Is it possible to make salt water fresh? How to make fresh water from sea water? Distillation of water by boiling
Once again to the question of obtaining suitable drinking water in extreme conditions.
What to do on a hike when drinking water is not provided along the way? For example, there will be swamps, lakes, rivers, estuaries, seas. But there will be no clean and fresh water.
Usually, when going on a hike, they take special water disinfection tablets with them; they remember their grandmother’s recipes about disinfecting water with herbs, disinfecting water with peroxide or potassium permanganate.
This is all well suited for fresh and relatively clear water. This, in principle, you can simply boil for about twenty minutes and cook with it calmly.
But what to do if the hike involves meeting only salt water? For example, the southern coast of Crimea and the Kerch Peninsula, where there is a lot to see, have very few sources of fresh water.
You can, of course, buy fresh water. But in such places it is quite expensive. What if the day is arranged far from civilization?
It turns out that there is only one way out - to carry water with you. And it’s somewhat inconvenient to add a five-liter bottle of water to a 15-kilogram backpack.
In order to avoid such inconveniences, an experiment was carried out to desalinate seawater using the distillation process.
What is distillation
Distillation- this is when the water boils, the steam above it collects, cools, and turns back into water in the receiving container.
Why does distillation remove salts from water? Because salts boil at more high temperature than water. Therefore, water evaporates first. No salts. This is what you need to do with sea water.
What happens if the water that is distilled contains substances that boil at a lower temperature? For example, volatile organic compounds? They will evaporate first. Therefore, one of the principles of distillation is not to direct the steam collector directly to the receiving device, but to allow the water to seriously boil.
Another important principle of distillation is good vapor cooling. The performance of the device directly depends on the cooling efficiency. If some of the steam does not cool, but evaporates, then, accordingly, less pure water will be obtained.
So, how we carried out the distillation of sea water in practice.
Distillation of sea water in practice
Location : beach north of the village of Podmayachnoe near the city of Kerch. It takes at least half an hour to get to the nearest store with water in the heat. Of course, it’s beautiful there - cliffs, layers of fossils, etc. But it's hot.
Water source: Sea of Azov. The sea is not very salty, salinity is about 10 grams per liter. In comparison, the salinity of the Black Sea is 16-20 grams per liter.
Materials and equipment :
* two bricks;
* bonfire;
* tourist kettle;
* curved aluminum tube;
* Glass bottle;
* hole in the sand;
* mug for sea water;
* a lot of wood and a lot of patience
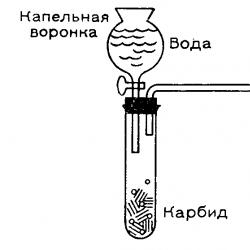
The distillation procedure is very simple. Sea water - into the kettle, kettle - on the fire. Hole in the ground. A glass bottle was buried in it at an angle. Only the neck protruded from the sand, into which the steam transmitting tube was inserted.



