The use of radioactive isotopes in industry. Radioactive isotopes and their applications
Municipal educational institution"Pobedinsky average comprehensive school» Shegarsky District Tomsk Region
STATE (FINAL) CERTIFICATION OF GRADUATES IX CLASSES
ABSTRACT ON PHYSICS
PHENOMENON OF RADIOACTIVITY. ITS SIGNIFICANCE IN SCIENCE, TECHNOLOGY, MEDICINE
Completed: Dadaev Aslan, 9th grade student
Supervisor: Gagarina Lyubov Alekseevna, teacher of physics
Pobeda 2010
1. Introduction……………………………………………………………...page 1
2. The phenomenon of radioactivity………..…………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
2.1. Discovery of radioactivity……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
2.2. Sources of radiation…………………………………………….. page 6
3. Production and use of radioactive isotopes……………..page 8
3.1. The use of isotopes in medicine……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………….
3.2. radioactive isotopes in agriculture………………page 10
3.3.Radiation chronometry……………………………………p.11
3.4. The use of radioactive isotopes in industry ... p. 12
3.5. The use of isotopes in science……………………………...page 12
4. Conclusion…………………………………………………………...page 13
5. Literature …………………………………………………………..page 14
INTRODUCTION
The idea of atoms as immutable smallest particles of matter was destroyed by the discovery of the electron, as well as the phenomenon of natural radioactive decay, discovered by the French physicist A. Becquerel. A significant contribution to the study of this phenomenon was made by the outstanding French physicists Maria Sklodowska-Curie and Pierre Curie.
Natural radioactivity has existed for billions of years, it is present literally everywhere. Ionizing radiation existed on Earth long before the origin of life on it and were present in space before the appearance of the Earth itself. Radioactive materials have been part of the Earth since its birth. Any person is slightly radioactive: in tissues human body one of the main sources of natural radiation are potassium - 40 and rubidium - 87, and there is no way to get rid of them.
Through implementation nuclear reactions when bombarding the nuclei of aluminum atoms with a - particles, the famous French physicists Frederic and Irene Curie - Joliot in 1934 managed to artificially create radioactive nuclei. Artificial radioactivity is fundamentally no different from natural and obeys the same laws.
Currently, artificial radioactive isotopes are produced in various ways. The most common is the irradiation of a target (future radioactive drug) in a nuclear reactor. It is possible to irradiate the target with charged particles in special installations, where the particles are accelerated to high energies.
Target: find out in which areas of life the phenomenon of radioactivity is used.
Tasks:
Study the history of the discovery of radioactivity.
Find out what happens to a substance when it is exposed to radiation.
· Find out how to obtain radioactive isotopes and where they will be used.
Develop the skill of working with additional literature.
· Perform a computer presentation of the material.
MAIN PART
2. The phenomenon of radioactivity
2.1 Discovery of radioactivity
Story radioactivity began with the fact that in 1896 the French physicist Henri Becquerel was engaged in luminescence and the study of X-rays.
The discovery of radioactivity, the clearest evidence of the complex structure of the atom .
Commenting on the discovery of Roentgen, scientists put forward the hypothesis that X-rays emitted during phosphorescence, regardless of the presence of cathode rays. A. Becquerel decided to test this hypothesis. Wrapping the photographic plate in black paper, he placed on it metal plate bizarre shape, covered with a layer of uranium salt. After a four-hour exposure to sunlight, Becquerel developed a photographic plate and saw on it the exact silhouette of a metal figure. He repeated the experiments with great variations, getting prints of the coin, the key. All experiments confirmed the tested hypothesis, which Becquerel reported on February 24 at a meeting of the Academy of Sciences. However, Becquerel does not stop experiments, preparing more and more new options.

Henri Becquerel Welhelm Conrad Roentgen
On February 26, 1896, the weather over Paris deteriorated and the prepared photographic plates with pieces of uranium salt had to be placed in a dark desk drawer until the sun came up. It appeared over Paris on March 1, and the experiments could be continued. Taking the plates, Becquerel decided to develop them. Having developed the plates, the scientist saw silhouettes of uranium samples on them. Understanding nothing, Becquerel decided to repeat the random experiment.
