What type of chemical bond is in a molecule. Types of chemical bonds
The most important characteristics of a bond include: length, polarity, dipole moment, saturation, directivity, strength, and multiplicity of bonds.
Communication length is the distance between the nuclei of atoms in a molecule. The bond length is determined by the size of the nuclei and the degree of overlap of electron clouds.
The bond length in HF is 0.92∙10 -10, in HCl - 1.28∙10 -10 m. The stronger the chemical bond, the shorter its length.
However, it follows from the theory that the bond strength of double bonds is twice that of single bonds, which is not true. Specialization? Mathematical - Informational? Why is water listed? Water plays an important role in sustaining life. There is no life. In the body of biological organs, tissues and fluids there is water. It dissolves the transport of assimilated and dissipated substances; constantly maintains concentration in the body.
Like air, it is vital for people. A person consumes an average of 2 liters of water per day. If a person cannot survive more than a few days. Water is the most abundant substance and represents three quarters of the earth's surface. Like air, it is the main factor in keeping earth on earth.
Bond angle (Valence angle) called the angle between imaginary lines passing through the nuclei of chemically bonded atoms. ∟HOH=104 0.5; ∟H 2 S \u003d 92.2 0; ∟H 2 S e \u003d 91 0.0.
The most important characteristic chemical bond is energy, defining it strength.
Quantitatively, the strength of a bond is characterized by the energy expended on breaking it, and is measured in kJ per 1 mol of a substance.
It can also be found in animal and plant organisms, as well as in crystalline hydrides in the form of water. Waters containing a small amount of water are called "soft waters", in contrast to "hard waters", which have a high percentage of calcium, especially calcium and magnesium.
We can talk about rain water, rivers and rivers, sea water etc. Pure water is obtained from natural water by repeated distillation under conditions where it is impossible to dissolve gases from the air or solids from the containers in which they are stored.
Therefore, the bond strength quantitatively characterizes the sublimation energy E subl. substances and the energy of dissociation of a molecule into atoms E diss. . Sublimation energy is understood as the energy expended for the transition of a substance from solid state into gaseous. For diatomic molecules, the binding energy is equal to the dissociation energy of the molecule into two atoms.
Preparation of the most important inorganic acids. Household consumption approx. ten% Agriculture OK. 50% industry 40%. It is a colorless, odorless, colorless liquid. In large quantities it is blue. Water at ordinary temperature reacts with: strong galvanic metals and forms bases plus hydrogen; metals with a lesser electronegative nature, leading to the formation of basic oxides and hydrogen.
There are very strong bonds between the atoms of a solid. Liquid mills, oceans, rivers Landmarks are as attractive as solid ones. Gases Vapors in the atmosphere For those in molecules together, very weak, so gases can flow. The bond between them is covalent and is a continuum in the form of vibrations.
For example, E diss. (and therefore E St.) in the H 2 molecule is 435 kJ / mol. In the molecule F 2 \u003d 159 kJ / mol, in the molecule N 2 \u003d 940 kJ / mol.
For not diatomic, but polyatomic molecules of the AB type, n is the average binding energy
due to AB n \u003d A + nB.
For example, the energy absorbed in the process
is equal to 924 kJ/mol.
Bond energy
E OH = = = = 462 kJ/mol.
In the solid state, it has almost twice as many hydrogen bonds as in liquid state. Each oxygen atom is linked by covalently bonded polar bonds of two hydrogen atoms of the molecule and two other hydrogen atoms coming from different neighboring molecules through hydrogen bonds. Two hydrogen atoms in a water molecule form two hydrogen bonds with oxygen atoms in two adjacent molecules. Thus, each oxygen atom is tetrahedrally surrounded by four oxygen atoms, just like the carbon atoms of diamond.
The action of water on metals Metals: potassium, calcium, sodium react with water, cold, with the formation of hydroxide and the release of hydrogen. Some metals corrode in the presence of water. The attack is stronger in the presence of oxygen and carbon dioxide. Action of water on oxides Water reacts with soluble oxides of metal hydroxides. One of the most important reactions is lime quenching, a strong exothermic reaction. The resulting calcium hydroxide is relatively poorly soluble in water. Therefore, when the lime is quenched, the resulting lime is obtained, which is a thin suspension of Ca 2 in a saturated solution of calcium hydroxide.
The conclusion about the structure of molecules and the structure of a substance is made according to the results obtained by different methods. In this case, the obtained information is used not only about the lengths and energies of bonds, bond angles, but also other properties of the substance, such as, for example, magnetic, optical, electrical, thermal, and others.
The totality of experimentally obtained data on the structure of a substance supplements and generalizes the results of quantum-chemical calculation methods that use the concept of the quantum-mechanical theory of chemical bonding. It is believed that the chemical bond is mainly carried out by valence electrons. For s- and p-elements, the valence electrons are the orbitals of the outer layer, and for d-elements, the electrons of the s-orbital of the outer layer and the d-orbital of the pre-outer layer.
When sulfur dioxide is dissolved in water, a chemical reaction results in an acid, sulfuric acid. The Importance of Water for Life Water plays an important role in sustaining life. It dissolves the transport of assimilated and dissipated substances; constantly maintains concentration in the body and, evaporating on the surface of the body, is involved in temperature regulation.
Water contributes to the osmotic phenomena of plants and is of particular importance in the process of photosynthesis. Drinking water is different from distilled water. The influence of water on religion and philosophy. Water is considered cleansing in most religions, for example, are baptisms performed in churches with water?
The nature of the chemical bond.
A chemical bond is formed only if, when the atoms approach each other total energy system (E kin. + E pot.) decreases.
Consider the nature of the chemical bond using the example of the molecular hydrogen ion H 2 + . (It is obtained by irradiating hydrogen molecules H 2 with electrons; in a gas discharge). For such a simple molecular system, the Schrödinger equation is most accurately solved.