That's it - the water boiled, passed through the tube into the bottle, and cooled there. For better cooling, the bottle was watered with cold (relatively, of course) sea water. As the salt water evaporated, it was added to the kettle.
Now the technology know-how:
I. The first thing that was adjusted was the seawater level in the kettle. Sea water should occupy less than half the volume, preferably one third. This is necessary to increase the intensity of vaporization. It is not known why more steam is formed in this case, but it turned out that this is so.
II. Further, the transmission tube must not be watered to cool the steam. The cooling water turned out to be insidious, and flowed down the tube (by the way, about 80 cm long) from below into a bottle with clean water. Accordingly, the taste of the purified water was not very good.
III. After, plastic bottle It is better not to take it for the receiving device. Because the steam makes her cringe. Although, if you don’t have a glass one at hand, a plastic one will do. In the area where the camp was located, both plastic and glass bottles were present in abundance.
results: in 30-40 minutes of distillation, about 350 milliliters of water are purified using the described method.
conclusions
The tested distiller perfectly purifies sea water. The salts in the resulting water cannot be tasted. Apparently, they simply aren’t there :) The cleaning process requires a fairly large amount of fuel. Thorough cooling of the receiving tank almost doubles the productivity of the distiller. A tested distiller, taking into account all sorts of adjustments and problems per day, can provide clean water to 2-3 people for 2-3 days. In order to provide clean water to a larger number of people for a longer period of time, either a more advanced distiller is needed (which will make its design heavier and make transportation difficult), or the tried-and-tested distiller must be made into personal equipment (every participant purifies the required amount of water per day).
Thus, the studies carried out showed excellent results in purifying sea water using the field method. The most important thing is that the weight of the kettle and steam wand did not exceed 500 grams.
12 13 988 0
We live in a unique place - the Earth, which, although it has a lot of land, is still mostly covered with water. We swim in it, move through it and, most importantly, drink from it. Unlike many animals, we are unable to get enough fluids simply from fruits and vegetables - we need to drink fluids regularly to stay hydrated. But the body of water has one more unique property - it is almost all salty. The percentage of fresh water is surprisingly small. Yes, we are accustomed to it, because this kind of water comes into our homes and is sold in stores. But what if we suddenly don’t have access to fresh water, if we only have sea water? Then it needs to be desalinated. Let's figure out how this can be achieved.
You will need:
This method is also called sublimation. It can be easily done even at home, although it will not provide a large amount of liquid.
Take an ordinary saucepan into which salted water is poured. Next, you need to cover this pan with a lid and put it on fire. Gradually, condensation will accumulate on its lid.
However, even when the lid is removed, most of the unleavened drops will flow back into the pan, so it is necessary to slightly improve this improvised device.
- A hole is drilled in the lid of the pan.
- A flexible tube is inserted into it, for example, a coil from a moonshine still.
- Its other end is lowered into an empty vessel.
- Next, you need to cover the tube with a damp cloth so that the steam in it cools.
- It will condense and fall into the empty vessel.
As a result, the heated pan will end up with only salt, and the second vessel will only have distilled water.
Keep in mind, however, that there will be no salt in such a liquid at all, and therefore your thirst will be poorly quenched.
It is better to pour a small amount of salt water into it. 
This method uses special precipitating reagents. They interact with salts contained in sea water and form compounds that are not soluble. Therefore, they settle and can be filtered out calmly and without problems.
This approach has its disadvantages, in particular, the high cost of reagents, the slowness of the reaction, and the large number of necessary reagents.
Therefore, this method is used very rarely, and in everyday life it is almost never used.

This method is predominantly industrial and has been used for a long time. It is based on the use of two semi-permeable membranes made of cellulose acetate or polyamide. Small water molecules can penetrate through them without any restrictions, while larger ions of salt, as well as other impurities, are retained and are not allowed further. 
It is difficult to achieve desalination of a large amount of liquid in this way, and this method is difficult to implement in everyday life - it is suitable for industrial enterprises.
This desalination method seems very simple in its idea, but in its implementation it is quite labor- and resource-intensive. The idea is based on the fact that salt does not get into the ice when frozen, because ice formation occurs only from water molecules.
The largest amount of fresh water in nature is contained in all kinds of glaciers.
Usually the Eskimos resort to this method. They expose a container of salt water to the cold, and then wait until ice crystals form there. This ice is collected and melted - and the water can be drunk. 
Issues discussed in the material:
- Why is there a need for desalination of seawater?
- What methods exist for desalinating seawater?
- How to desalinate sea water at home
- What problems are inherent in the process of seawater desalination?
Purification and desalination of sea water is an industrial process, as a result of which salts are removed from it and a product is obtained that is suitable for domestic use and consumption. Our article will talk about methods and technologies for desalination of sea water.

60% of the earth's surface consists of areas where there are either no sources of fresh water at all, or there are but a very small amount. Since many arid areas have few freshwater bodies, watering problems arise. They could be solved thanks to the possibility of using desalinated sea water for these purposes. There are significant reserves of such water on Earth, but due to its high salt content it cannot be used for economic purposes.
To grow crops, it is necessary to water them with water with a very low salt content. If plants receive more than 0.25% salts with moisture, they simply will not grow. They will also be negatively affected by the presence of alkalis in the water. Many countries, including Russia, are looking for ways to desalinate salty water sources, which would help cope with drought problems in areas located near the sea.
In countries with well-developed industry, there is an increasingly acute shortage of fresh water supplies. In particular, this applies to the USA and Japan, where required for industry, Agriculture and domestic needs, the volumes of water have long exceeded those available.
The amount of fresh water does not meet the needs in developed countries with low rainfall, such as Israel and Kuwait.