He put two plates in an opaque box, poured uranium salt on them, first placing glass on one of them, and an aluminum plate on the other. Five hours all this was in a dark room, after which Becquerel developed photographic plates. And what - the silhouettes of the samples are again clearly visible. This means that some rays are formed in uranium salts. They look like X-rays, but where do they come from? One thing is clear that there is no connection between X-rays and phosphorescence.
He reported this at a meeting of the Academy of Sciences on March 2, 1896, completely confusing all its members.
Becquerel also established that the intensity of the radiation of the same sample does not change with time, and that new radiation is capable of discharging electrified bodies.
Most of the members of the Paris Academy, after the next report of Becquerel at a meeting on March 26, believed that he was right.
The phenomenon discovered by Becquerel is called radioactivity, at the suggestion of Maria Sklodowska-Curie.


Maria Sklodowska - Curie
Radioactivity - the ability of atoms of some chemical elements to spontaneous radiation.
In 1897, while doing her doctoral dissertation, Maria, having chosen a topic for research - the discovery of Becquerel (Pierre Curie advised her wife to choose this topic), decided to find the answer to the question: what is the true source of uranium radiation? To this end, she decides to examine a large number of samples of minerals and salts and find out if only uranium has the property to radiate. Working with samples of thorium, she discovers that, like uranium, it gives the same rays and about the same intensity. This means that this phenomenon turns out to be a property not only of uranium, and it should be given a special name. Uranium and thorium were called radioactive elements. Work continued with new minerals.
Pierre, as a physicist, feels the importance of work and, leaving the study of crystals for a while, begins to work with his wife. As a result of this joint work, new radioactive elements were discovered: polonium, radium, etc.
In November 1903, the Royal Society awarded Pierre and Marie Curie one of England's highest scientific awards, the Davy Medal.
On November 13, the Curies, together with Becquerel, receive a telegram from Stockholm about the award of Nobel Prize in physics for outstanding discoveries in the field of radioactivity.
The case started by the Curies was picked up by their students, among whom was daughter Irene and son-in-law Frederic Joliot, who in 1935 won the Nobel Prize for the discovery artificial radioactivity .

Irene and Frederic Curie - Joliot
English physicists E. Rutherford and F. Soddy it was proved that in all radioactive processes mutual transformations of atomic nuclei of chemical elements take place. The study of the properties of the radiation that accompanies these processes in the magnetic and electric fields, showed that it is divided into a-particles, b-particles and g-rays ( electromagnetic radiation very short wavelength).


E. Rutherford F. Soddy
Some time later, as a result of the study of various physical characteristics and properties of these particles ( electric charge, masses, etc.) it was possible to establish that b-particle is an electron, and a-particle is a fully ionized atom chemical element helium (i.e. a helium atom that has lost both electrons).
In addition, it turned out that radioactivity- this is the ability of some atomic nuclei to spontaneously transform into other nuclei with the emission of particles.
So, for example, several varieties of uranium atoms were found: with masses of nuclei approximately equal to 234 a.m.u., 235 a.m.m., 238 a.m.u. and 239 amu Moreover, all these atoms had the same chemical properties. They entered in the same way chemical reactions, forming the same compounds.
In some nuclear reactions, strongly penetrating radiation is produced. These rays penetrate through a layer of lead several meters thick. This radiation is a stream of particles charged neutrally. These particles are named neutrons.
In some nuclear reactions, strongly penetrating radiation is produced. These rays are different types and have different penetrating power. For example, neutron flux penetrates through a layer of lead several meters thick.
2.2. Sources of radiation
Radiation is very numerous and diverse, but about seven its main sources.
The first source is our Earth. This radiation is explained by the presence of radioactive elements in the Earth, the concentration of which varies widely in different places.
second origin radiation - space, from where a stream of high-energy particles constantly falls to the Earth. The sources of cosmic radiation are stellar explosions in the Galaxy and solar flares.
Third source radiation is radioactive natural materials used by man for the construction of residential and industrial premises. On average, the dose rate inside buildings is 18% - 50% higher than outside. A person spends three quarters of his life indoors. A person who is constantly in a room built of granite can receive - 400 mrem / year, from red brick - 189 mrem / year, from concrete - 100 mrem / year, from wood - 30 mrem / year.
Fourth the source of radioactivity is little known to the population, but no less dangerous. These are radioactive materials that a person uses in daily activities.
The composition of inks for printing bank checks includes radioactive carbon, which provides easy identification of forged documents.
Uranium is used to produce paint or enamel on ceramics or jewelry.