In the hydrogen ion H 2 + one electron moves in the field of two nuclei - protons. The distance between the nuclei is 0.106 nm, the binding energy (dissociation into H atoms and H + ion) is 255.7 kJ/mol. That is, the particle is strong.
AT molecular ion H 2 + electrostatic forces of two types act - the forces of attraction of the electron to both nuclei and the forces of repulsion between the nuclei. The repulsive force manifests itself between the positively charged nuclei H A + and H A +, which can be represented as the following fig. 3. The repulsive force tends to separate the nuclei from each other.

Rice. 3. Force of repulsion (a) and attraction (b) between two nuclei, arising when they approach each other at distances of the order of the size of atoms.
Attractive forces act between the negatively charged electron e - and the positively charged nuclei H + and H +. A molecule is formed if the resultant of the forces of attraction and repulsion is zero, that is, the mutual repulsion of the nuclei must be compensated by the attraction of the electron to the nuclei. Such compensation depends on the location of the electron e - relative to the nuclei (Fig. 3 b and c). Here we mean not the position of an electron in space (which cannot be determined), but the probability of finding an electron in space. The location of the electron density in space, corresponding to Fig. 3.b) contributes to the convergence of the nuclei, and the corresponding fig. 3.c) - repulsion of the nuclei, since in this case the forces of attraction are directed in one direction and the repulsion of the nuclei is not compensated. Thus, there is a binding region when the electron density is distributed between the nuclei and a loosening or anti-bonding region when the electron density is distributed behind the nuclei.
If an electron enters the bonding region, then a chemical bond is formed. If the electron enters the region of loosening, then the chemical bond is not formed.
Depending on the nature of the electron density distribution in the binding region, there are three main types of chemical bonding: covalent, ionic, and metallic. These bonds do not occur in their pure form, and usually a combination of these types of bonds is present in compounds.
Link types.
In chemistry, the following types of bonds are distinguished: covalent, ionic, metallic, hydrogen bonds, van der Waals bonds, donor-acceptor bonds, and dative bonds.
covalent bond
When a covalent bond is formed, atoms share electrons with each other. An example of a covalent bond is a chemical bond in a Cl 2 molecule. Lewis (1916) first suggested that in such a bond each of the two chlorine atoms shares one of its outer electrons with the other chlorine atom. For overlapping atomic orbitals two atoms should come as close to each other as possible. A shared pair of electrons forms a covalent bond. These electrons occupy the same orbital, and their spins are directed in opposite directions.
Thus, a covalent bond is carried out by the socialization of electrons from different atoms as a result of the pairing of electrons with opposite spins.
A covalent bond is a widely used type of bond. A covalent bond can occur not only in molecules, but also in crystals. It occurs between identical atoms (in H 2, Cl 2, diamond molecules) and between different atoms (in H 2 O, NH 3 ...)
The mechanism of the occurrence of a covalent bond
Let us consider the mechanism using the example of the formation of the H 2 molecule.
H + H \u003d H 2, ∆H \u003d -436 kJ / mol
The nucleus of a free hydrogen atom is surrounded by a spherically symmetric electron cloud formed by a 1s electron. When atoms approach each other up to a certain distance, their electron clouds (orbitals) partially overlap (Fig. 4).
Rice. 4. The mechanism of bond formation in the hydrogen molecule.
If the distance between the nuclei of the hydrogen atoms approaching before touching is 0.106 nm, then after the overlap of the electron clouds, this distance is 0.074 nm.
As a result, a molecular two-electron cloud appears between the centers of the nuclei, which has the maximum electron density in the space between the nuclei. Density increase negative charge between the nuclei favors a strong increase in the forces of attraction between the nuclei, which leads to the release of energy. The stronger the chemical bond, the greater the overlap of electron orbitals. As a result of the occurrence of a chemical bond between two hydrogen atoms, each of them reaches the electronic configuration of a noble gas atom - helium.
There are two methods that explain from the quantum mechanical point of view the formation of an overlap region of electron clouds, and the formation of a covalent bond, respectively. One of them is called the VS method ( valence bonds), another MO (molecular orbitals).
In the method of valence bonds, the overlapping of atomic orbitals of a selected pair of atoms is considered. In the MO method, the molecule is considered as a whole and the distribution of electron density (from one electron) is spread over the entire molecule. From the position of MO 2H in H 2 are connected due to the attraction of the nuclei to the electron cloud located between these nuclei.
Depiction of a covalent bond
Links are depicted in different ways:
one). Using electrons as dots
In this case, the formation of a hydrogen molecule is shown by the diagram
H∙ + H∙ → H: H
2). Using square cells (orbitals), like placing two electrons with opposite spins in one molecular quantum cell

This diagram shows that the molecular energy level is lower than the original atomic levels, which means that the molecular state of matter is more stable than the atomic state.
3). A covalent bond is represented by a bar
For example, N - N. this feature symbolizes a pair of electrons.
If one covalent bond has arisen between atoms (one common electron pair), then it is called single, if more, then a multiple double(two common electron pairs), triple(three shared electron pairs). A single bond is represented by one line, a double bond by two, and a triple bond by three.
A dash between atoms shows that they have a generalized pair of electrons.
Classification of covalent bonds
Depending on the direction of overlapping electron clouds, σ-, π-, δ-bonds are distinguished. The σ-bond arises when electron clouds overlap along the axis connecting the nuclei of interacting atoms.
Examples of σ-bond:

Rice. 5. Formation of a σ-bond between s-, p-, d- electrons.
An example of the formation of a σ-bond when s-s clouds overlap is observed in a hydrogen molecule.
π-bond is carried out by overlapping electron clouds on both sides of the axis, connecting the nuclei of atoms.
Rice. 6. Formation of a π-bond between p-, d- electrons.
The δ-bond occurs when two d-electron clouds located in parallel planes overlap. The δ bond is less strong than the π bond, and the π bond is less strong than the σ bond.