Russia ranks first in the world in terms of terrestrial freshwater resources. Baikal alone is enough to satisfy today's needs Russian population and industries in fresh water. This lake is so deep that if you direct the flows of all the rivers into its basin globe, then it will take almost 300 days to fill.
However, most of Russia’s water resources are concentrated in practically uninhabited and undeveloped areas of Siberia, the North and Far East. To the highly developed central and southern regions with high level industry, agriculture and population density account for only 20% of freshwater reserves.
Certain countries of Central Asia (Turkmenistan, Kazakhstan), as well as the Caucasus, Donbass and the south-eastern part of the Russian Federation, have enormous mineral resources, but do not have freshwater sources.
In Russia there are a large number of underground springs, the level of mineralization of which ranges from 1 to 35 g/l. They cannot be used for the needs of the population, since they contain a large amount of salts, but after desalination they can be used.
In the process of desalination of seawater, an important parameter is its salinity, which is understood as the mass of dry salts in grams per 1 kg of substance. The amount of salts per unit volume of liquid can vary significantly depending on the sea. For example, the Black, Caspian and Azov seas are characterized as slightly salty. The average salinity of the World Ocean is 35g/kg.
In addition to table salt (NaCl), sea water also contains a number of other chemical elements, mainly in the form of ions that can be obtained from it in industrial scale: K+, Mg2+, Ca2+, Sr2+, Br-, F-, H3BO3. In total, about 50 chemical elements in varying concentrations were discovered in the marine subsoil, including lithium (Li), rubidium (Rb), phosphorus (P), iodine (J), iron (Fe), zinc (Zn) and molybdenum (Mo).

Marine water reserves contain more than 50 chemical elements. The concentration of each of them is extremely small, but their total mass determines the salinity of the liquid. Only water that contains no more than 0.001 g/ml of salts can be suitable for food. In order to achieve such a concentration, various seawater desalination technologies are used. Experts are trying to develop desalination systems that would consume little energy, but at the same time purify the water as much as possible for use by the population.
Today they apply following methods desalination of sea water: distillation, reverse osmosis, ionization and electrodialysis.

In the southern regions, solar desalination plants are actively used, in which seawater is heated and evaporated. There is also the opposite method, in which salt water is frozen and then fresh water is separated from it, since it freezes faster.

A seawater desalinator is a device that can remove dissolved salts from water. After the purification procedure, water is obtained that can be used not only for household needs, but also for drinking. The design of the device is convenient and practical in operation.
However, desalinated water is not clean at the same time, because it also retains other components, the density of which determines the area of its application. So, on sea vessels it is required different types water reserves:
- drinking water, which is used only for cooking and drinking;
- water for personal hygiene and deck washing;
- water for steam generators, or nutrient water;
- process water, which is used as a coolant for engines;
- distilled water.
To obtain all these types, different ship-based desalination plants are used.
Desalination technologies include the following:
- Distillation, in which a desalination plant heats and evaporates seawater. The resulting steam is “caught” and brought to the required temperature.
- Filtration, in which the device operates on the principle of reverse osmosis. Salt water is purified without changing from one state to another. The operation of such a device is based on bringing the concentration of dissolved impurities to the optimum. Very high pressure allows you to “squeeze out” excess salt particles.
The largest desalination plant on the planet is located in the Israeli city of Hadera. This unit is comparable in size to an entire plant. It desalinates about thirty-three billion gallons of seawater each year. The desalination plant operates on the principle of reverse osmosis, as a result of which Mediterranean waters are not subjected to heat treatment.

The installation is completely sealed, creating a greenhouse effect without allowing vapors to escape outside. As a result, the pure aqueous residue is retained in a larger volume. At the end, the plug is unscrewed and the purified liquid is poured into a container.
Similar devices are used in the navy. They use the heat of the liquid, which serves to cool the main and auxiliary diesel engines. Purified water, heated to 60 °C, enters the inlet through the pipes of the heating battery. Upon exiting, the temperature of the liquid drops to approximately 10 °C.
Vacuum desalination plant produces about 800 liters of distilled water per hour. It can satisfy all fresh water needs without wasting fuel energy, and full automation saves on maintenance costs. Since the evaporation temperature is quite low, the water desalination plant can operate for six to twelve months without requiring cleaning.
It is known that the Israeli population suffers from a serious shortage of drinking supplies. The operation of the apparatus described above makes it possible to cover almost two-thirds of the water needs of the entire country.
Today, a variety of equipment is used to desalinate seawater, including unique solar-powered desalination plants. They are filled with water, which, under the influence of solar heat, turns into steam, condenses on the walls of the housing and then settles at the bottom of the device.