Uranium and thorium are used in the manufacture of glass.
Porcelain artificial teeth are reinforced with uranium and cerium. At the same time, radiation to the mucous membranes adjacent to the teeth can reach 66 rem / year, while the annual norm for the whole organism should not exceed 0.5 rem (i.e., 33 times more)
The TV screen emits 2-3 mrem/year per person.
Fifth source - enterprises for the transportation and processing of radioactive materials.
sixth nuclear power plants are the source of radiation. At the nuclear power plant
In addition to solid waste, there are also liquid (contaminated water from reactor cooling circuits) and gaseous waste contained in the carbon dioxide used for cooling.
Seventh the source of radioactive radiation is medical installations. Despite the common use of them in everyday practice, the risk of exposure from them is much greater than from all the sources discussed above and sometimes reaches tens of rem. One of the most common diagnostic methods is an x-ray machine. So, with radiography of teeth - 3 rem, with fluoroscopy of the stomach - the same amount, with fluorography - 370 mrem.
What happens to matter when exposed to radiation?
Firstly, the amazing constancy with which radioactive elements emit radiation. During the day, months, years, the radiation intensity does not noticeably change. It is not affected by heating or an increase in pressure, the chemical reactions in which the radioactive element entered, also did not affect the intensity of the radiation.
Secondly, radioactivity is accompanied by the release of energy, and it is released continuously over a number of years. Where does this energy come from? With radioactivity, a substance undergoes some profound changes. It was suggested that the atoms themselves undergo transformations.
Having the same chemical properties means that all these atoms have the same number of electrons in electron shell, which means that same charges nuclei.
If the charges of the nuclei of atoms are the same, then these atoms belong to the same chemical element (despite the differences in their masses) and have the same serial number in the table of D.I. Mendeleev. Varieties of the same chemical element that differ in the mass of atomic nuclei are called isotopes .
3. Obtaining and using radioactive isotopes
Radioactive isotopes found in nature are called natural. But many chemical elements occur in nature only in a stable (i.e. radioactive) state.
In 1934, French scientists Irene and Frédéric Joliot-Curie discovered that radioactive isotopes could be created artificially as a result of nuclear reactions. These isotopes are called artificial .
To obtain artificial radioactive isotopes, one usually uses nuclear reactors and accelerators elementary particles. There is a branch of industry specialized in the production of such elements.
Subsequently, artificial isotopes of all chemical elements were obtained. In total, approximately 2000 radioactive isotopes are currently known, and 300 of them are natural.
At present, radioactive isotopes are widely used in various fields of scientific and practical activity: technology, medicine, agriculture, communications, the military field, and some others. In this case, the so-called labeled atom method.
3.1 Use of isotopes in medicine
Application of isotopes, one of the most outstanding studies carried out with the help of "tagged atoms" was the study of metabolism in organisms.
With the help of isotopes, the mechanisms of development (pathogenesis) of a number of diseases were revealed; they are also used to study metabolism and diagnose many diseases.
Isotopes are introduced into the human body in extremely small quantities (safe for health), not capable of causing any pathological changes. They are distributed unevenly throughout the body by blood. The radiation arising from the decay of an isotope is recorded by devices (special particle counters, photography) located near the human body. As a result, you can get an image of any internal organ. From this image, one can judge the size and shape of this organ, an increased or decreased concentration of the isotope in
its various parts. It is also possible to evaluate the functional state (i.e. work) of the internal organs by the rate of accumulation and excretion of the radioisotope by them.
So, the state of cardiac circulation, blood flow velocity, the image of the cavities of the heart is determined using compounds, including isotopes of sodium, iodine, technetium; to study pulmonary ventilation and diseases of the spinal cord, isotopes of technetium and xenon are used; macroaggregates of human serum albumin with an iodine isotope are used to diagnose various inflammatory processes in the lungs, their tumors, and in various diseases of the thyroid gland.
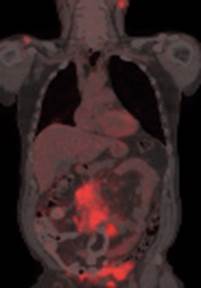
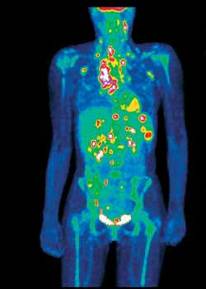
Use of isotopes in medicine
The concentration and excretory functions of the liver are studied using bengal-rose paint with an isotope of iodine, gold. An image of the intestine, stomach is obtained using the isotope of technetium, of the spleen using erythrocytes with the isotope of technetium or chromium; with the help of an isotope of selenium, diseases of the pancreas are diagnosed. All these data allow us to make the correct diagnosis of the disease.
