crystallization formula. Solidification of crystalline bodies
The transition of a substance from a solid to a liquid state is called melting, and the transition from liquid state into a solid - by solidification or crystallization.
When melting solid the distances between the particles forming the crystal lattice increase, and the lattice itself is destroyed. This means that during the melting process, the molecular potential energy substances. Thus, the melting of a substance cannot occur spontaneously, since energy must be expended on this process.
During crystallization, particles approach each other, which form a lattice, and their potential energy decreases. Therefore, crystallization can only occur when the liquid gives up its energy to some external bodies.
So the unit of mass liquid substance has more internal energy than a unit mass of the same substance in the solid state, even if their temperature is the same.
The region in which matter is homogeneous in all physical and chemical properties, is called the phase of the state of this substance. Note that the solid and liquid phases of a substance at the same temperature can remain in equilibrium for an arbitrarily long time if the solid phase cannot receive energy, and the liquid phase cannot give it away. For example, ice can float in water for a long time if the temperature of all surrounding bodies is the same and equal to 0°C.
Let there be only a solid phase of matter, which receives energy from other bodies. Then, at first, both the molecular potential and molecular kinetic energies of this substance will increase, since the distances between the particles in the crystal lattice and the speed of their movement will increase. Then, at a certain temperature, the destruction of the crystal lattice will begin. Until the entire substance melts, its temperature remains unchanged, and all the energy received by the substance goes only to work on overcoming the forces of molecular cohesion. When only the liquid phase remains, then, continuing to receive energy, it will already heat up, i.e., its molecular kinetic energy will begin to increase.
If the liquid phase gives up its energy to the surrounding bodies, then all the described processes will be repeated in reverse order.
On fig. 12.1 shows graphs of changes in the temperature of a substance during melting and solidification. The segment (Fig. 12.1, a) expresses the amount of heat received by the substance when heated in the solid state (from T to the segment during melting and the segment - when heated in the liquid state. The segment Q (Fig. 12.1, b) expresses the amount of heat given off by the substance when cooled in the liquid state (from to), cut - when solidifying and cut - when cooled in the solid state.Experience shows that the melting and solidification of a certain substance occurs at the same temperature, which does not change as long as the solid and liquid phases of the substance coexist. This temperature is called the melting point.
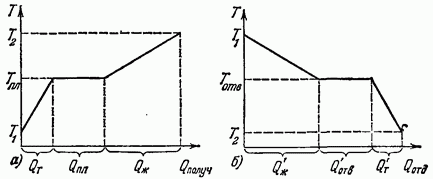
Note that during the melting and solidification of a substance, there is always a sharp boundary between the solid and liquid phases.
As experience shows, melting and solidification processes are not observed in amorphous substances. When heated, they gradually soften, and when cooled, they gradually thicken. The temperature of amorphous substances in these cases changes continuously, and there is no boundary between the solid and liquid phases, since their entire mass has a uniform appearance.
So, melting and crystallization can be observed only in crystalline bodies.
In crystalline, as well as from one crystalline state to another (recrystallization, or secondary crystallization); first kind. Crystallization from a liquid or gas phase is an exothermic process in which heat is released phase transition, or heat of crystallization; while the change in most cases is [in J / (mol. K)]: for simple substances 5-12, for inorganic compounds 20-30, for organic compounds 40-60. Recrystallization can proceed with the release or absorption of heat. In industry and lab. In practice, crystallization is used to obtain products with a given composition, impurity content, size, shape and defectiveness (see ... Crystals), as well as for fractional separation of mixtures, growing single crystals, etc.
