, relatively small, should include such radioactive isotopes that are mixed with stable elements that are actively involved in the metabolism and ensure the functioning of all organs and systems of living matter (for example, 40 K, 14 C, 3 H). In this regard, the content of isotopes of this group in organisms depends on the degree of accumulation of stable elements. For example, peas contain 0.9% potassium, and butter - 0.014%, so the specific activity of peas due to 40 K is 274 Bq / kg, and butter - 3.7 Bq / kg.
Other radioactive isotopes (for example, 238 U, 226 Ra, 232 Th, 210 Pb, 210 Po) can be assigned to such a group, the significance of which in metabolic processes is currently not well understood. The results of many studies indicate that the content of this group of isotopes in plant and animal organisms depends on their concentration in the environment. So, in the ashes of plants grown on ordinary soil, the uranium content is on average 3×10 -4 g/kg, and in the ashes of plants growing on soil enriched with uranium, it is 2×10 -3 g/kg. In addition, it should be noted that the relative efficiency of accumulation of radioactive isotopes of this group decreases with a sharp increase in their content in the environment.
Of the first group of isotopes, the potassium isotope, 40 K, occupies the main place in terms of the magnitude of the generated activity. The amount of potassium in plant organisms is 3-10 times less than its content in the earth's crust. Even less than in the rocks, potassium (10-15 times) in the body of animals. In table. 33 shows the potassium content and specific activity at 40 K of some food products of plant and animal origin.
Table 33Potassium content and specific radioactivity at 40 K of individual food products of plant and animal origin
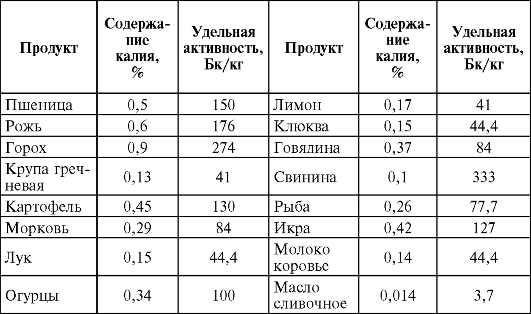
The specific radioactivity of the biomass for carbohydrate is an order of magnitude lower than for 40 K, and the activity for tritium is negligible.
The main isotopes of the second group contained in plants and animals are 226 Ra, 210 Pb, 210 Rho, as well as uranium and thorium isotopes. The specific activity of 210 Rb and 210 Rho in plant food ranges from 0.02 to 0.37 Bq/kg. The different content of these nuclides in products of plant origin is due to the different sorption surface of plants. The content of 210 Pb and 210 Rho in tea is especially high (up to 30.5 Bq/kg). In food products of animal origin, the specific activity of 210 Pb ranges from 13.7 mBq (milk) to 0.18 Bq/l, and 210 Rho - from 3.3 (milk) to 0.13 mBq/kg (beef). On average, the daily diet of an inhabitant of the middle latitudes of Russia contains about 0.22 Bq 210 Rho, with a ratio of 210 Rho / 210 Pb equal to 0.73.
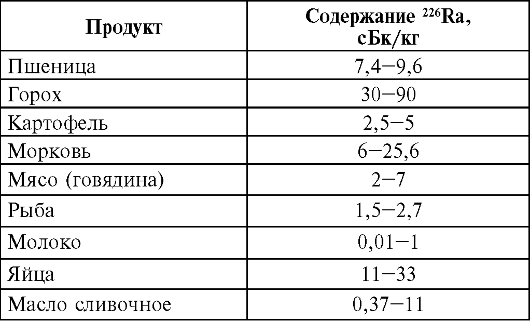
According to domestic scientists, the content of uranium is an order of magnitude higher in food products of plant origin than animal. So, in wheat bread, the uranium content averages 4.1 × 10 -7%, in buckwheat - 4.2 × 10 -7%, in beef - 1.4 × 10 -8%, in fish - 1.1 ?10 -8%, in milk - 4?10 -9%.
The total radioactivity of plants and animal tissues due to α-emitters is 0.37 and 0.037 Bq/kg, respectively.
Thus, the main source of natural radionuclides entering the human body is the diet, which is dominated by products of plant origin.
The radioactivity of the human body
The radioactivity of the human body is due to the presence in the body of all those radioactive isotopes that are found in the biosphere. The approximate content of the most common radionuclides is given in Table. 34.
The radioactivity of the entire series of uranium and thorium with daughter products is about 10 times higher. When assessing the content of radionuclides in individual human organs and systems, it is necessary to consider first of all the radioactivity due to the presence of isotopes (potassium, carbon and hydrogen), which are necessarily part of living structures and without which the existence of an organism is impossible.
Table 34The content of natural radionuclides in the human body

The total content of potassium in the body of an adult (weighing 70 kg) is 0.19% (130 g). Tissues and organs with high functional activity are especially rich in potassium; skeletal muscles, nervous tissue, heart, liver, spleen, etc. The main depot of potassium in the body is muscle tissue. In view of the fact that 40 K occurs in nature in a mixture with stable isotopes in an amount of 0.0119%, the specific radioactivity of organs and tissues of the human body at 40 K is determined by the content of the stable isotope in them (Table 35).