With the help of the “tagged atoms” method, various deviations in the work of the circulatory system are also examined, tumors are detected (since it is in them that some radioisotopes accumulate). Thanks to this method, it was found that in a relatively short time the human body is almost completely renewed. The only exception is iron, which is part of the blood: it begins to be absorbed by the body from food only when its reserves run out.
When choosing an isotope, the question of the sensitivity of the method of isotopic analysis, as well as the type of radioactive decay and radiation energy, is of great importance.
In medicine, radioactive isotopes are used not only for diagnosis, but also for the treatment of certain diseases, such as cancer, Graves' disease, etc.
In connection with the use of very small doses of radioisotopes, radiation exposure to the body during radiation diagnostics and treatment does not pose a danger to patients.
3.2. Radioactive isotopes in agriculture
Increasingly, radioactive isotopes are being used in agriculture. Irradiation of plant seeds (cotton, cabbage, radish, etc.) with small doses of gamma rays from radioactive preparations leads to a noticeable increase in yield. Large doses of radiation cause mutations in plants and microorganisms, which in some cases leads to the appearance of mutants with new valuable properties ( radioselection). Thus, valuable varieties of wheat, beans and other crops have been bred, and highly productive microorganisms used in the production of antibiotics have been obtained.
Gamma radiation from radioactive isotopes is also used to control harmful insects and for conservation food products. "Tagged atoms" are widely used in agricultural technology. For example, to find out which of the phosphorus fertilizers is better absorbed by the plant, various fertilizers are labeled with radioactive phosphorus. By examining the plants for radioactivity, one can determine the amount of phosphorus absorbed by them from different varieties of fertilizer.
An interesting application for determining the age of ancient objects of organic origin (wood, charcoal, fabrics, etc.) was received by the method of radioactive carbon. In plants, there is always a beta - radioactive isotope of carbon with a half-life of T = 5700 years. It is formed in the Earth's atmosphere in a small amount from nitrogen under the action of neutrons. The latter arise due to nuclear reactions caused by fast particles that enter the atmosphere from space (cosmic rays). Combining with oxygen, this carbon forms carbon dioxide, which is absorbed by plants, and through them, by animals.
Isotopes are widely used to determine physical properties soil
and reserves of plant food elements in it, to study the interaction of soil and fertilizers, the processes of assimilation of nutrients by plants, the entry of mineral food into plants through leaves. Isotopes are used to identify the effect of pesticides on the plant organism, which makes it possible to establish the concentration and timing of their treatment of crops. Using the isotope method, the most important biological properties of agricultural crops (when evaluating and selecting breeding material) are studied: productivity, early maturity, and cold resistance.
AT animal husbandry study the physiological processes occurring in the body of animals, analyze feed for the content of toxic substances (small doses of which are difficult to determine by chemical methods) and trace elements. With the help of isotopes, techniques are being developed to automate production processes, for example, the separation of root crops from stones and clods of soil when harvesting with a combine on stony and heavy soils.
3.3 Radiation chronometry
Some radioactive isotopes can be successfully used to determine the age of various fossils ( radiation chronometry). The most common and effective method of radiation chronometry is based on the measurement of the radioactivity of organic substances, which is due to radioactive carbon (14C).
Studies have shown that in every gram of carbon in any organism, 16 radioactive beta decays occur per minute (more precisely, 15.3 ± 0.1). After 5730 years, in each gram of carbon, only 8 atoms per minute will decay, after 11,460 years - 4 atoms.
One gram of carbon from young forest samples emits about fifteen beta particles per second. After the death of the organism, its replenishment with radioactive carbon stops. The available amount of this isotope decreases due to radioactivity. By determining the percentage of radioactive carbon in organic remains, one can determine their age, if it lies in the range from 1000 to 50,000 and even up to 100,000 years.
The number of radioactive decays, i.e., the radioactivity of the samples under study, is measured by radioactive radiation detectors.