Physical and chemical bases of the process. The conditions under which crystallization is possible are determined by the type. In order for crystallization to proceed at a finite rate, the initial phase must be supercooled (overheated), supersaturated with a crystallizing substance, or introduced into an external phase. field that reduces the solubility of the crystallizing phase. In the supercooled (overheated) or supersaturated phase, crystallization centers are formed, which turn into crystals and grow, as a rule, changing the shape, the content of impurities and the imperfection. Crystallization centers appear homogeneously in the volume of the initial phase and heterogeneously on the surfaces of foreign solid particles (primary nucleation), as well as near the surface of the previously formed new phase (secondary nucleation). Total number crystallization centers that have arisen in a unit volume of a solution or in 1 s, or the total intensity of their primary and secondary formation, is found by the formula:
where a is the kinetic coefficient of primary nucleation, which is considered within the framework kinetic theory formation of a new phase; R -
. T is the crystallization temperature; y - specific surface free energy crystals; V t - molar volume of the new phase; Dm \u003d DHS and S \u003d (T 0 -7) / T 0 for melts, am \u003d RT1n (S + 1) and S \u003d (c-c 0) / c 0 for solutions; DH-enthalpy of crystallization; c - crystallizing substance; T 0 and c 0 - respectively. temperature of the substance and saturated solution; E act - transition from the environment to the centers of crystallization; I at - the intensity of secondary nucleation in the volume of the initial phase. To measure a, E act and I watt, the dependence of the intensity of formation of crystallization centers on temperature, supersaturation and the concentration of foreign solid particles is found.
The value of I and passes through one or non-crystallization maxima (Fig. 1) with increasing supercooling (supersaturation) and increases with mech. influences (mixing,
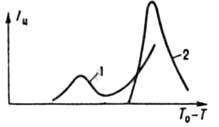
Rice. I Dependence of the rate of nucleation on supercooling of InSb: I a melt weighing 16 g was overheated in a quartz crucible by 15 K above the temperature for 9 min and then cooled at a rate of 1 deg/min; 2 the same, at 55 K for 20 s under the influence of . During growth, the crystallizing substance is first adsorbed on the surface of the formed crystal, and then embedded in its crystal lattice: with strong supercooling, it is equiprobable on any part of the surface (normal growth), with weak supercooling, in layers tangentially on steps formed by helical or two-dimensional nuclei (layer-by-layer growth). If supercooling is below a certain value, it is called the limit of morphological stability, a normally growing crystal repeats the shape (usually rounded) of a thermal or concentration field around it, and a layer-by-layer growing crystal has the shape of a polyhedron. When this limit is exceeded, tree-like crystals (dendrites) grow. Quantitatively, growth is characterized by a linear speed equal to the speed of movement of their surface in the direction normal to it. In industry, the effective linear growth rate is used (an increase of 1 s in the radius of the ball, the volume of which is equal to the volume of the crystal): I eff \u003d bS n exp (E p / RT), where b is the kinetic growth coefficient (10 -5 -10 -14 m /s), n-growth parameter (usually 1-3), E p - growth (10-150 kJ/mol). Parameters b, n and E p are found by measuring I eff at different temperatures and supersaturation of the solution or supercooling of the melt. With an increase in supercooling, I eff passes through a maximum similar to I m .
The growth rate can be limited by mass and with the environment (respectively, external diffusion and heat exchange modes of growth), the rate chemical interaction crystallizing component with other components of the medium (external kinetic regime) or processes on the surface (adsorption-kinetic regime). In the external kinetic mode, I eff increases with increasing concentrations of reagents and . in the external diffusion and heat exchange modes - with an increase in the intensity of mixing, in the adsorption-kinetic mode - with an increase in surface defects and a decrease in the concentration of surfactants.
At high growth rates, crystals acquire a significant number of nonequilibrium defects (vacancies, dislocations, etc.). When the limit of morphological stability is exceeded, three-dimensional inclusions of the medium, immured between the branches of dendrites (occlusion), get into the volume. Due to occlusion, the composition approaches the composition of the medium the more, the higher I eff. During their growth, crystals capture any impurity present in the medium, and the trapped impurity depends on the growth rate. If crystallization occurs in solution and the crystals continue to contact the medium after growth is completed, then the nonequilibrium trapped impurity is ejected from the medium, and their structure is improved (structural recrystallization). At the same time, additional structural defects appear in the mixed medium during collisions with each other and with the walls of the mold. Therefore, a stationary defectiveness of crystals is gradually established in the system, which depends on the intensity of mixing. In max. In the common case of the formation of a plurality during crystallization (mass crystallization), the precipitated phase is polydisperse, which is due to the non-simultaneity of nucleation and fluctuations in their growth. Small crystals are more soluble than large ones; therefore, with decreasing supersaturation, there comes a moment when the medium, remaining supersaturated with respect to the latter, becomes

Rice. 2. Size distribution function (usual r and most probable r A) during isothermal (298 K) periodic crystallization from an aqueous solution in a crystallizer with a stirrer (Re=10 4 number): 1 BaSO 4 , initial supersaturation S 0 =500. r A =7.6 µm; 2 - K 2 SO 4 , salting out with methanol (1.1) r A = 1 µm; t process time.