Table 35The content of potassium and 40 K in individual organs and tissues of a person
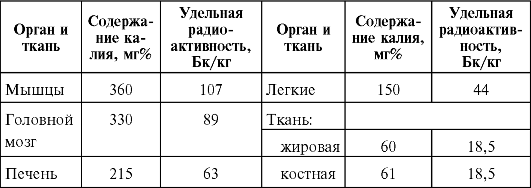
As the results of many studies have shown, the content of potassium, and consequently, 40 K in the human body depends on gender, age, body weight, the nature of muscle activity, etc. The content of potassium in the muscles is usually higher in men than in women, higher in persons performing heavy physical work. dystrophic changes in soft tissues during aging
nizma are accompanied by a decrease in the level of potassium. Thus, deviations in the concentration of potassium in the organs and systems of individual individuals compared with the above data can be quite significant and reach 150-200% or more.
The total carbon content in the body of an adult reaches 18%, i.e. about 12.6 kg. Taking into account the uniform distribution of carbon in the tissues, it can be assumed that their specific radioactivity for 14 C is 52 Bq/kg.
The amount of tritium in the body is almost constant and is determined by the content of the stable isotope (about 10.2% in muscles and 6.4% in bones). The specific activity of soft tissues of the human body due to 3 N is 0.55 Bq/kg, bones - 0.34 Bq/kg.
In conclusion, it should be noted that the activity of the human body, due to the presence of 40 K, 14 C and 3 H, primarily depends on the number of stable elements, the content of which is dictated by the requirements of the constancy of the internal environment, determined by the functional state of the body. Possible significant fluctuations in the radioactivity of food rations due to these isotopes in this case are not significant.
The biological role of radioactive isotopes present in the body in a negligibly small amount, conditionally assigned to group II, is still unknown. Selective accumulation in individual organs and systems of isotopes of this group or their uniform distribution can be explained by chemical properties close to those of biologically necessary stable elements. Of the radionuclides of this group, radium is the most studied in terms of content in the body. This isotope, like calcium and other osteotropic elements, accumulates mainly in bone tissue. The content of radium in individual tissues and organs is presented below.
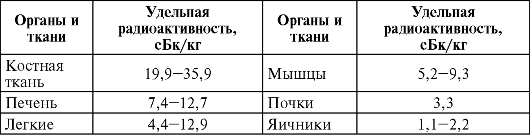
The main part of 210 Pb (up to 70%) is contained in the skeleton. With its long half-life, equal to 2000 days, the accumulation of an equilibrium amount of 210 Pb is possible. The specific activity of 210 Pb in bone tissue is 15 Bq/kg, in soft tissues - 6.4 Bq/kg. With air during the day, about 0.7 scBq 210 Rho enters the lungs of a person, in a person who smoked 1 pack of cigarettes per day, 10 times more (up to 0.07 Bq) enters the lungs.
The total content of uranium in the body is low and amounts to 8?10 -6 -1?10 -5 g/g. Thorium and its α-active daughter products account for up to 40% of the total α-activity of the human body. In addition, radon has some significance in the radioactivity of tissues and organs, at a concentration of 0.01 Bq/l in the inhaled air, the activity of soft tissues due to α-emitters can reach 0.05 Bq/kg.
The above content in the human body of radium, uranium and other radioactive isotopes assigned to group II is approximate, and it is very difficult to present the average specific activity of organs and tissues in this case. This is due to the fact that the degree of radioactivity of individual organs and tissues of the human body, on the one hand, is affected by the rate of metabolic processes and the functional state of the body, and, on the other hand, by a certain significance of the content of this group of isotopes in the diet. With a constant intake of radionuclides with the diet, a balance is established between their intake and excretion from the body. At the same time, an equilibrium concentration is created in individual organs and tissues. If we take into account that the content of radionuclides varies not only in different food products, but also in the same product grown in different geographical areas, the significance of the ethnic and economic characteristics of the nutrition of the population becomes clear. As an example, we can consider the radioecological chain lichen - reindeer - man. Significant sorption capacity of lichens and a long period of life (up to 300 years) contribute to a significant accumulation of 210 Pb and 210 Rho in them - up to 5.9 sBq/kg of air-dry mass on average. The level of accumulation of 210 Pb and 210 Rho in the body of a reindeer significantly depends on the grazing season of the animal. The content of these nuclides is maximum in
in the spring (food base - lichen) - 17 Bq/kg of raw meat, in the summer period (food base - annual grasses) the specific activity of polonium decreases by about 5 times (there is no such dependence on the season for 210 Pb). Accumulation of 210 Pb and 210 Rho in the bone tissue of indigenous reindeer herders (4.8 Bq/kg of raw tissue) is observed, exceeding their content in the skeleton by 10 times or more compared to people of other professions.
From the materials presented above, it can be seen that the main source of radioactive elements entering the human body are food products. In this regard, water is of secondary importance, and only with an increase in radium activity to 0.037 Bq/l and higher does its role in the formation of the radioactivity of the human body increase.
Thus, radionuclides are dispersed in the biosphere and are ubiquitous in earth rocks, water, air, foodstuffs and the human body. The importance of this phenomenon is primarily due to the doses of background radiation to which the population of our planet is exposed.
9.2. Human background exposure
Background exposure of the human body, depending on the sources of ionizing radiation, can be external and internal.
Sources of external exposure include cosmic rays, γ-radiation of radionuclides contained in rocks, soil and building materials, as well as in the air; β-radiation in this case can be ignored, due to the fact that the level of air ionization due to β-particles is low, the effective solid angle of body irradiation is less than 2π, and organic substances on the ground surface and facing materials in rooms, having a low specific β- activity, absorb β-flows from minerals and building structures.