Thus, by measuring the number of radioactive decays per minute in a certain weight of the material of the sample under study and recalculating this number per gram of carbon, we can determine the age of the object from which the sample was taken. This method is used to find out the age of Egyptian mummies, the remains of prehistoric fires, etc.
3.4. The use of radioactive isotopes in industry
One example is the following method for monitoring piston ring wear in engines internal combustion. By irradiating the piston ring with neutrons, they cause nuclear reactions in it and make it radioactive. When the engine is running, particles of the ring material enter the lubricating oil. By examining the level of radioactivity of the oil after a certain time of engine operation, the wear of the ring is determined. Radioactive isotopes make it possible to judge the diffusion of metals, processes in blast furnaces, etc. Powerful gamma radiation from radioactive preparations is used to study the internal structure of metal castings in order to detect defects in them.
Isotopes are also used in nuclear physics equipment for the manufacture of neutron counters, which makes it possible to increase the counting efficiency by more than 5 times, in nuclear power as neutron moderators and absorbers.
3.5. Use of isotopes in science
The use of isotopes in biology led to a revision of previous ideas about the nature of photosynthesis, as well as about the mechanisms that ensure the assimilation of inorganic substances by plants of carbonates, nitrates, phosphates, etc. organism. By introducing a label into organisms with food or by injection, it was possible to study the speed and migration routes of many insects (mosquitoes, flies, locusts), birds, rodents, and other small animals and obtain data on the size of their populations.
In the area of plant physiology and biochemistry With the help of isotopes, a number of theoretical and applied problems were solved: the routes of entry of mineral substances, liquids and gases into plants, as well as the role of various chemical elements, including microelements, in plant life were clarified. It has been shown, in particular, that carbon enters plants not only through the leaves, but also through the root system, and the ways and speeds of movement of a number of substances from the root system to the stem and leaves and from these organs to the roots have been established.
In the area of physiology and biochemistry of animals and humans arrival rates studied various substances in their tissues (including the rate of incorporation of iron into hemoglobin, phosphorus into nervous and muscle tissues, calcium into bones). The use of "labeled" food led to a new understanding of the rates of absorption and distribution of nutrients, their "fate" in the body and helped to trace the influence of internal and external factors (starvation, asphyxia, overwork, etc.) on metabolism.
CONCLUSION
Outstanding French physicists Maria Sklodowska - Curie and Pierre Curie, their daughter Irene and son-in-law Frederic Joliot and many other scientists not only made a great contribution to the development nuclear physics but were passionate fighters for peace. They did significant work on the peaceful use of atomic energy.
In the Soviet Union, work on atomic energy began in 1943 under the guidance of the outstanding Soviet scientist I. V. Kurchatov. In the difficult conditions of an unprecedented war, Soviet scientists solved the most complex scientific and technical problems associated with the mastery of atomic energy. On December 25, 1946, under the leadership of I.V. Kurchatov, for the first time on the continent of Europe and Asia, chain reaction. In the Soviet Union began era of the peaceful atom.
In the course of my work, I found out that radioactive isotopes obtained artificially have found wide application in science, technology, agriculture, industry, medicine, archeology and other fields. This is due to the following properties of radioactive isotopes:
a radioactive substance continuously emits a certain type of particles and the intensity does not change over time;
radiation has a certain penetrating power;
Radioactivity is accompanied by the release of energy;
under the influence of radiation, changes in the irradiated substance can occur;
· Radiation can be detected in different ways: with special particle counters, photography, etc.
LITERATURE
1. F.M. Diaghilev "From the history of physics and the life of its creators" - M.: Enlightenment, 1986.
2. A.S. Enokhin, O.F. Kabardin and others. "Reader in Physics" - M.: Enlightenment, 1982.
3. P.S. Kudryavtsev. "History of Physics" - M .: Education, 1971.
4. G.Ya. Myakishev, B.B. Bukhovtsev "Physics grade 11" - M.: Enlightenment, 2004.
5. A.V. Peryshkin, E.V. Gutnik "Physics grade 9" - M.: Bustard, 2005.
6. Internet - resources.
Review
for the examination abstract in physics “The phenomenon of radioactivity. Its significance in science, technology, medicine.
The author sees the relevance of the chosen topic in the possibility of using nuclear energy for peaceful purposes. Radioactive isotopes obtained artificially have found wide application in various fields of scientific and practical activity: science, technology, agriculture, industry, medicine, archeology, etc.