From this moment, their dissolution and growth of large ones (Oswald ripening) begin, as a result of which the average size increases, and their number decreases. At the same time, in a stirred medium, the crystals break up during collisions and after a while acquire a stationary dispersion, determined by the intensity of the mechanical action. Main quantities, mass crystallization characteristic - size distribution function f(r,t)=dN/dr , where N is the number of crystals, the size of which is less than the current size r, per unit volume at time t. This function often has a bell-shaped appearance (Fig. 2); its ascending branch is sensitive mainly to the nucleation, growth, splitting and dissolution (during maturation) of crystals, the descending branch is sensitive to the growth and formation of their aggregates. If the standard deviation of the size from the average does not exceed half, the latter, the mentioned function is called. narrow, if exceeding - wide. Function change f(r,t) at crystallization is described by the equation:

where a is the fluctuation coefficient of the crystal growth rate; D to and v to - resp. diffusion coefficient and speed of movement in the medium; I ar and I p - respectively. the intensity of formation of a given size due to the adhesion of smaller particles and the splitting of crystals.
The system of equations of material and heat balances, equations (2), as well as equations relating the size and growth rate with their shape, defectiveness and impurity content, are the basis for modeling and calculating mass crystallization and choosing the optimal conditions for its implementation. Mass crystallization is carried out periodically or continuously. During periodic crystallization, the melt or saturated solution (steam) is cooled, the solvent is evaporated, salting out agents are added (see below), or portions of the reagents that form production crystals are mixed. In continuous crystallization, flows of melt, supersaturated solution or reagents are introduced into the crystallizer and the crystalline product is continuously withdrawn.
With periodic process, the crystallization rate, determined by the formula:
![]() ,
,
where r and V - resp. the density of the solid phase and the volume of the system, first slowly increases (induction period), then increases sharply as a result of a simultaneous increase in r and f and, having passed through a maximum, decreases (Fig. 3) due to a decrease in I eff. During periods of induction and an increase in the rate of crystallization, the nucleation and growth of crystals predominate in the system, during a period of a decrease in the rate, their growth, aggregation and splitting, and then Oswald maturation and structural recrystallization. The induction period is shortened under the influence of factors that accelerate the nucleation and growth of crystals. So, when cooling melts, this period first decreases with increasing cooling intensity, and then
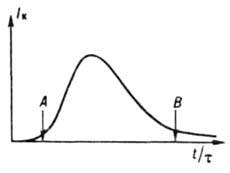
Rice. 3. Typical change in the rate of periodic crystallization: t - process time; t is the duration of the induction period; A is the moment of appearance of a new phase; B - the beginning of the stage of structural recrystallization and Oswald ripening increases due to the extreme dependence of the nucleation and growth rates on supercooling; if the cooling rate is high enough, the melt solidifies while remaining amorphous (see Fig. glassy state). To reduce the induction period, product crystals (seed) are added to the system, which grow, which leads to an increase in the crystallization rate. As a result of the release with increasing heat, crystallization decreases supercooling and slows down nucleation. At low supercoolings (supersaturations), nuclei do not appear at all, and the seed introduced into the system in the form of single crystals can grow into a single crystal, and in the form of a powder, into the so-called monodisperse product with a narrow function f(r, t).
With continuous crystallization, the function f(r, t) under comparable conditions is wider than with periodic crystallization, which is explained by the spread of residence times in continuous crystallizers. To narrow this function, the crystallization mode is brought closer to the ideal displacement mode, in order to expand - to the ideal mode (see Fig. Thread structure). At a low supersaturation of the system, continuous crystallization is stable to fluctuations in the external. conditions; at high supersaturation, its value and size fluctuate during crystallization.