Properties of a covalent bond
a). Polarity.
There are two types of covalent bonds: non-polar and polar.
In the case of a non-polar covalent bond, the electron cloud formed by a common pair of electrons is distributed in space symmetrically with respect to the nuclei of atoms. An example is diatomic molecules consisting of atoms of one element: H 2 , Cl 2 , O 2 , N 2 , F 2 . Their electron pair equally belongs to both atoms.
In the case of a polar bond, the electron cloud forming the bond is displaced towards the atom with a higher relative electronegativity.
Examples are molecules: HCl, H 2 O, H 2 S, N 2 S, NH 3, etc. Consider the formation of the HCl molecule, which can be represented by the following scheme
![]()
The electron pair is shifted to the chlorine atom, because the relative electronegativity of the chlorine atom (2.83) is greater than that of the hydrogen atom (2.1).
b). Saturability.
The ability of atoms to participate in the formation of a limited number of covalent bonds is called the saturation of a covalent bond. The saturation of covalent bonds is due to the fact that only external electrons participate in the chemical interaction. energy levels, that is, a limited number of electrons.
in) . Orientation and hybridization of the covalent bond.
A covalent bond is characterized by orientation in space. This is explained by the fact that electron clouds have a certain shape and their maximum overlap is possible with a certain spatial orientation.
The direction of the covalent bond determines the geometric structure of the molecules.
For example, for water, it has a triangular shape.
Rice. 7. Spatial structure of the water molecule.
It has been experimentally established that in the water molecule H 2 O the distance between the nuclei of hydrogen and oxygen is 0.096 nm (96 pm). The angle between the lines passing through the nuclei is 104.5 0 . Thus, the water molecule has an angular shape and its structure can be expressed in the form of the presented figure.
Hybridization
As experimental and theoretical studies(Slater, Pauling) during the formation of some compounds, such as BeCl 2 , BeF 2 , BeBr 2 , the state of the valence electrons of an atom in a molecule is described not by pure s-, p-, d- wave functions, but by their linear combinations. Such mixed structures are called hybrid orbitals, and the mixing process is called hybridization.
As quantum-chemical calculations show, the mixing of s- and p-orbitals of an atom is a favorable process for the formation of a molecule. In this case, more energy is released than in the formation of bonds involving pure s- and p-orbitals. Therefore, the hybridization of the electronic orbitals of an atom leads to a large decrease in the energy of the system and, accordingly, to an increase in the stability of the molecule. A hybridized orbital is more elongated on one side of the nucleus than on the other. Therefore, the electron density in the overlapping region of the hybrid cloud will be greater than the electron density in the overlapping region of the s- and p-orbitals separately, as a result of which the bond formed by the electrons of the hybrid orbital is characterized by greater strength.
There are several types of hybrid states. When the s- and p-orbitals hybridize (called sp hybridization), two hybrid orbitals arise, located at an angle of 180 0 relative to each other. In this case, a linear structure is formed. This configuration (structure) is known for most alkaline earth metal halides (for example, BeX 2 where X=Cl, F, Br), i.e. the connection angle is 180 0 С.

Rice. 8. sp hybridization
Another type of hybridization, called sp 2 hybridization (formed from one s and two p orbitals), leads to the formation of three hybrid orbitals, which are located at an angle of 120 0 to each other. In this case, a trigonal structure of a molecule (or a regular triangle) is formed in space. Such structures are known for compounds BX 3 (X=Cl, F, Br).



Rice. 9. sp 2 hybridization.
No less common is sp 3 hybridization, which is formed from one s and three p orbitals. In this case, four hybrid orbitals are formed, oriented in space symmetrically to the four vertices of the tetrahedron, that is, they are located at an angle of 109 0 28 ". This spatial position is called tetrahedral. Such a structure is known for molecules NH 3, H 2 O and in general for elements of period II. Schematically its view in space can be displayed in the following figure

Rice. 10. Spatial arrangement of bonds in the ammonia molecule,
projected onto a plane.
The formation of tetrahedral bonds due to sp 3 hybridization can be represented as follows (Fig. 11):
Rice. 11. Formation of tetrahedral bonds during sp 3 hybridization.
The formation of tetrahedral bonds during sp 3 hybridization is shown in fig. 12.

Fig.12. Formation of tetrahedral bonds during sp 3 - hybridization into CCl 4 molecules
Hybridization concerns not only s- and p-orbitals. To explain the stereochemical elements of III and subsequent periods, it becomes necessary to construct hybrid orbitals simultaneously including s-, p-, d-orbitals.
Substances with a covalent bond include:
1. organic compounds;
2. solid and liquid substances, in which bonds are formed between pairs of halogen atoms, as well as between pairs of hydrogen, nitrogen and oxygen atoms, for example, H 2;
3. elements of group VI (for example, spiral chains of tellurium), elements of group V (for example, arsenic), elements of group IV (diamond, silicon, germanium);
4. compounds that obey the 8-N rule (such as InSb, CdS, GaAs, CdTe), when the elements that form them are located in the periodic table of Mendeleev in groups II-VI, III-V.
AT solids with a covalent bond can form for the same substance different crystal structures, the binding energy of which is almost the same. For example, the ZnS structure can be cubic (zinc blende) or hexagonal (wurtzite). The arrangement of the nearest neighbors in zinc blende and wurtzite is the same, and the only and slight difference in the energies of these two structures is determined by the arrangement of atoms following the nearest ones. This ability of some substances is called allotropy or polymorphism. Another example of allotropy is silicon carbide, which has a number of polytites of various structures from purely cubic to hexagonal. These numerous crystalline modifications of ZnS, SiC exist at room temperature.
Ionic bond
An ionic bond is an electrostatic force of attraction between ions with opposite charges (i.e. + and −).