Today, two desalination methods are widely used in industry: membrane (mechanical) and thermal (distillation). In the first case, reverse osmosis technology is used. Sea water is passed through semi-permeable membranes under pressure significantly higher than the difference in pressure between fresh and sea water (for the latter it is 25-50 atm.).
The microscopic pores of the filters freely allow only small water molecules to pass through, trapping larger ions of salt and other impurities. The material for such membranes is polyamide or cellulose acetate; they are produced in the form of hollow fibers or rolls.
The method of deep reverse osmism water desalination has a number of advantages compared to other methods. Firstly, the devices are simple and compact, and secondly, they do not require large amounts of energy. In addition, the reverse osmosis system is controlled in semi-automatic and automatic mode.
But still, this method also has its disadvantages. The quality of cleaning here depends on how effective the pre-treatment was. In addition, the resulting drinking water still contains a fairly large amount of salt (500 mg/m3 total concentration salts). This method also requires increased operating costs, since it requires regular purchase of related chemicals and replacement of membrane filters.
Wonthaggi Desalination Plant is the world's largest membrane water desalination plant, located in Melbourne. It is capable of processing 440 thousand cubic meters of water per day. In the Israeli city of Ashkelon there is a plant where water is purified from salts using reverse osmosis. It processes 330 thousand cubic meters of water per day.

The essence of the thermal method (distillation) is that at a seawater desalination station, the liquid is boiled, and the resulting steam is accumulated and condensed. This creates a distillate – fresh water. You can evaporate water without bringing it to a boil. In this case, it is heated at a higher pressure than in the evaporation chamber. The heat of the water itself is used to create steam. At the same time, it is cooled to the saturation temperature of the remaining brine. The disadvantages of this method are cost, high energy intensity, and the presence of an external source of steam. However, it is this that provides the largest volume of fresh water per unit of time. For example, the Shoaiba 3 plant (Saudi Arabia) produces up to 880 thousand cubic meters of fresh water per day using the distillation method.
The two methods can be compared in several key ways:
|
Options |
Reverse osmosis |
Thermal method |
|
Physico-chemical principle |
Membrane diffusion |
Thermal evaporation and condensation |
|
Energy consumption (including consumption of auxiliary devices) |
Electricity: 3.5-4.5 kW-h/m3 |
Electricity: 2.5-5 kW-h/m3, thermal 40-120 kW-h/m3 |
|
Highest temperature during desalination process |
Sea water temperature |
|
|
Water quality (salt content mg/l) |
||
|
Average productivity of one desalination module |
6000-24000 m3/day |
120000 m3/day |
|
Basic devices |
Pumps, membranes |
Pumps, valves, vacuum units |
|
total cost |
||
|
Production automation level |
||
|
Possibility of changing the composition of sea water |
Medium-high |
|
|
Maintenance Requirements |
||
|
Scaling potential |
Medium-low |
|
|
Space requirements |
||
|
Most needed improvements |
Improving water pre-treatment, improving membrane properties |
Cheaper materials and heat transfer methods |

Difficulties with fresh water supply arose in Crimea after the well-known events in 2014. Then Ukraine blocked the canal through which fresh water flowed to the peninsula, resulting in a shortage of technical and drinking water supplies.
There is information about the planned installation of a desalination system in Kerch, which will produce about 50 tons of water per hour. Water resources purified from salts will be used mainly for technical needs: feeding heating networks and steam boilers. This will help reduce the load on the general water supply.
Water purification at this installation will take place in several stages. It is planned to use a combined membrane technology for clarification, a reverse osmosis method for purification from salts, and an ion exchange method for polishing softening.
The system will operate automatically; only one operator will be needed to control the process.
Today, the profitability of irrigating crops with desalinated seawater is a big question: unfortunately, existing technologies do not make it possible to obtain both high-quality and cheap fresh water from salt water. But different countries the world are constantly working in this direction, because ecological problems desalination of sea water concerns all humanity and requires permission.
Scientists have high hopes for using nuclear energy to purify water resources, which would make desalination technologies much cheaper.
Do-it-yourself desalination of sea water at home and in extreme conditions