The power of γ-radiation from radionuclides contained in the water of the seas and oceans reaches 0.05 μR/h. In areas with an increased amount of radioactive elements (some areas in Brazil, India, France, Russia), the intensity of γ-radiation is especially high. So, in the region of monazite sands of Brazil, it reaches 1 μSv / h, in India - up to 3 μSv / h, in mountainous regions
France - 0.2-0.4 µSv/h; in Pyatigorsk (Northern Caucasus) - up to 2-3 μSv / h.
In table. 36 shows the power of exposure doses of γ-radiation depending on the content of the main natural radionuclides in the rocks.
Table 36Dose rate of external γ-radiation from natural radionuclides contained in rocks

As can be seen from the table, depending on the content of these elements in the rocks, the radiation power can vary widely. As a rule, sedimentary rocks contain less natural radionuclides than igneous rocks, thereby creating a lower (2-3 times) level of radiation power.
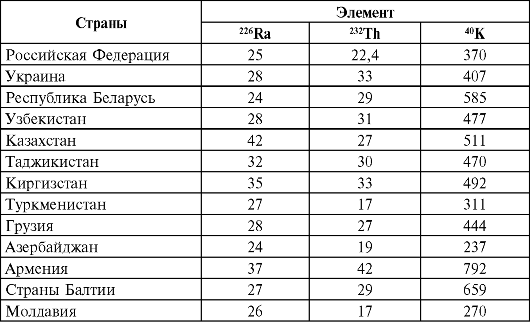
In table. 37 and 38 shows the content of 226 Ra, 236 Th and 40 K in the main building materials for different countries and Russia.
Table 37Natural radioactivity of building materials in some countries, Bq/kg

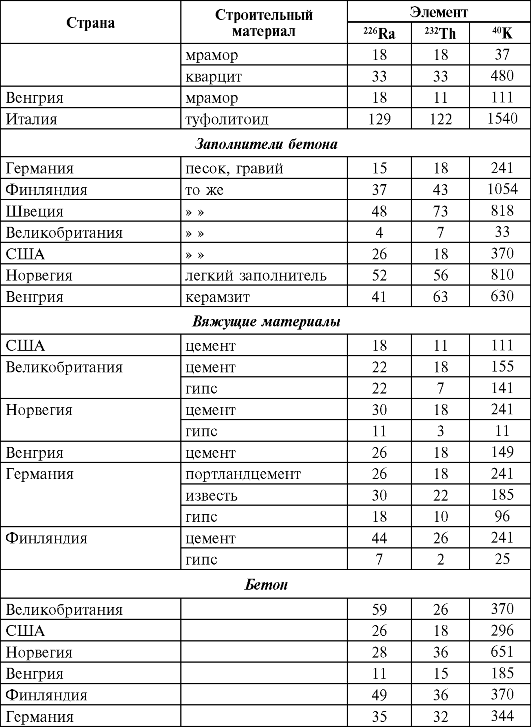
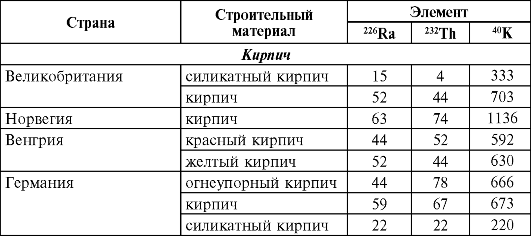
Table 38Natural radioactivity of building materials in different regions, Bq/kg
Of particular interest is the level of γ-radiation in residential buildings. The fact is that, on the one hand, the geometry of human body irradiation changes indoors (on the street it approaches 2π, indoors - to 4π), and on the other hand, the power of γ-radiation depends on the content of radionuclides in building materials. The equivalent dose in buildings built of wood has the lowest power - up to 0.5 mSv / year, large doses - in brick buildings - up to 1 mSv / year and reinforced concrete - up to 1.7 mSv / year.
The equivalent dose rate in different cities of the world outdoors is given below.
Equivalent dose rate in different cities of the world outdoors

When assessing the dose created by cosmic radiation, firstly, it is assumed that cosmic radiation has a high degree of hardness, therefore, the practically absorbed dose in any tissues and organs of the human body should be the same. Secondly, background fluctuations due to different levels of solar activity, as well as its changes depending on latitude, are not taken into account. To calculate the dose created by cosmic radiation, it is necessary to refer to the ionization of air due to this component of background radiation. The most reliable value of air ionization for middle latitudes is considered to be the ionization rate equal to 1.94 pairs of ions in 1 cm 3 /s. Knowing this value, you can find the dose created in the tissues of the human body using the following formula:
where D to - absorbed dose due to cosmic radiation; 1.94 - the number of pairs of ions that occur in 1 cm 3 of air due to
cosmic rays; 3.6?10 3 - the number of seconds in 1 hour; 24 - the number of hours in 1 day; 365 - the number of days in a year; 1.93?10 9 - the number of pairs of ions that occur at a dose of 1 R; 0.87?10 -2 - coefficient of dose conversion from P to Gy.
Thus, 0.28 mGy/year is the average dose received by the population of our planet due to cosmic radiation. When assessing the possible biological effect of this type of ionizing radiation, it is necessary to know the RBE for each component of cosmic rays.