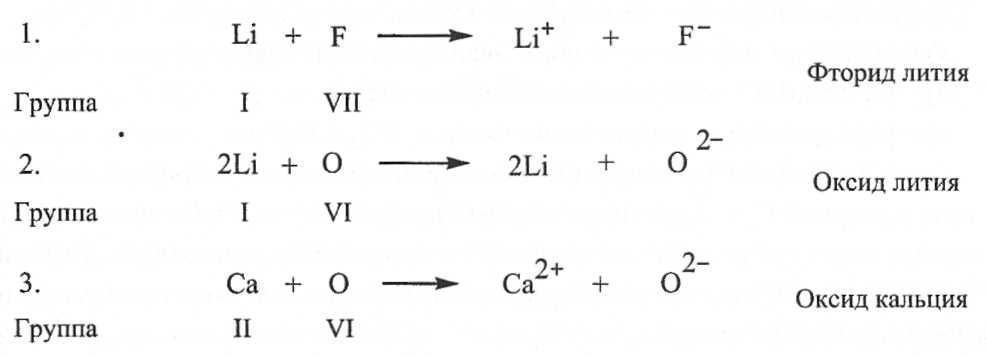
The idea of the ionic bond was formed on the basis of the ideas of V. Kossel. He suggested (1916) that when two atoms interact, one of them gives up and the other accepts electrons. Thus, an ionic bond is formed as a result of the transfer of one or more electrons from one atom to another. For example, in sodium chloride, an ionic bond is formed as a result of the transfer of an electron from a sodium atom to a chlorine atom. As a result of this transfer, a sodium ion with a charge of +1 and a chlorine ion with a charge of -1 are formed. They are attracted to each other by electrostatic forces, forming a stable molecule. The electron transfer model proposed by Kossel makes it possible to explain the formation of such compounds as lithium fluoride, calcium oxide, and lithium oxide.
The most typical ionic compounds consist of metal cations belonging to groups I and II of the periodic system, and anions of non-metallic elements belonging to groups VI and VII.
The ease of formation of an ionic compound depends on the ease of formation of its constituent cations and anions. The ease of formation is higher, the lower the ionization energy is the atom that donates electrons (electron donor), and the atom that accepts electrons (electron acceptor) has a greater affinity for the electron. electron affinity is a measure of an atom's ability to accept an electron. It is quantitatively defined as the change in energy that occurs when one mole of singly charged anions is formed from one mole of atoms. This is the so-called concept of "first electron affinity". The second electron affinity is the change in energy that occurs when one mole of doubly charged anions is formed from one mole of singly charged anions. These concepts, that is, the ionization energy and electron affinity, refer to gaseous substances and are characteristics of atoms and ions in gaseous state. But it should be borne in mind that most ionic compounds are most stable in the solid state. This circumstance is explained by the existence of a crystal lattice in their solid state. The question arises. Why, after all, ionic compounds are more stable in the form of crystal lattices, and not in a gaseous state? The answer to this question is the calculation of the energy of the crystal lattice, based on the electrostatic model. In addition to this, this calculation is also a test of the ionic bond theory.
To calculate the energy of the crystal lattice, it is necessary to determine the work that must be spent on the destruction of the crystal lattice with the formation of gaseous ions. For the calculation, the concept of the forces of attraction and repulsion is used. The expression for the potential energy of interaction of singly charged ions is obtained by summing the energy of attraction and the energy of repulsion
E \u003d E inc + E out (1).
As E prit, the energy of the Coulomb attraction of ions of opposite signs is taken, for example, Na + and Cl - for the NaCl compound
E int \u003d -e 2 / 4πε 0 r (2),
since the distribution of the electronic charge in the filled electron shell is spherically symmetrical. Due to the repulsion that occurs due to the Pauli principle when the filled shells of the anion and cation overlap, the distance at which the ions can approach is limited. The repulsive energy changes rapidly with internuclear distance and can be written as the following two approximate expressions:
E otm \u003d A / r n (n≈12) (3)
E otm \u003d B ∙ exp (-r / ρ) (4),
where A and B are constants, r is the distance between ions, ρ is a parameter (characteristic length).
It should be noted that none of these expressions corresponds to a complex quantum mechanical process that leads to repulsion.
Despite the approximation of these formulas, they allow one to accurately calculate and, accordingly, describe the chemical bond in the molecules of such ionic compounds as NaCl, KCl, CaO.
Since the electric field of the ion has spherical symmetry (Fig. 13), the ionic bond, unlike the covalent bond, does not have directionality. The interaction of two oppositely charged ions is compensated by repulsive forces only in the direction connecting the centers of the ion nuclei; in other directions, the electric fields of the ions are not compensated. Therefore, they are able to interact with other ions. Thus, an ionic bond does not have saturation.
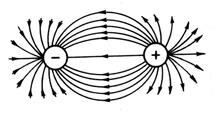
Rice. 13. Spherical symmetry electrostatic field
oppositely charged charges.
Due to the non-directionality and non-saturation of the ionic bond, it is energetically most favorable when each ion is surrounded by maximum number ions of the opposite sign. Due to this, the most preferred form of existence of an ionic compound is a crystal. For example, in a NaCl crystal, each cation has six anions as nearest neighbors.
Only when high temperatures in the gaseous state, ionic compounds exist in the form of non-associated molecules.
In ionic compounds, the coordination number does not depend on the specifics of the electronic structure of atoms, as in covalent compounds, but is determined by the ratio of the sizes of the ions. With a ratio of ionic radii in the range of 0.41 - 0.73, octahedral coordination of ions is observed, with a ratio of 0.73-1.37 - cubic coordination, etc.
Thus, under normal conditions, ionic compounds are crystalline substances. The concept of two-ion molecules, for example, NaCL, CsCl is not applicable to them. Each crystal is made up of a large number of ions.