However, the "Introduction" section does not indicate the relevance and interest of the author in the chosen topic of the abstract.
Accessible, logically spelled out the discovery of radioactivity; studies carried out with the help of "tagged atoms".
The design of the abstract does not in all cases meet the requirements:
· Not numbered pages;
· Each section is not printed from a new page;
There are no references to illustrations in the text;
· In the section "Literature" sites of Internet resources are not indicated.
In general, despite minor shortcomings in the compilation and design, we can say that the abstract “The phenomenon of radioactivity. Its importance in science, technology, medicine” deserves a “good” rating.
Physics teacher, Pobedinskaya secondary school: ___________ / L.A. Gagarina/
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Hosted at http://www.allbest.ru/
nucleus radioactive isotope analysis
Radioactive isotopes and their applications
Isotopes are varieties of the same chemical element that are similar in their physical and chemical properties, but have different atomic mass.
Radioactivity - the transformation of atomic nuclei into other nuclei, accompanied by the emission of various particles and electromagnetic radiation.
In nature, there are both stable isotopes and unstable - radioactive, the nuclei of atoms of which are subject to spontaneous transformation into other nuclei with the emission of various particles (or processes of radioactive decay). About 270 stable isotopes are now known. The number of unstable isotopes exceeds 2000, the vast majority of them obtained artificially as a result of various nuclear reactions. The number of radioactive isotopes in many elements is very large and can exceed two dozen. The number of stable isotopes is much less. Some chemical elements consist of only one stable isotope (beryllium, fluorine, sodium, aluminum, phosphorus, manganese, gold and a number of other elements). Largest number stable isotopes - 10 found in tin, in iron, for example, they are 4, in mercury - 7.
With the help of nuclear reactions, it is possible to obtain radioactive isotopes of all chemical elements. Get them on electron particle accelerators and nuclear reactors. They are also called "tagged atoms".
Radioisotope diagnostics - the use of radioactive isotopes and labeled compounds for the study of human organs and systems in order to recognize diseases. The main method of radioisotope diagnostics is the method of radioactive indication, i.e., the method of monitoring the radioactive substances introduced into the body.
Radioactive isotopes of a number of chemical elements are sources of ionizing radiation, which can be registered with the help of special radiometric and recording devices after the introduction of the isotope into the human body with to a large extent accuracy. Modern radiological equipment makes it possible to capture and study extremely small amounts of radioactive compounds (the so-called indicator amounts), which are practically harmless to the body of the subject. By registering the distribution, movement, transformation and excretion of radioactive tracers from the body, the doctor is able to judge the participation of the relevant elements in the biochemical and physiological processes in the body. Among the numerous methods of radioisotope diagnostics, laboratory radiometry, clinical radiometry, clinical radiography and scanning are the most widely used. Radioisotope scanning of internal organs makes it possible to determine the location in the body of the organ under study, to establish its shape and size, and to identify the presence of a number of pathological changes in it. The main advantage of radioisotope research methods is their complete painlessness and practical safety for the patient with high accuracy of diagnostic results.
One of the most outstanding studies was the study of metabolism in organisms. It has been proven that in a relatively short time the body undergoes an almost complete renewal. Its constituent atoms are replaced by new ones. Only iron, as experiments on the isotopic study of blood have shown, is an exception to this rule. Radioactive isotopes are used in medicine for both diagnosis and therapeutic purposes. Radioactive sodium, introduced in small amounts into the blood, is used to study blood circulation, iodine is intensively deposited in the thyroid gland, especially in Graves' disease. By monitoring the deposition of radioactive iodine with a counter, a diagnosis can be made quickly. Large doses of radioactive iodine cause partial destruction of abnormally developing tissues, and therefore radioactive iodine is used to treat Graves' disease. Intense cobalt gamma radiation is used in the treatment of cancer (cobalt gun).
No less extensive are the applications of radioactive isotopes in industry. One example of this is the following method for monitoring piston ring wear in internal combustion engines. By irradiating the piston ring with neutrons, they cause nuclear reactions in it and make it radioactive. When the engine is running, particles of the ring material enter the lubricating oil. By examining the level of radioactivity of the oil after a certain time of engine operation, the wear of the ring is determined. Radioactive isotopes make it possible to judge the diffusion of metals, processes in blast furnaces, etc.