When calculating the dose received by a person due to external exposure, the average time spent outdoors and inside them is taken into account, outdoor stay is taken equal to 0.2, while the annual effective equivalent dose due to terrestrial γ-radiation outdoors will be 6 × 10 - 5 Sound Based on the ratio of wood, brick and concrete buildings around the world, UNSCEAR estimates the globally averaged absorbed dose rate in indoor air to be about 610 -8 Gy/h. The time spent by a person in the premises is 80%, therefore it can be calculated that the annual effective equivalent dose indoors will be equal to 2.9 × 10 -4 Sv, and the total annual effective equivalent dose due to external exposure to radionuclides of terrestrial origin will be 3.5 × 10 -4 Sound
The radiation of natural radionuclides contained in the atmosphere causes air ionization by about 2 orders of magnitude less than the γ-radiation of rocks and soil, so it has an insignificant contribution to the total effect.
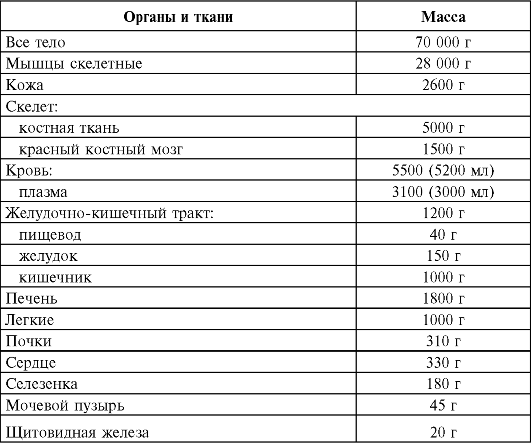
Internal exposure of the human body is created by 40 K, 14 C, 226 Ra, 222 Rn, 210 Po and other radioactive elements contained in the body. When calculating the dose rate created by one or another isotope, they proceed from its average content in the body of a “standard” person, whose organ mass is presented below.
The mass of organs and tissues of a "standard" person
An example of these calculations is the calculation of the soft tissue dose rate delivered by 40 K using the following formula:
where 4440 is the total activity of the soft tissues of a "standard" person at 40 K, Bq; 0.6 - average energy of β-particles, MeV; 1.6?10 -6 - the number of ergs in 1 MeV; 3.6?10 3 - the number of seconds in 1 hour; 24 - the number of hours in 1 day; 365 - the number of days in a year; 70?10 3 - the mass of a "standard" person, g; 10 -4 - coefficient of transition from erg/g to Gy.
With uneven distribution of radionuclides in the body, the value of specific activity is used. It should also be remembered
about the possibility of a certain contribution to the irradiation of daughter decay products; Thus, when calculating the dose rate in bone tissue created by 226 Ra, the dose from daughter products - 222 Rn, RaA, RaB, RaC is also taken into account.
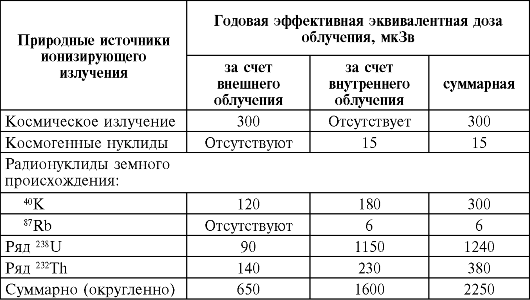
In table. 39 shows data on human background exposure.
Table 39Annual effective equivalent exposure doses due to natural sources of ionizing radiation in regions with a normal radiation background (temperate climate zone)
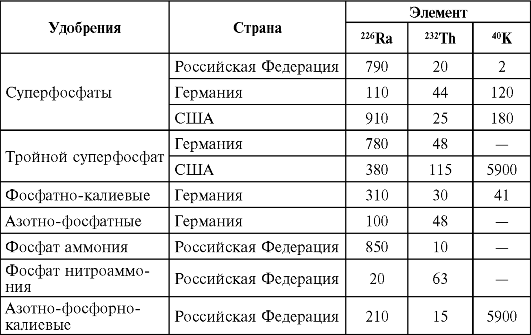
As a result of human activity, the radiation background is gradually changing, which is associated with the use for construction purposes of various wastes in the form of ash and slag from energy facilities, ferrous and non-ferrous metallurgy, as well as the chemical industry, the use of fertilizers obtained from natural mineral raw materials (Tables 40, 41 ). At present, the contribution of this component of the natural radiation background to the population exposure dose, as a rule, does not exceed 3-5%. At the same time, there is a need to take this factor into account in areas with intensive industrial waste used as a basis for the manufacture of building materials. Permissible levels of natural radionuclides in building materials and fertilizers are presented in Chapter 5.