An ionic bond can be represented as a limiting polar bond, for which the effective charge of an atom is close to unity. For a purely covalent non-polar bond, the effective charge of the atoms is zero. In real substances, purely ionic and purely covalent bonds are rare. Most compounds have an intermediate nature of the bond between non-polar covalent and polar ionic. That is, in these compounds, the covalent bond has a partially ionic character. The nature of ionic and covalent bonds in real substances is shown in Figure 14.

Rice. 14. Ionic and covalent nature of the bond.
The proportion of the ionic nature of the bond is called the degree of ionicity. It is characterized by the effective charges of the atoms in the molecule. The degree of ionicity increases with an increase in the difference in the electronegativity of its constituent atoms.
metal connection
In metal atoms, external valence electrons are held much weaker than in non-metal atoms. This causes the loss of the connection of electrons with individual atoms for a sufficiently long period of time and their socialization. A socialized ensemble of external electrons is formed. The existence of such an electronic system leads to the emergence of forces that keep the positive metal ions in a close state, despite their similar charge. Such a bond is called a metallic bond. Such a bond is characteristic only for a metal and exists in the solid and liquid state of matter. A metallic bond is a type of chemical bond. It is based on the socialization of external electrons, which lose their connection with the atom and are therefore called free electrons (Fig. 15).
Rice. 15. Metal connection.
The following facts confirm the existence of a metallic bond. All metals have high thermal conductivity and high electrical conductivity, which is provided by the presence of free electrons. In addition, the same circumstance determines the good reflectivity of metals to light irradiation, their brilliance and opacity, high plasticity, and a positive temperature coefficient of electrical resistance.
The stability of the crystal lattice of metals cannot be explained by such types of bonds as ionic and covalent. Ionic bonding between metal atoms located at the nodes of the crystal lattice is impossible, since they have the same charge. A covalent bond between metal atoms is also unlikely, since each atom has 8 to 12 nearest neighbors, and the formation of covalent bonds with so many shared electron pairs is unknown.
Metal structures are characterized by the fact that they have a rather rare arrangement of atoms (internuclear distances are large) and big number nearest neighbors of each atom in the crystal lattice. Table 1 lists three typical metal structures.
Table 1
Characteristics of the structures of the three most common metals
We see that each atom participates in the formation of a large number of bonds (for example, with 8 atoms). Such a large number of bonds (with 8 or 12 atoms) cannot be simultaneously localized in space. Communication must be carried out due to resonance oscillatory motion external electrons of each atom, which results in the collectivization of all external electrons of the crystal with the formation of an electron gas. In many metals, it is enough to take one electron from each atom to form a metallic bond. This is exactly what is observed for lithium, which has only one electron in the outer shell. A lithium crystal is a lattice of Li + ions (balls with a radius of 0.068 nm) surrounded by an electron gas.

Rice. 16. Various types of crystalline packing: a-hexagonal close packing; b - face-centered cubic packing; B-cubic packing.
There are similarities between metallic and covalent bonds. It lies in the fact that both types of bond are based on the socialization of valence electrons. However, a covalent bond connects only two neighboring atoms, and the shared electrons are in close proximity to the connected atoms. In a metallic bond, several atoms participate in the socialization of valence electrons.
Thus, the concept of a metallic bond is inextricably linked with the idea of metals as a set of positively charged ionic cores with large gaps between ions filled with electron gas, while at the macroscopic level the system remains electrically neutral.
In addition to the above types of chemical bonds, there are other types of bonds that are intermolecular: hydrogen bond, van der Waals interaction, donor-acceptor interaction.
Donor-acceptor interaction of molecules
The mechanism of formation of a covalent bond due to a two-electron cloud of one atom and a free orbital of another is called donor-acceptor. An atom or particle that provides a two-electron cloud for communication is called a donor. An atom or particle with a free orbital that accepts this electron pair is called an acceptor.
The main types of intermolecular interaction. hydrogen bond
Between valence-saturated molecules, at distances exceeding the particle size, electrostatic forces of intermolecular attraction can appear. They are called van der Waals forces. The van der Waals interaction always exists between closely spaced atoms, but plays an important role only in the absence of stronger bonding mechanisms. This weak interaction with a characteristic energy of 0.2 eV/atom takes place between neutral atoms and between molecules. The name of the interaction is associated with the name of van der Waals, since it was he who first suggested that the equation of state, taking into account the weak interaction between gas molecules, describes the properties of real gases much better than the equation of state ideal gas. However, the nature of this attractive force was explained only in 1930 by London. At present, the following three types of interactions are attributed to Van der Waals attraction: orientational, induction, dispersion (London effect). The van der Waals attraction energy is determined by the sum of the orientation, induction and dispersion interactions.
E int = E op + E ind + E disp (5).
Orientation interaction (or dipole-dipole interaction) is manifested between polar molecules, which, when approached, turn (orient) towards each other with opposite poles so that potential energy the system of molecules became minimal. The energy of the orientational interaction is the more significant, the larger the dipole moment of the molecules μ and the smaller the distance l between them:
E op \u003d - (μ 1 μ 2) 2 / (8π 2 ∙ε 0 ∙l 6) (6),
where ε 0 is an electrical constant.
The inductive interaction is associated with the processes of polarization of molecules by surrounding dipoles. It is the more significant, the higher the polarizability α of the non-polar molecule and the greater the dipole moment μ of the polar molecule
E ind \u003d - (αμ 2) / (8π 2 ∙ε 0 ∙l 6) (7).
The polarizability α of a non-polar molecule is called deformation polarizability, since it is associated with the deformation of the particle, while μ characterizes the displacement of the electron cloud and nuclei relative to their previous positions.