In chem. and related industries, as well as in laboratories, crystallization from melts and solutions is mainly used, less often - crystallization from vapor and solid phases. Crystallization from melts is used mainly for solidifying molten substances and, in addition, for their fractional separation and. Solidification of substances in the form of castings (blocks) is carried out in special forms. In small-scale production (for example, reagents), separate molds of a certain size or configuration are usually used, in which the melt is cooled by natural heat exchange with environment; in large-capacity industries (naphthalene, etc.), crystallization is carried out in sectioned, tubular, conveyor and other crystallizers with built-in molds, forcibly cooled with water, liquid NH 3, freons, etc.
To obtain products in the form of thin plates or flakes, continuously operating belt, roller and disk molds are used, where curing occurs much more intensively than in molds. In a ribbon mold (Fig. 4), the initial melt
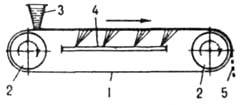
Rice. 4. Tape mold: 1 tape; 2 drive drums; 3 feeding hopper; 4 cooling device; 5, the cured product is fed in a thin layer onto a moving metal belt, on which it is cooled until it is completely solidified. In the roller apparatus (Fig. 5), the product crystallizes on the outer surface of a rotating hollow drum (roller) cooled from the inside, partially immersed in a bath of melt; the crystals are removed from the drum with a fixed knife. In disc devices, products are cured on the surface of rotating discs cooled from the inside.

Rice. 5. Roller mold: 1 drum; 2 bath; 3 knife; 4 refrigerant supply pipe; 5 nozzle; 6 melt; 7 cured product.
When preparing products for granulation, the melt is dispersed directly into a gaseous refrigerant stream, mainly air (production of ammonium nitrate, urea, etc.), or liquid, such as water or oil (production of plastics, sulfur, etc.) in hollow towers or apparatuses with fluidized layer, where small droplets crystallize
Crystallization from solutions is mainly used to isolate valuable components from solutions, as well as to concentrate them and to purify substances from impurities. Substances whose solubility strongly depends on temperature (for example, KNO 3 in water) crystallize by cooling hot solutions, while the initial amount of solvent contained in the mother liquor does not change in the system (isohydric crystallization). In small-scale production, capacitive batch molds are used, equipped with cooled jackets. In such devices, the solution is cooled with continuous stirring according to a certain program. To prevent intense encrustation of cooling surfaces, the temperature difference between the solution and the refrigerant should be no more than 8-10°C. In large-scale production, as a rule, scraper, screw, disk, drum and rotary continuous-action molds are used. Scrapers usually consist of non-crystallization tubular sections connected in series, each of which has a shaft with scrapers and which are equipped with a common or individual cooling jackets. When the shaft rotates, the scrapers clean the inside. the surface of the cooled pipes from settled on them and contribute to the transportation of the resulting thickened suspension from section to section. In screw molds, the solution is mixed and moved using solid or belt screws.
Disc molds are equipped with fixed or rotating discs. In the first case (Fig. 6), a drive shaft with scrapers is located along the axis of the apparatus for cleaning the surfaces of the disks from deposited crystals; the initial solution is fed into the mold from above, and the resulting suspension sequentially passes in the space between the cooled disks and is discharged through the lower fitting. In the second case, the shaft with disks is placed inside a trough or a horizontal cylindrical vessel; the crystals are removed from the surface of the disks by fixed scrapers.

The main element of the drum mold is a hollow drum with support bandages, installed at an angle of 15° to the horizontal axis and rotating at a frequency of 5-20 min -1 . The solution, cooled by a water jacket or air (which is pumped by a fan through the internal cavity of the drum), enters from one end of it, and the suspension is discharged from the other.
Viscous solutions (for example, fatty acids) are often cooled in rotary crystallizers - cylindrical apparatus, inside which high speed the rotor with knives rotates. The latter, under the action of centrifugal force, are pressed against the inner surface of the mold, clearing it of settled crystals. The solution will usually be fed into the apparatus under positive pressure. To increase the residence time in the crystallizer of the solution and its greater supercooling, several devices are connected in series.