Table 40Natural radioactivity of building materials made from industrial waste, Bq/g
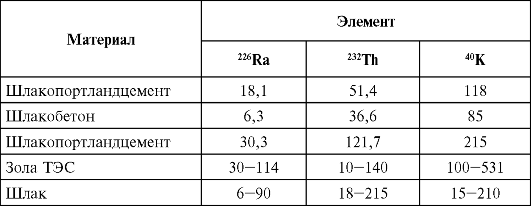
Table 41Natural radioactivity of phosphate fertilizers, Bq/kg
test questions
1. What sources of ionizing radiation form the natural radiation background?
2. Give a description of cosmic radiation.
3. What groups of radioactive elements are conditionally distinguished in natural radioactivity?
4. What radionuclides determine the radioactivity of the air?
5. What factors determine the radioactivity of natural waters?
6. What radionuclides cause the radioactivity of flora and fauna, the human body?
7. What is the level of equivalent doses in buildings constructed from various building materials?
8. What is the average level of human exposure due to natural background radiation?
“Radiation background is normal” - this phrase is usually used when assessing situations related to the operation of nuclear power plants. The normal radiation background is up to 0.20 µSv/h (20 µR/h). The safety threshold for people is 0.30 µSv/hour (30 µR/hour). Sanitary norms and rules prescribe not to exceed the annual effective radiation dose of 1 mSv when performing X-rays. But you will not find the normative value of natural radiation in any international or domestic regulatory document. Why?
Where does natural radiation come from?
The natural radiation background of the Earth is associated with its history and the evolution of the biosphere. Since the birth of our planet, it has been under the constant influence of cosmic radiation. A colossal amount of cosmogenic radionuclides was involved in the formation of the earth's crust. Scientists believe that tectonic processes, molten magma, the formation of mountain systems owe their appearance to radioactive decay and heating of the bowels. In places of faults, shifts and stretching of the earth's crust, oceanic depressions, radionuclides came to the surface and places with powerful ionizing radiation appeared. The formation of supernovae also had an impact on the Earth - the level of cosmic radiation increased tenfold on it. True, supernovae were born about once every hundreds of millions of years. Gradually, the radioactivity of the Earth decreased.
At present, the Earth's biosphere is still affected by cosmic radiation, radionuclides dispersed in solid earth rocks, oceans, seas, groundwater, air and living organisms. The totality of the listed components of the radiation background (ionizing radiation) is commonly called the natural radioactive background. Natural radioactivity includes several components:
- cosmic radiation;
- radioactive substances in the earth's interior;
- radionuclides in water, food, air and building materials.
Natural radiation is an integral part natural environment a habitat. The honor of its discovery belongs to the French scientist A. Becquerel, who accidentally discovered the phenomenon of natural radioactivity in 1896. And in 1912, the Austrian physicist W. Hess discovered cosmic rays by comparing the ionization of air in the mountains and at sea level.
The power of cosmic radiation is not uniform. Closer to the earth's surface, it decreases due to the shielding atmospheric layer. Conversely, it is stronger in the mountains, since the protective screen of the atmosphere is weaker. For example, in an airplane that flies in the sky at an altitude of 10,000 meters, the radiation level exceeds ground radiation by almost 10 times. The strongest source of radioactive radiation is the Sun. And here the atmosphere serves as our protective screen.
Natural radiation background in various places of the world
Permissible radiation background in different parts of the world is significantly different. In France, for example, the annual dose of natural radiation is 5 mSv, in Sweden - 6.3 mSv, and in our Krasnoyarsk only 2.3 mSv. On the golden beaches of Guarapari in Brazil, where more than 30,000 people vacation every year, the radiation level is 175 mSv / year due to the high content of thorium in the sand. In the hot springs of the town of Ram-Ser in Iran, the radiation level reaches 400 mSv / year. The famous resort of Baden-Baden also has an increased radiation background, as well as some other popular resorts. The radiation background in cities is controlled, but this is an average figure. How not to get into trouble if you do not want to put your health to the test with an increased dose of natural radionuclides? The radioactivity indicator will become your reliable travel expert.
Under background radiation It is customary to understand the ionizing radiation of natural sources of cosmic and terrestrial origin, as well as artificial radionuclides dispersed in the biosphere as a result of human activity. RF consists of the following components:
Natural radiation background(ERF) is ionizing radiation from natural sources of extraterrestrial (cosmic) and terrestrial origin, affecting a person on the surface of the Earth.
Technogenically modified radiation background(TIRF) is the ionizing radiation of sources and radionuclides created or dispersed in the biosphere as a result of human activities.
2. Natural radiation background, characteristics of natural sources of ionizing radiation of terrestrial and extraterrestrial origin
The source of ionizing radiation NRF of extraterrestrial origin is primary cosmic radiation , which in the vicinity of the Earth consists of galactic cosmic radiation(generated in still exactly unknown, but remote from the Earth objects) and solar cosmic rays. The average energy of cosmic particles is about 10 8 - 10 9 eV. Primary cosmic radiation consists mainly of protons (90%) and alpha particles, there are nuclei of lithium, beryllium, boron and others. The electron flux is about 1.5% of the flux of all cosmic particles, positrons are 5 times less, and gamma quanta were also found in a small amount.
The Earth's magnetic field noticeably affects the primary radiation, preventing low-energy particles from entering the atmosphere. There are "traps" in the Earth's magnetic field, i.e. areas of space characterized by the fact that charged particles can neither fly into them from the outside, nor fly out of them. Magnetic traps is a natural reservoir for the accumulation of charged particles (mainly protons and electrons). Such zones are called Earth's radiation belts.
Primary cosmic radiation interacts (or, more precisely, is absorbed) with the atmosphere, resulting in the formation of secondary cosmic radiation (which is made up of pions, protons, neutrons, muons, electrons and photons) and cosmogenic radionuclides that affect a person.
The intensity of secondary cosmic radiation depends on the thickness of the atmosphere. Cosmic radiation at sea level is about 100 times less intense than at the boundary of the atmosphere and consists mainly of muons, and the North and South Poles receive more ionizing radiation than the equatorial regions (due to the Earth's magnetic field).