Dispersion interaction (London effect) occurs in any molecules, regardless of their structure and polarity. Due to the instantaneous mismatch of the centers of gravity of the charges of the electron cloud and nuclei, an instantaneous dipole is formed, which induces instantaneous dipoles in other particles. The motion of instantaneous dipoles becomes coordinated. As a result, neighboring particles experience mutual attraction. The dispersion interaction energy depends on the ionization energy E I and the polarizability of molecules α
E disp \u003d - (E I 1 ∙ E I 2) ∙ α 1 α 2 / (E I 1 + E I 2) l 6 (8).
The hydrogen bond has an intermediate character between the valence and intermolecular interactions. The hydrogen bond energy is low, 8-80 kJ/mol, but it is higher than the van der Waals interaction energy. The hydrogen bond is characteristic of liquids such as water, alcohols, acids and is due to a positively polarized hydrogen atom. The small size and the absence of internal electrons allow the hydrogen atom present in a liquid in any compound to enter into additional interaction with a negatively polarized atom of another or the same molecule that is not covalently bound to it.
A δ- - H δ+ .... A δ- - H δ+ .
That is, there is an association of molecules. The association of molecules leads to a decrease in volatility, an increase in the boiling point and heat of vaporization, an increase in viscosity and permittivity liquids.
Water is a particularly suitable substance for hydrogen bond formation, since its molecule has two hydrogen atoms and two lone pairs at the oxygen atom. This causes a high dipole moment of the molecule (μ D = 1.86 D) and the ability to form four hydrogen bonds: two as a proton donor and two as a proton acceptor
(N 2 O .... N - O ... N 2 O) 2 times.
It is known from experiments that with a change in molecular weight in the series hydrogen compounds elements of the third and subsequent periods, the boiling point rises. If this pattern is applied to water, then its boiling point should not be 100 0 C, but 280 0 C. This contradiction confirms the existence of a hydrogen bond in water.
Experiments have shown that molecular associates are formed in liquid and especially in solid water. Ice has a tetrahedral crystal lattice. In the center of the tetrahedron there is an oxygen atom of one water molecule, at four vertices there are oxygen atoms of neighboring molecules, which are connected by hydrogen bonds with their nearest neighbors. In liquid water, hydrogen bonds are partially broken; in its structure, a dynamic equilibrium is observed between associates of molecules and free molecules.
Valence bond method
The theory of valence bonds, or localized electron pairs, assumes that each pair of atoms in a molecule is held together by one or more shared electron pairs. In the representation of the theory of valence bonds, a chemical bond is localized between two atoms, that is, it is two-center and two-electron.
The method of valence bonds is based on the following main provisions:
Each pair of atoms in a molecule is held together by one or more shared electron pairs;
A single covalent bond is formed by two electrons with antiparallel spins located in the valence orbitals of the bonding atoms;
When a bond is formed, the wave functions of electrons overlap, leading to an increase in the electron density between atoms and a decrease in total energy systems;
Each atom has a certain number of electrons.
Entering into chemical reactions, atoms donate, acquire, or socialize electrons, reaching the most stable electronic configuration. The configuration with the lowest energy is the most stable (as in noble gas atoms). This pattern is called the "octet rule" (Fig. 1).
Rice. one.
This rule applies to all connection types. Electronic bonds between atoms allow them to form stable structures, from the simplest crystals to complex biomolecules that eventually form living systems. They differ from crystals in their continuous metabolism. However, many chemical reactions proceed according to the mechanisms electronic transfer, which play an important role in the energy processes in the body.
A chemical bond is a force that holds together two or more atoms, ions, molecules, or any combination of them..
The nature of the chemical bond is universal: it is an electrostatic force of attraction between negatively charged electrons and positively charged nuclei, determined by the configuration of the electrons in the outer shell of atoms. The ability of an atom to form chemical bonds is called valency, or oxidation state. The concept of valence electrons - electrons that form chemical bonds, that is, those located in the most high-energy orbitals. Accordingly, the outer shell of an atom containing these orbitals is called valence shell. At present, it is not enough to indicate the presence of a chemical bond, but it is necessary to clarify its type: ionic, covalent, dipole-dipole, metallic.
The first type of connection isionic connection
According to Lewis and Kossel's electronic theory of valency, atoms can achieve a stable electronic configuration in two ways: first, by losing electrons, becoming cations, secondly, acquiring them, turning into anions. As a result of electron transfer, due to the electrostatic force of attraction between ions with charges of the opposite sign, a chemical bond is formed, called Kossel " electrovalent(now called ionic).
In this case, anions and cations form a stable electronic configuration with a filled outer electron shell. Typical ionic bonds are formed from cations of T and II groups of the periodic system and anions of non-metallic elements of groups VI and VII (16 and 17 subgroups - respectively, chalcogens and halogens). The bonds in ionic compounds are unsaturated and non-directional, so they retain the possibility of electrostatic interaction with other ions. On fig. 2 and 3 show examples of ionic bonds corresponding to the Kossel electron transfer model.
Rice. 2.
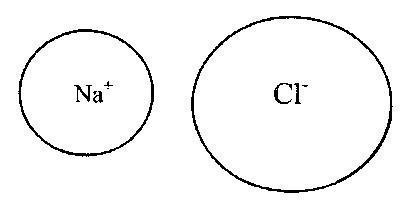
Rice. 3. Ionic bond in the sodium chloride (NaCl) molecule
Here it is appropriate to recall some of the properties that explain the behavior of substances in nature, in particular, to consider the concept of acids and grounds.
Aqueous solutions of all these substances are electrolytes. They change color in different ways. indicators. The mechanism of action of indicators was discovered by F.V. Ostwald. He showed that the indicators are weak acids or bases, the color of which in the undissociated and dissociated states is different.
Bases can neutralize acids. Not all bases are soluble in water (for example, some organic compounds that do not contain -OH groups are insoluble, in particular, triethylamine N (C 2 H 5) 3); soluble bases are called alkalis.
Aqueous solutions of acids enter into characteristic reactions:
a) with metal oxides - with the formation of salt and water;
b) with metals - with the formation of salt and hydrogen;
c) with carbonates - with the formation of salt, CO 2 and H 2 O.