If you need to purify seawater from salts while camping, the best option for this is a homemade distiller, similar in design to well-known distillation apparatuses.
The essence of the process in a conventional desalination plant is as follows: a salty liquid is heated to a boil, then the resulting steam is accumulated in a container and cooled. After the procedure, cooled droplets of water purified from salt impurities settle on the walls of the chamber.
Salts are released from the mixture because the boiling point of the brine solution is slightly higher than that of pure water. Therefore, the fresh component evaporates faster and settles in the collection container.
To desalinate sea water in camping conditions you will need:
- first of all, the water itself, which is always in abundance on the shore of the sea or salt lake;
- a pot or kettle as a heating container;
- an aluminum tube, which should be prepared before the start of the hike;
- a deep hole dug in the sand: it will serve as a cooling device;
- another container (glass bottle, stainless steel jar, etc.) where water purified from impurities will be collected.
On the shore of a lake or sea, you should dig a hole up to a meter deep, place a container (bottle) in it at a slight angle, and insert a tube into the neck of it.
Have a rubber gasket in advance: with its help you will reliably seal the junction of the aluminum tube with the neck of the bottle.
Then the structure should be covered with sand so that only the upper part of the neck with the inserted tube remains open. The end of the tube will need to be placed over a pot or open kettle of sea water. In this case, the fire is lit a short distance from the bottle with the pipe.
After the fire flares up, the water in the container will heat up and begin to bubble, and the steam will gradually spread through the tube into a bottle buried in the sand, where it will settle as condensation. Gradually, up to 200-300 grams of clean fresh liquid forms at the bottom of the container.

The most in a simple way To purify water from salt at home is the use of a system consisting of a number of filters connected in a certain sequence. But even a complex multi-stage combination cannot remove absolutely all harmful impurities from water. Therefore, long-known home desalination methods are very popular among people.
For example, water is poured into a bottle and placed in the freezer, where after a while the pure component freezes. The part that does not freeze contains all the harmful impurities, so it is drained. The frozen aqueous residue, when it melts at room temperature, can be used for drinking and other needs.
There are two more ways to purify water from salt, which can be easily implemented at home. The first is long boiling, as a result of which salt settles on the walls in the form of scale. The second is filtration using activated carbon. In this case, the amount of material used will depend on the salt concentration.

Today, of all desalination methods, reverse osmosis technology is the most in demand. But its use requires large costs for the production and operation of membranes, as well as significant energy capacity. In addition, after desalination using this method, a highly concentrated salt solution remains, which is returned to the sea or ocean, which increases the salinity of water resources. Because of this, the purification process becomes even more complex, and the cost of desalination of seawater only increases every year.
In addition, only 1/3 of the world's freshwater reserves are found in soil (2/3 are frozen in snow covers and glaciers). And they are used by humans so quickly that nature does not have time to replenish what is lost.
In this regard, the shortage of fresh water is increasing on a global scale.
Experts predict that more than two billion people will experience water shortages by 2030. This problem is further aggravated by the fact that each country uses different amounts of fresh water.
For example, an American spends on average about 400 liters per day, while a resident of an underdeveloped country uses only 19 liters. Half of the world's population has no running water at all. All this will one day lead to people paying special attention to the oceans as sources of water.
The main task in seawater desalination is to minimize energy and equipment costs. This is especially important because a country that has a greater need for purified water must also withstand economic competition with countries that have cheaper and more abundant freshwater sources.
Based on the results of design developments, it turns out that only for a small number of consumers it will be cheaper to transport water from a natural reservoir over a distance of 400-500 km than to desalinate it. Assessing underground reserves of varying degrees of salinity in arid areas, we can conclude that desalination is the only economically viable method of water supply for them, given their remoteness from natural freshwater sources.
Desalination methods used today can be productively used to return used water resources to nature without degrading the condition of fresh water bodies.
If the water quality leaves much to be desired...
The problem of dirty water in the house can be partially solved by installing a high-quality filter, but in such systems it is periodically necessary to replace components, because this directly determines how well the drinking liquid will be purified.
At the same time, the question remains unresolved: how to ensure that our workplace or child has water at school best quality? The best solution is to buy it with delivery.
The Iceberg company offers favorable conditions for servicing its clients:
- free delivery of water to your home or office: buyers pay only the cost of the product;
- the wells from which our water is drawn have registration documents in the State Water Cadastre of the Russian Federation;
- Advanced technologies are used to extract and bottle water, which helps preserve and enhance its quality and natural purity;
- We also sell modern water coolers and other equipment manufactured by well-known European brands, taking into account existing quality standards. The sizes of pumps and racks for bottles vary, allowing the devices to be installed even in small spaces;
- delivery of drinking water to your home or office is carried out at a minimal price, thanks to constant promotions from our company;
- Along with water, you can purchase disposable tableware, tea, coffee and other auxiliary products.