When using scraper, screw, rotary and sometimes disk molds, small crystals (0.1-0.15 mm) are often formed, which leads to an increase in caking and adsorption contamination of the product, and also worsens its filterability. Therefore, in order to enlarge the product, after the mentioned apparatuses, so-called crystal dissolvers are installed, in which the concentrated suspension is kept under slow cooling, which leads to growth up to 2-3 mm.
To obtain coarse-grained homogeneous products, fluidized-bed crystallizers are often used (Fig. 7). The initial solution, together with the circulating clarified mother liquor, is pumped into the heat exchanger, where, as a result of cooling, the solution is supersaturated and flows through the circulation pipe to the lower part of the crystal dissolver, in which the crystals are kept in suspension by the upward flow of the solution. Crystallization occurs mainly on ready-made crystallization centers, while large crystals are deposited on the bottom of the apparatus, from where they are removed in the form of a thickened suspension. The clarified mother liquor is divided into two parts: one is removed from the upper part of the apparatus, the other is fed to recirculation.

Rice. 7. Fluidized bed crystallizer: I pump: 2 heat exchanger: 3 circulation pipe; 4 crystal solvent.
In a number of cases, the crystallization of solutions is carried out by direct mixing them with liquid, gaseous and evaporating refrigerants in mixing, bubbling, spraying, and other apparatuses. If the solubility of a substance changes little with temperature (for example, NaCl in water), crystallization is carried out by partial or almost complete evaporation of the solvent by evaporating a saturated solution at almost constant temperature(isothermal crystallization). By design, evaporator crystallizers largely resemble evaporators and can have internal or external (Fig. 8) heating chambers. In such a crystallizer, the initial and circulating solutions, passing through the chamber, are heated to the boiling point. The resulting vapor-liquid mixture enters the separator, where the vapor is separated from the solution. The crystals deposited in the separator, together with the mother liquor, are sent to a special apparatus, in which they are separated from it and removed in the form of a concentrated suspension;
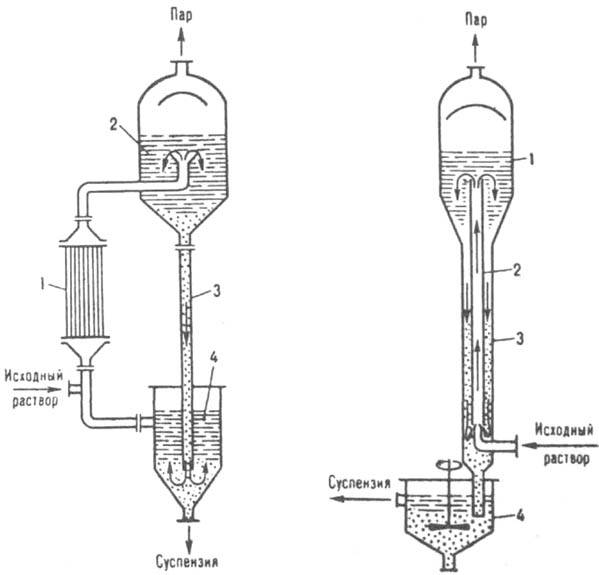
Rice. 8. Evaporating crystallite mountains: 1 remote heating chamber: 2 separator: 3 circulation pipe; 4 crystal separator.
Rice. 9. Vacuum crystallizer: 1 - separator: 2 - circulation pipe: 3 barometric pipe; 4 water seal
the clarified mother liquor is returned to the chamber. To prevent incrustation (fouling) of heating surfaces, the solution must circulate in the mold at a sufficiently high speed (up to 3 m/s), which is often achieved by using axial pumps.
With simultaneous cooling and evaporation of the solvent, crystallization is carried out in batch or continuous vacuum crystallizers, with forced or natural circulation of the solution. The solution is cooled due to the adiabatic evaporation of part of the solvent when a certain vacuum is created in such an apparatus. The amount of solvent evaporated is usually 8-10% of the total mass of the solution. In a crystallizer with natural circulation (Fig. 9), the initial solution is fed into the lower part of the circulation pipe and, together with the circulating suspension, rises up, where it boils as a result of a decrease in pressure. The resulting vapors pass through the separator and enter the barometric condenser. The supersaturated solution and the precipitated crystals move down the barometric tube, from where the crystals, together with part of the mother liquid, are discharged into the hydraulic seal. Vacuum pumps or steam jet injectors are used to maintain the vacuum. In large-scale production, multi-vessel vacuum crystallization plants with 4-24 housings are widely used, in which the depth of rarefaction gradually increases from the first housing to the last. Vacuum crystallizers are more efficient and economical than evaporator crystallizers.