When cosmic rays act on the atmosphere, various nuclear reactions occur in its upper layers, resulting in the formation of cosmogenic radionuclides. Of these, tritium (H-3), C-14, P-32, S-35, Be-7, Na-22 and Na-24 are of primary importance.
In general, a person living at sea level receives 0.315 mSv/year from sources of ionizing radiation of extraterrestrial origin, including 0.3 mSv from external exposure and 0.015 mSv from internal exposure.
The levels of terrestrial radiation are not the same for different places on the globe and depend on the concentration of radionuclides in one or another part of the earth's crust. Rocks of volcanic origin - granite, basalt - are characterized by an increased content of radionuclides; much less radioactive elements in sedimentary rocks - limestone, sandstone.
Most high levels terrestrial radiation is observed in Brazil (on the beaches of the Guarapari seaside resort - up to 175 mSv / year), in southwestern India (monazite sands rich in thorium). Other places with high levels of radiation are also known, for example, in France, in Nigeria, in Madagascar. The territory of the Scandinavian countries and England is distinguished by an increased content of radionuclides of the uranium series.
According to UNSCEAR calculations, the average effective dose of external exposure that a person receives per year from terrestrial sources of natural radiation is 0.35 mSv, including 0.09 mSv due to uranium series radionuclides and 0.14 mSv due to thorium series radionuclides. The decay products of uranium and thorium through food chains, as well as with air and water, enter the human body, causing internal exposure: due to the uranium family, the effective dose is 0.95 mSv / year, due to the thorium family - 0.19 mSv / year. When radioactive elements are ingested, it is important to take into account their solubility and, accordingly, the absorption coefficient.
Natural sources of ionizing radiation of terrestrial origin are represented by radionuclides of two groups:
A. Radionuclides included in the radioactive series;
B. Radionuclides not included in the radioactive series.
3. Radon-222 (Rn-222) is the main contributor to the natural radioactivity of atmospheric air and human exposure levels due to natural sources of radiation. Radon undergoes alpha decay with the formation of Po-218, T 1/2 Rn-222 - 3.8 days. Radon and short-lived products of its decay enter the body mainly through the respiratory organs, but can enter through the gastrointestinal tract (when drinking radon water) and through the skin (when taking radon baths). Removal of radon from the body, regardless of the method of its intake, is carried out mainly through the lungs.
Radon is a colorless, invisible, tasteless and odorless inert gas, about 7.5 times heavier than air. It is formed in the process of radioactive decay of radionuclides of the uranium and thorium series. There are three natural (natural) isotopes of radon:
radon-222 (T 1/2 - 3.8 days, decay series U -238);
radon-220 or thoron (T 1/2 - 55 seconds, decay series Th-232);
radon-219 or actinon (T 1/2 - 4 seconds, U-235 decay series).
All radon isotopes are alpha emitters, and further decay of their daughter products is accompanied by the emission of both alpha and beta particles. Most of the radon and thoron are physically bound to the material in which their precursors are found. However, some may diffuse from the site of formation into another environment. Due to the relatively long half-life Rn-222 can diffuse over long distances (within a few meters). Actinon migration is limited to a few millimeters and usually does not reach the surface of the material. A small part of the thoron can stand out and migrate within a few centimeters. Therefore, with the exception of places rich in thorium, the concentrations Rn-219 and 220 are negligible compared to Rn-222.
The main sources of radon are soil, building materials, groundwater, natural gas, coal, mines, dumps formed during the extraction of phosphate fertilizers, plants, geothermal power plants, and nuclear fuel cycle enterprises. The main sources of radon in the atmosphere are soil and ground rocks. In general, the concentration of radon and its daughter decay products in the air depends on the place, time of year and day, altitude and meteorological conditions. From a geological point of view, about 40% of the territory of the Republic of Belarus are potentially radon hazardous. This is due to the shallow occurrence of granite rocks and widespread active zones of tectonic faults.
The concentration of radon in indoor air depends mainly on four factors:
active and passive diffusion of radon from the soil through the foundation and basement surfaces of buildings;
radon exhalation from building materials and products from which the building is built;
exhalations of radon from water and gas;
influence of climate, lifestyle, degree of ventilation of the room.
Measures aimed at reducing the concentration of radon in indoor air can be thorough isolation of residential premises from soil and soil, ordinary painting (reduces radon exhalation from building materials by 32-87%) and wallpapering walls, improving ventilation of residential premises and active ventilation of cellars , the use of materials that meet the requirements of radiation safety. Radon and its decay products make a significant contribution to human exposure. The main part of the dose a person receives indoors. It is believed that the concentration of radon indoors in temperate zones is on average 8 times higher than in the outdoor air. The concentration of daughter decay products exceeds the concentration of radon by more than 200 times. The inhalation route of entry into the body of radon isotopes and their daughter decay products is considered the most dangerous.
Most important factors that affect the formation of the dose to the respiratory tract due to radon and its decay products are:
indoor radon concentration;
factor of equilibrium of decay products;
characterization of aerosols, their retention and purification in the respiratory tract;
the amount of breathing;
home depreciation time.