The properties of acids and bases are described by several theories. In accordance with the theory of S.A. Arrhenius, an acid is a substance that dissociates to form ions H+ , while the base forms ions HE- . This theory does not take into account the existence of organic bases that do not have hydroxyl groups.
In line with proton Bronsted and Lowry's theory, an acid is a substance containing molecules or ions that donate protons ( donors protons), and the base is a substance consisting of molecules or ions that accept protons ( acceptors protons). Note that in aqueous solutions, hydrogen ions exist in a hydrated form, that is, in the form of hydronium ions H3O+ . This theory describes reactions not only with water and hydroxide ions, but also carried out in the absence of a solvent or with a non-aqueous solvent.
For example, in the reaction between ammonia NH 3 (weak base) and hydrogen chloride in the gas phase, solid ammonium chloride is formed, and in an equilibrium mixture of two substances there are always 4 particles, two of which are acids, and the other two are bases:
This equilibrium mixture consists of two conjugated pairs of acids and bases:
1)NH 4+ and NH 3
2) HCl and Cl ‑
Here, in each conjugated pair, the acid and base differ by one proton. Every acid has a conjugate base. A strong acid has a weak conjugate base, and a weak acid has a strong conjugate base.
The Bronsted-Lowry theory makes it possible to explain the unique role of water for the life of the biosphere. Water, depending on the substance interacting with it, can exhibit the properties of either an acid or a base. For example, in reactions with aqueous solutions of acetic acid, water is a base, and with aqueous solutions of ammonia, it is an acid.
1) CH 3 COOH + H 2 O ↔ H 3 O + + CH 3 SOO- . Here, an acetic acid molecule donates a proton to a water molecule;
2) NH3 + H 2 O ↔ NH4 + + HE- . Here the ammonia molecule accepts a proton from the water molecule.
Thus, water can form two conjugated pairs:
1) H 2 O(acid) and HE- (conjugate base)
2) H 3 O+ (acid) and H 2 O(conjugate base).
In the first case, water donates a proton, and in the second, it accepts it.
Such a property is called amphiprotonity. Substances that can react as both acids and bases are called amphoteric. Such substances are often found in nature. For example, amino acids can form salts with both acids and bases. Therefore, peptides readily form coordination compounds with the metal ions present.
Thus, the characteristic property of an ionic bond is the complete displacement of a bunch of binding electrons to one of the nuclei. This means that there is a region between the ions where the electron density is almost zero.
The second type of connection iscovalent connection
Atoms can form stable electronic configurations by sharing electrons.
Such a bond is formed when a pair of electrons is shared one at a time. from each atom. In this case, the socialized bond electrons are distributed equally among the atoms. An example of a covalent bond is homonuclear diatomic H molecules 2 , N 2 , F 2. Allotropes have the same type of bond. O 2 and ozone O 3 and for a polyatomic molecule S 8 and also heteronuclear molecules hydrogen chloride HCl, carbon dioxide CO 2, methane CH 4, ethanol FROM 2 H 5 HE, sulfur hexafluoride SF 6, acetylene FROM 2 H 2. All these molecules have the same common electrons, and their bonds are saturated and directed in the same way (Fig. 4).
For biologists, it is important that the covalent radii of atoms in double and triple bonds are reduced compared to a single bond.
![]()
Rice. four. Covalent bond in the Cl 2 molecule.
Ionic and covalent types of bonds are two limiting cases of the set existing types chemical bonds, and in practice most of the bonds are intermediate.
Compounds of two elements located at opposite ends of the same or different periods of the Mendeleev system predominantly form ionic bonds. As the elements approach each other within a period, the ionic nature of their compounds decreases, while the covalent character increases. For example, the halides and oxides of the elements on the left side of the periodic table form predominantly ionic bonds ( NaCl, AgBr, BaSO 4 , CaCO 3 , KNO 3 , CaO, NaOH), and the same compounds of the elements on the right side of the table are covalent ( H 2 O, CO 2, NH 3, NO 2, CH 4, phenol C6H5OH, glucose C 6 H 12 O 6, ethanol C 2 H 5 OH).
The covalent bond, in turn, has another modification.
In polyatomic ions and in complex biological molecules, both electrons can only come from one atom. It is called donor electron pair. An atom that socializes this pair of electrons with a donor is called acceptor electron pair. This type of covalent bond is called coordination (donor-acceptor, ordative) communication(Fig. 5). This type of bond is most important for biology and medicine, since the chemistry of the most important d-elements for metabolism is largely described by coordination bonds.
![]()
Pic. 5.
As a rule, in a complex compound, a metal atom acts as an electron pair acceptor; on the contrary, in ionic and covalent bonds, the metal atom is an electron donor.
The essence of the covalent bond and its variety - the coordination bond - can be clarified with the help of another theory of acids and bases, proposed by GN. Lewis. He somewhat expanded the semantic concept of the terms "acid" and "base" according to the Bronsted-Lowry theory. The Lewis theory explains the nature of the formation of complex ions and the participation of substances in nucleophilic substitution reactions, that is, in the formation of CS.
According to Lewis, an acid is a substance capable of forming a covalent bond by accepting an electron pair from a base. A Lewis base is a substance that has a lone pair of electrons, which, by donating electrons, forms a covalent bond with Lewis acid.
That is, the Lewis theory expands the range of acid-base reactions also to reactions in which protons do not participate at all. Moreover, the proton itself, according to this theory, is also an acid, since it is able to accept an electron pair.