Powerful gamma radiation of radioactive preparations is used to study the internal structure of metal castings in order to detect defects in them.
Radioactive isotopes are being used more and more widely in agriculture. Irradiation of plant seeds (cotton, cabbage, radish, etc.) with small doses of gamma rays from radioactive preparations leads to a noticeable increase in yield. Large doses of radiation cause mutations in plants and microorganisms, which in some cases leads to the appearance of mutants with new valuable properties (radioselection). Thus, valuable varieties of wheat, beans and other crops have been bred, and highly productive microorganisms used in the production of antibiotics have been obtained. Gamma radiation from radioactive isotopes is also used to control harmful insects and to preserve food. Radioactive isotopes have been widely used in agricultural technology. For example, to find out which of the phosphate fertilizers is better absorbed by the plant, various fertilizers are labeled with radioactive phosphorus 15 32P. By examining the plants for radioactivity, one can determine the amount of phosphorus absorbed by them from different varieties of fertilizer.
Radiocarbon analysis is a physical method of dating biological remains, objects and materials of biological origin by measuring the content of the radioactive isotope 14C in relation to stable carbon isotopes. An interesting application of radioactivity is the method of dating archaeological and geological finds by the concentration of radioactive isotopes. An unstable carbon isotope occurs in the atmosphere due to nuclear reactions caused by cosmic rays. A small percentage of this isotope is found in air along with the usual stable isotope. Plants and other organisms consume carbon from the air and accumulate both isotopes in the same proportion as they do in air. After the death of plants, they cease to consume carbon and the unstable isotope, as a result of α-decay, gradually turns into nitrogen with a half-life of 5730 years. By accurately measuring the relative concentration of radioactive carbon in the remains of ancient organisms, it is possible to determine the time of their death. This method is used to find out the age of Egyptian mummies, the remains of prehistoric fires, etc.
The radioactive method of analyzing a substance makes it possible to determine the content of various metals in it, from calcium to zinc, in extremely low concentrations - up to 1-10 g. (this requires only 10-12g of the substance). Radioactive drugs are widely used in medical practice for the treatment of many diseases, including malignant tumors. Isotopes of plutonium-238, curium-224 are used for the production of small-capacity batteries for heart rate stabilizers. For their continuous operation for 10 years, only 150-200 mg of plutonium is sufficient (conventional batteries last up to four years).
Radioisotope energy sources are devices of various designs that use the energy released during radioactive decay to heat the coolant or convert it into electricity. A radioisotope energy source is fundamentally different from a nuclear reactor in that it uses not a controlled chain reaction, but the energy of the natural decay of radioactive isotopes. Radioisotope energy sources are used where it is necessary to ensure the autonomy of equipment operation, significant reliability, low weight and dimensions. At present, the main areas of application are space (satellites, interplanetary stations, etc.), deep-sea vehicles, remote territories (the far north, the open sea, Antarctica). In general, simply speaking, the study of "deep space" without radioisotope generators is impossible, since at a considerable distance from the Sun, the level of solar energy that can be used by means of photocells is small. For example, in the orbit of Saturn, the illumination of the Sun at the zenith corresponds to the earth's twilight. In addition, at a significant distance from the Earth, very high power is required to transmit radio signals from a space probe. Thus, the only possible source of energy for spacecraft under such conditions, in addition to the nuclear reactor, it is the radioisotope generator that acts. Existing applications:
· Interstellar probes: Electrical heat supply of space vehicles.
Medicine: power supply for pacemakers, etc.
· Power supply of lighthouses and buoys.
Promising areas of application:
· Android robots: Electric heating supply. as the main source of energy.
· Space-based combat lasers: Laser pumping and electrical heat supply.
Fighting vehicles: Powerful engines with a long resource (unmanned reconnaissance vehicles - aircraft and mini-boats, power supply for combat helicopters and aircraft, as well as tanks and autonomous launchers).
· Deep-sea hydroacoustic stations: long-term power supply of non-returnable vehicles.
Radioactive isotopes and compounds labeled with radioactive isotopes are widely used in various fields of human activity. Industry and technological control, agriculture and medicine, communications and Scientific research-- it is practically impossible to cover the entire spectrum of applications of radioactive isotopes, although they all arose in a little more than 100 years.