It is currently believed that a radon concentration of 20 Bq/m 3 increases the radiation dose by 1 mSv. From this value, the problem of radon becomes apparent. Moreover, it has been found that the dose to the respiratory tract is highly dependent on age. At the age of about 6 years, it has a maximum and is approximately 2.5 times greater than the dose formed at the age of 30 years. Mouth breathing in a child leads to a greater intake of radon than breathing through the nose, which makes it necessary to sanitize the upper respiratory tract in children. It has been shown that radon inhalation is accompanied by an uneven distribution of the radiation dose in human organs and tissues. There are epidemiological data on the association of radon with the incidence of lung cancer and leukemia.
Let's determine the estimated annual dose of radiation that a person receives constantly, which is provided by the radiation background. And let's figure out whether it is worth being afraid of an x-ray, flying on an airplane, etc.
Around us there are a huge number of sources of radiation, both natural and man-made origin, with which we live every day. Here are the main sources and what radiation they provide.
Radiation in the air
The most significant source of natural background radiation is in the air and is radon, a radioactive gas. Radon and its isotopes, parent radionuclides, decay products provide an average inhalable dose of 1,260 microsieverts (µSv) per year. In Russia, the average individual exposure dose according to the data for 2001-2010 is 1,980 μSv per year. This makes up a large part of the total dose of radiation that a person receives on average from natural and man-made sources. Radon is distributed unevenly and its concentration depends on various factors. It is a decay product of uranium, which is quite common in the earth's crust, its highest concentrations are concentrated in ore-bearing rocks. Radon leaks from these rocks into the atmosphere, into groundwater, or into buildings. When breathing, it and its decay products enter the lungs, where they remain for certain period time. There are areas where radon is a significant health hazard. In buildings in Scandinavia, the United States, the Czech Republic and Iran, the concentration of radon was recorded, exceeding the average value by more than 500 times.
This is radiation from space, from the Sun and other stars. It is partially delayed by the Earth's atmosphere. Therefore, the higher the altitude, the less air is available to trap it and the greater the cosmic radiation. The radiation dose varies from approximately 250 μSv per year at sea level to 500 μSv per year at 1 km altitude. Approximately we will take a dose of radiation in the amount of 390 μSv.
When flying on an airplane, a person receives a slightly increased dose of radiation, usually it is 5 μSv per hour of flight. The average additional dose of radiation for flight personnel is 2190 μSv per year.
Radiation background of the Earth
The presence of the radiation background of the Earth is associated with the radiation of uranium, thorium and other radioactive substances that are found in the soil. The average value is about 480 µSv per year, and this value is much lower along the coasts.
In India and Brazil, which have high levels of thorium in the soil, doses can be much higher. In the states of Kerala, India and Minas Gerais, Brazil, the background is about 10,000 μSv per year.
radiation in food
Products naturally contain carbon-14, which is radioactive, the radioactive isotope potassium-40, and other radioactive isotopes. With food and water, a person receives about 290 μSv per year. In addition, some plants and animals accumulate more radioactive substances in themselves, therefore, when they are consumed, the dose is higher. Potatoes, beans, nuts, sunflower seeds have above average levels of radiation. A person contains potassium-40 (30 mg), carbon-14 (10-8 g) and other radionuclides. This leads to the fact that each person also has a radiation background.
Technogenic radiation background
According to some estimates, the global average human exposure to artificial radiation is 600 μSv, primarily due to medical procedures. The amount of radiation received depends to a large extent on the equipment and the specifics of medical care. AT different countries it is different. In the US, for example, the average amount of exposure received is much higher, at 3,000 µSv per year. In Russia, it is much less.
- Typical x-ray chest- 30 - 300 µSv.
- Dental x-ray - 5 to 10 µSv.
Other anthropogenic causes of radiation exposure: smoking, radioactive building materials, historical testing nuclear weapons, accidents at nuclear power plants and the operation of nuclear power plants.
Consumer goods
Cigarettes, building materials, etc. also have background radiation. Cigarettes contain polonium-210, a decay product of radon found in tobacco leaves. Very active smokers who smoke 1.5 packs a day receive a radiation dose of 60,000 μSv per year. Since the dose of radiation is received by the smoker locally in the bronchi of the lungs, it cannot be compared with the allowable radiation rates, since they are designed for the effect of radiation on the body as a whole.
Some estimates put consumer products at 130 μSv per year.
Use of nuclear weapons
Elevated nuclear explosions between 1940 and 1960 led to the release of a significant amount of radioactive substances. Some pollution is local, some has spread throughout the world. In 1963 this pollution reached its peak. They gave a background of about 150 μSv per year. And they accounted for about 7% of the average radiation background of all sources. By the year 2000, the global radiation background associated with these pollutions had dropped to 5 μSv per year.
Accidents at nuclear power plants
Under normal conditions nuclear reactors release small amounts of radioactive gases that create negligible levels of radiation. Large releases of radioactivity from nuclear power plants are extremely rare. So far, there have been two major accidents at nuclear power plants - this is an accident at Chernobyl nuclear power plant and Fukushima I. These accidents resulted in significant pollution environment.
Residents of the affected areas from the Chernobyl accident received a total dose of 10,000 to 50,000 µSv over 20+ years, with most of the dose received in the first years after the disaster. The liquidators received a dose of more than 100,000 μSv. Due to acute radiation sickness, 28 people died. Now the dose of radiation around the world from the Chernobyl accident is about 2 μSv.
Residents of the affected areas from the Fukushima I accident received a total dose between 1,000 and 15,000 μSv. 167 liquidators received doses above 100,000 µSv, and 6 of them received doses above 250,000 µSv.