Clean water is valuable, but it should not be worth its weight in gold. Our mission is to provide every home and workplace with high-quality drinking water, so we have prepared the most favorable conditions for our clients.
Today, the problem with drinking water is becoming more and more urgent in the world - there is quite a bit of it. Africa, for example, is provided with this resource by only 30 percent of the required amount.
Other countries on this continent carry out
delivery of drinking waterif possible, but this is still not enough. It was this situation that prompted scientists to think about whether it is possible to make drinking water from sea water? In fact, perhaps even at home, although it is a long process. In this case, you will need a distillation cube or a moonshine still. In this case, the law of physics is used, according to which salts cannot completely dissolve in water. That is, after evaporation, minerals remain at the bottom.Passing sea water
By driving sea water through a moonshine still, after boiling it, you will get ready-to-drink drinking water with a minimum amount of impurities. In its composition, it is more similar to distilled water, which does not conduct electricity. Therefore, it is quite difficult to get drunk on it. But pharmacies sell so-called “fortifiers”; by adding just a few drops you can get the water that the human body needs. So, in total, the production of drinking water from sea water costs a little more than the production of mineral water.
How to make drinking water from sea water in natural conditions?
It is not difficult to turn sea water into drinking water if you create a kind of moonshine still from available materials. To do this, you will need a hole, which is wrapped from the inside with film, several large stones and hay. Water poured into the hole is covered with hay. Stones are placed on top, which are also covered with film. After the water heats up, it will begin to evaporate, and when it gets cool, it will condense on the stones. Of course, there will be very little water, but enough to at least quench your thirst.
Sailors and shipbuilders were the first to think about how to desalinate the water of the seas and oceans. After all, for seafarers, fresh water is the most valuable cargo on board. You can survive a storm, endure the severe heat of the tropics, survive separation from the land, eat corned beef and crackers for months. But how can you live without water? And hundreds of barrels of ordinary fresh water were loaded into the holds. Paradox! After all, there is an abyss of water overboard. Yes, water, but salty, and to such an extent that it is 50-70 times saltier than potable water. It is natural, therefore, that the idea of desalination is as old as the world.
Even the ancient Greek scientist and philosopher Aristotle (384-322 BC) wrote: “By evaporating, salt water forms fresh water...” The first experience of artificial desalination of water recorded in written sources dates back to the 4th century BC.
Legend has it that Saint Basil, shipwrecked and left without water, figured out how to save himself and his comrades. He boiled sea water, soaked it in steam sea sponges, squeezed them out and got fresh water... Centuries have passed since then and people have learned to create desalination plants. The history of water desalination in Russia began in 1881. Then, in a fortress on the shores of the Caspian Sea, near present-day Krasnovodsk, a desalination plant was built to supply the garrison with fresh water. It produced 30 square meters of fresh water per day. This is very little! And already in 1967, an installation was created there that provided 1,200 square meters of water per day. Currently, there are more than 30 desalination plants operating in Russia, their total capacity is 300,000 square meters of fresh water per day.
The first large plants for producing fresh water from sea water appeared, of course, in desert areas of the world. More precisely, in Kuwait, on the shores of the Persian Gulf. One of the largest oil and gas fields in the world is located here. Since the early 1950s, several seawater desalination plants have been built in Kuwait. A powerful distillation plant in combination with a thermal power plant operates on the island of Aruba in the Caribbean Sea. Now desalinated water is already used in Algeria, Libya, Bermuda and the Bahamas, and in some areas of the United States. There is a seawater desalination plant in Kazakhstan on the Mangyshlak Peninsula. Here, in the desert, in 1967, a man-made oasis grew up - the city of Shevchenko. Among its main attractions are not only the world-famous powerful nuclear power plant, a large seawater desalination plant, but also a carefully thought-out water supply system. There are three water supply lines in the city. One carries high-quality fresh drinking water, the second carries slightly brackish water, which can be used for washing and watering plants, and the third carries ordinary sea water, used for technical needs, including sewage.
Water desalination plant at the nuclear power plant in the city of Shevchenko (1982).
More than 120 thousand people live in the city, and each person has no less water than Muscovites or Kiev residents. Enough water for plants too. But giving them water is not such a simple matter: an adult tree drinks 5-10 liters per hour. But nevertheless, for each resident there is 45 square meters of area occupied green spaces. This is almost 1.5 times more than in Moscow, 2 times more than in Vienna, famous for its parks, about 5 times more than in New York and London, 8 times more than in Paris.