Hosted on Allbest.ru
Similar Documents
Basic concepts and terminology. Detection and quantitative measurements of radionuclides. Autoradiography. scintillation counters. Immigers. Basic radionuclides in life science. Technical characteristics of labeled compounds. Radionuclide 3H (tritium).
abstract, added 09/18/2007
Isotopes in medicine. Basic characteristics of radionuclides for use in diagnostic purposes. State-of-the-art mammography system with low radiation dose and high resolution. Isotopes in industry and agriculture.
presentation, added 06/08/2012
Physical foundations nuclear reaction: nucleon binding energy and nuclear fission. Release of nuclear energy. Features of the use of energy released during the fission of heavy nuclei at nuclear power plants, nuclear icebreakers, aircraft carriers and submarines.
presentation, added 04/05/2015
Isotopes are varieties of the same chemical element that are similar in their physical and chemical properties, but have different atomic masses. The structure of the atom, description of the proton-neutron model of the nucleus. Discovery and application of isotopes, their radioactivity.
presentation, added 12/27/2010
Interaction between nucleons. Features of nuclear forces. Methods for the release of nuclear energy: the fission of heavy nuclei and the synthesis of light nuclei. A device in which the reaction of their fission is supported. Accumulation of radioactive elements in the human body.
presentation, added 12/16/2014
History of the development of the method of labeled atoms. Isotope tracers, stable and radioactive isotopes. Isotopic tracers in medicine, biology and agriculture. Centillary radiation counters. Introduction of a radioactive label into biological preparations.
abstract, added on 12/14/2013
The main sources of radioactive contamination: industrial decontamination caused by an explosion nuclear weapons, emergency objects. Types of decontamination work at nuclear power plants, the procedure for their implementation and evaluation of practical effectiveness.
test, added 05/26/2015
Analysis of natural and artificial radioactive substances. Methods of analysis based on the interaction of radiation with substances. Radiotracer methods of analysis. An analysis method based on the elastic scattering of charged particles on the absorption of P-particles.
abstract, added 03/10/2011
Application of thermonuclear fusion energy. radioactive decay. Obtaining nuclear energy. The splitting of the atom. Nuclear division of heavy elements, obtaining new neurons. transformation kinetic energy into warmth. Discovery of new elementary particles.
presentation, added 04/08/2015
Charge, mass, size and composition atomic nucleus. Binding energy of nuclei, mass defect. Nuclear forces and radioactivity. Density of nuclear matter. The concept of nuclear reactions and their main types. Fission and fusion of nuclei. quadrupole electric moment kernels.
"The use of radioactive isotopes"- By examining the level of radioactivity of the oil after a certain time of engine operation, the wear of the ring is determined. Brachytherapy is not a radical, but practically an outpatient operation, during which we introduce titanium grains containing an isotope into the affected organ. Gamma radiation from radioactive isotopes is also used to control harmful insects and to preserve food.
"Natural Radioactivity"- Using the periodic table, determine which element arose as a result of the collapse of the parent nucleus. Substances capable of spontaneous spontaneous emission. natural radioactivity. Properties of radioactive radiation. Element. Decay. Missing words. radioactive decay.
"Discovery of radioactivity" This is how the biological action of radioactivity was discovered for the first time. Then Becquerel began to test different salts of uranium (including those lying in the dark for years). The plate is constantly illuminated. The history of the discovery of radioactivity. By placing a metal cross between the salt and the plate, Becquerel obtained the weak contours of the cross on the plate.
"Radioactivity and Radiation"- Penetrating power of radiation. displacement rule. Isotopes. Three types of radiation. half-lives. A completely new kind of substance is formed. Discovery of Becquerel. radioactive transformations. Radioactivity. The nature of radioactive radiation. Law of radioactive decay.
"Discovery of the phenomenon of radioactivity"- "Unknown" rays. Ernest Rutherford. Maria was an excellent mother. The technical equipment is primitive. Knowledge. The structure of the atom. No accidental discovery. Humanity will derive more benefit from new discoveries. Action of radium on animals. Science is organized knowledge. The history of the discovery of radioactivity.
"Radioactivity of an element"- Welcome to the lesson. Ionized atom of a chemical element. Properties of radioactive radiation. electromagnetic phenomena. Henri Becquerel. Electron. radioactive elements emit radiation. Democritus. Physical quantities. Formulas. Water. Problem solving. Circuit. Periodic law discovered. Explain the experience.
In total there are 14 presentations in the topic