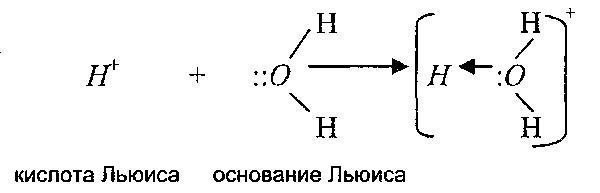
Therefore, according to this theory, cations are Lewis acids and anions are Lewis bases. The following reactions are examples:
It was noted above that the subdivision of substances into ionic and covalent ones is relative, since there is no complete transfer of an electron from metal atoms to acceptor atoms in covalent molecules. In compounds with an ionic bond, each ion is in the electric field of ions of the opposite sign, so they are mutually polarized, and their shells are deformed.
Polarizability determined electronic structure, charge and size of the ion; it is higher for anions than for cations. The highest polarizability among cations is for cations of a larger charge and smaller size, for example, for Hg 2+ , Cd 2+ , Pb 2+ , Al 3+ , Tl 3+. Has a strong polarizing effect H+ . Since the effect of ion polarization is two-way, it significantly changes the properties of the compounds they form.
The third type of connection isdipole-dipole connection
In addition to the listed types of communication, there are also dipole-dipole intermolecular interactions, also known as van der Waals .
The strength of these interactions depends on the nature of the molecules.
There are three types of interactions: permanent dipole - permanent dipole ( dipole-dipole attraction); permanent dipole - induced dipole ( induction attraction); instantaneous dipole - induced dipole ( dispersion attraction, or London forces; rice. 6).

Rice. 6.
Only molecules with polar covalent bonds have a dipole-dipole moment ( HCl, NH 3, SO 2, H 2 O, C 6 H 5 Cl), and the bond strength is 1-2 debye(1D \u003d 3.338 × 10 -30 coulomb meters - C × m).
In biochemistry, another type of bond is distinguished - hydrogen connection, which is a limiting case dipole-dipole attraction. This bond is formed by the attraction between a hydrogen atom and a small electronegative atom, most often oxygen, fluorine and nitrogen. With large atoms that have a similar electronegativity (for example, with chlorine and sulfur), the hydrogen bond is much weaker. The hydrogen atom is distinguished by one essential feature: when the binding electrons are pulled away, its nucleus - the proton - is exposed and ceases to be screened by electrons.
Therefore, the atom turns into a large dipole.
A hydrogen bond, unlike a van der Waals bond, is formed not only during intermolecular interactions, but also within one molecule - intramolecular hydrogen bond. Hydrogen bonds play an important role in biochemistry, for example, for stabilizing the structure of proteins in the form of an a-helix, or for the formation of a DNA double helix (Fig. 7).
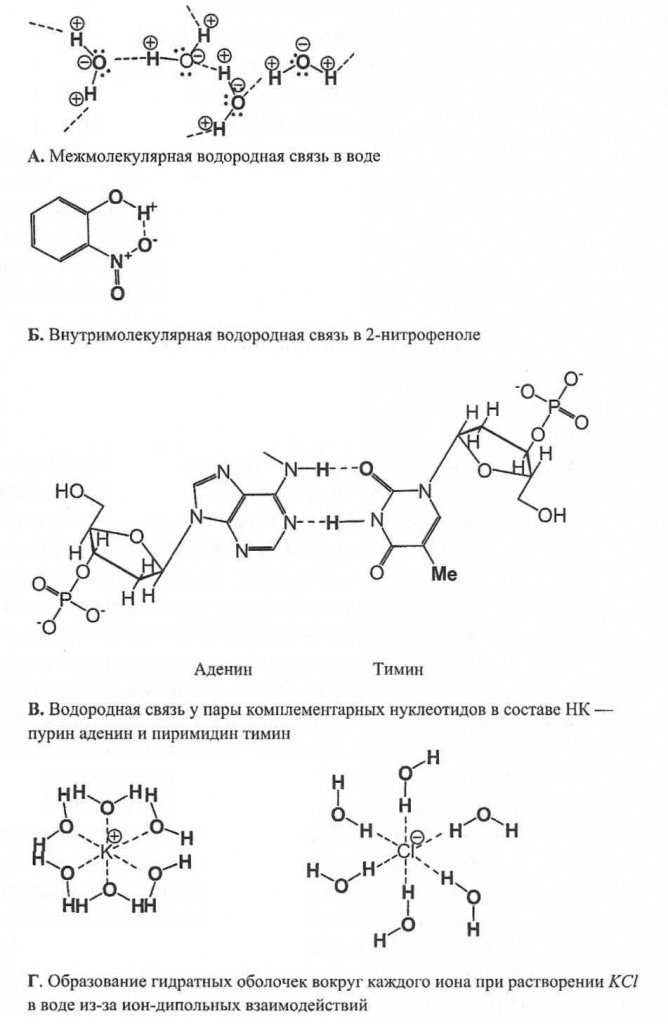
Fig.7.
Hydrogen and van der Waals bonds are much weaker than ionic, covalent, and coordination bonds. The energy of intermolecular bonds is indicated in Table. one.
Table 1. Energy of intermolecular forces
Note: The degree of intermolecular interactions reflect the enthalpy of melting and evaporation (boiling). Ionic compounds require much more energy to separate ions than to separate molecules. The melting enthalpies of ionic compounds are much higher than those of molecular compounds.
The fourth type of connection -metallic bond
Finally, there is another type of intermolecular bonds - metal: connection positive ions lattices of metals with free electrons. This type of connection does not occur in biological objects.
From overview types of bonds, one detail is clarified: an important parameter of an atom or ion of a metal - an electron donor, as well as an atom - an electron acceptor is its the size.
Without going into details, we note that the covalent radii of atoms, the ionic radii of metals, and the van der Waals radii of interacting molecules increase as their atomic number in the groups of the periodic system increases. In this case, the values of the ion radii are the smallest, and the van der Waals radii are the largest. As a rule, when moving down the group, the radii of all elements increase, both covalent and van der Waals.
The most important for biologists and physicians are coordination(donor-acceptor) bonds considered by coordination chemistry.
Medical bioinorganics. G.K. Barashkov