The average dose from the accident at the Three Mile Island nuclear power plant was 10 μSv.
In addition to the civilian accidents mentioned above, there were several accidents related to military installations, such as the accident at Windscale, the contamination of the Techa River with nuclear waste from the Mayak production association, and the Kyshtym accident.
Radiation background at operating nuclear power plants
Near a nuclear power plant, as a rule, an additional background of the order of 0.1 μSv per year is created (very small), the average dose received by people living near a coal-fired CHP plant is three times higher!
Radiation in the workplace
The International Commission on Radiological Protection (ICRP) recommends limiting radiation exposure at the workplace to 50,000 µSv per year, and 100,000 µSv over 5 years.
There are also other man-made sources, such as watching TV, which gives about 10 μSv per year. Let's leave 1% for other sources.
As a result, it turns out that the radiation background is about 3,300 µSv per year without taking into account the impact of medical procedures (0.38 µSv per hour) and 3,900 µSv, taking into account the impact of medical procedures. But it must be taken into account that these values are highly dependent on the conditions of the terrain, altitude, etc., therefore, everywhere there is a radiation background.

Are x-rays and flying on an airplane dangerous?
Radiation levels up to about 0.5 μSv per hour are considered safe. But people can tolerate radiation of 10 μSv per hour for several hours without much harm to their health. Therefore, flying on an airplane, which gives an additional 5 μSv per hour, does not cause much harm to a person, but it is not recommended to fly more than 72 hours a month. The absorbed dose of radiation accumulated in the body during life should not exceed 100,000 -700,000 µSv.
Should I be afraid of x-rays? If you do it once a year, then the dose of radiation is small compared to the effects of other sources of radiation, and the body can tolerate it. Especially if the study is carried out with modern equipment that creates a minimum radiation dose of 30 µSv. And often x-rays avoid much more harm than this procedure can cause.
What is really to be feared is the high concentration of radon in the premises, so they must be well ventilated, especially in areas where its concentration is increased.
 Environmental problems ocean. 5 threats to the future
Environmental problems ocean. 5 threats to the future
All living beings inhabiting our planet are constantly exposed to ionizing radiation through external and internal exposure from natural (and natural radioactive substances) and artificial (nuclear industry waste, radioactive used in biology, medicine, agriculture etc.) sources of ionizing radiation. Those. The development of life on Earth has taken place and is taking place in the presence of background radiation.
Under background radiation It is customary to understand from natural (natural) sources of cosmic and terrestrial origin, as well as from artificial radionuclides dispersed in the biosphere as a result of human activity. The radiation background is due to environmental factors and does not include those that work with sources of ionizing radiation, as well as radiation used for diagnostic and therapeutic purposes.
There are natural background radiation, artificial radiation background, technologically modified (increased) radiation background. All sources of background radiation are divided into two main groups: natural and artificial.
Natural background radiation (NRF) is the main component of background radiation. NRF sources are those that act on a person on the surface of the Earth from external natural sources of unearthly origin (cosmic radiation), external natural sources of terrestrial origin (present in the earth's crust, water, air), as well as from internal sources (i.e. natural radionuclides origin, which are contained in the human body). Most natural sources are such that it is completely impossible to avoid exposure from them. We receive 78% of our exposure from natural sources of radiation.
A person is exposed to radiation in two ways:
1. External exposure - exposure from sources of radioactive radiation outside the body. It can be produced by all types of radiation, but only gamma and x-ray radiation, fast and slow neutrons, are of practical importance. beta radiation. Alpha radiation in view of the negligible penetrating power of practical importance is not.
2. Internal exposure - originating from a source of radioactive exposure (radioactive substance) located inside the body. It continues continuously until the radioactive substance in the body decays or is removed from the body. largely depends on the distribution of the radioactive substance in the body, on the nature of the radiation (L-, β-, γ - emitter), radiation energy, half-life and half-life.
The natural radiation background is an integral factor external environment and plays a significant role in human life. natural radioactive elements have been part of the Earth since its formation. Evolutionary development shows that under conditions of natural background radiation, optimal conditions are provided for the vital activity of plants, animals and humans. The ability of radioactive radiation to cause mutations was probably one of the main reasons for evolution species to improve their organization.
The natural radiation background on the Earth's surface is not strictly constant value. Its changes are associated with both global and local anomalies. They are caused by cyclic fluctuations in the cosmic background and similar processes that have acquired the character of global catastrophes.
Local anomalies are observed in certain regions of India, Brazil, Iran, Egypt, as well as in the United States, France, CIS countries (including Ukraine). They are the result of geological processes, when, as a result of intense volcanic activity and mountain building, heavy natural radionuclides, primarily uranium and thorium, as well as their decay products, moved from the bowels to the surface of the Earth. Therefore, some of the inhabitants of the Earth receive more significant doses than others, depending on where they live. Where radioactive rocks occur, the level of radiation (radiation background) is significantly higher than the average values, in other places it can be correspondingly lower than the average values. In Belarus, the average exposure from natural sources is 2.4 mSv/year. In some regions of Brazil, this dose reaches 10 mSv per year, and in the state of Keralla (India) even up to
28 mSv/year.
The radiation dose also depends on the lifestyle of people. The use of certain building materials (asbestos), the use of natural gas for cooking, and the sealing of rooms all increase exposure from natural sources.