Some substances can be crystallized by salting out. When isolating inorganic compounds, organic substances are used (for example, Na 2 SO 4 is crystallized by adding methanol, ethanol or NH 3 to its aqueous solution) or containing the same ion with the compound being isolated (for example, FeSO 4 is crystallized from etching solutions by adding concentrated H 2 SO4); when selecting org. compounds - water, aqueous solutions of inorganic salts, etc. The introduction of organic substances into the solution as salting out agents usually increases the cost of the process due to the complexity of their regeneration. Vapor phase crystallization allows the crystallization of substances with a high partial pressure vapors above the solid phase and capable of directly passing from a gaseous state to a crystalline one (for example, iodine, phthalic anhydride). Such crystallization is used to isolate valuable components from vapor-gas mixtures, obtain aerosols, deposit thin crystalline layers on the surface of various bodies (for example, in the production of semiconductor materials), etc. Crystallization of the amorphous solid phase and recrystallization is carried out, as a rule, at temperatures close to the temperatures of the crystallized substances. In this case, as a result of thermal diffusion processes, the primary crystalline structure of the substance changes or nucleation and growth from the amorphous phase occur. Such crystallization is used to obtain substances and materials with a given crystal structure or degree of crystallinity (thermoplastic polymers, glass, etc.). Lit.: Mullin, J.W. Crystallization, trans. from English, M., 1965; Magusevich LN. Crystallization from solutions in the chemical industry, M., 1968; Bamfort A V, Industrial crystallization, per. from English, M., 1969; Ponomarenko VG Tkachenko crystallization P., Kurlyand Yu. A., Crystallization in a fluidized bed. crystallization, 1972; Melikhov I.V., Merkulova M.S. Cocrystallization, M.. 1975; Gelperin N. I., Nosov G. A., Fundamentals of the technology of crystallization of melts, M., 1975; Kidyarov B. I., Kinetics of Education
SOLIDIFICATION OF CRYSTAL BODIES
As the temperature decreases, a substance can change from a liquid state to a solid state.
This process is called curing or crystallization.
During the solidification of a substance, the same amount of heat is released, which is absorbed during its melting.

The calculation formulas for the amount of heat during melting and crystallization are the same.

The melting and solidification temperatures of the same substance, if the pressure does not change, are the same.
Throughout the process of crystallization, the temperature of the substance does not change, and it can simultaneously exist in both liquid and solid states.
LOOK AT THE BOOKSHELF!
WOW, INTERESTING PHENOMENA!
Colored ice?
If you add a little paint or tea leaves to a plastic glass with water, stir and, having received color solution, wrap the glass on top and put it in the cold, then a layer of ice will begin to form from the bottom to the surface. However, don't expect to get colored ice!

Where the freezing of water began, there will be an absolutely transparent layer of ice. Its upper part will be colored, and even stronger than the original solution. If the concentration of paint was very high, then a puddle of its solution may remain on the surface of the ice.
The fact is that transparent fresh ice is formed in solutions of paint and salts. growing crystals displace any foreign atoms and molecules of impurities ,
trying to build the perfect grid as long as possible. Only when the impurities have nowhere to go, does the ice begin to build them into its structure or leave them in the form of capsules with a concentrated liquid. Therefore, sea ice is fresh, and even the dirtiest puddles are covered with transparent and clean ice.
At what temperature does water freeze?
Always at zero degrees?
But if boiled water is poured into an absolutely clean and dry glass and placed outside the window in frost at a temperature of minus 2-5 degrees C, covered with clean glass and protected from direct sunlight, then in a few hours the contents of the glass will cool below zero, but will remain liquid.
If you then open a glass and throw a piece of ice or snow or even just dust into the water, then literally before your eyes the water will instantly freeze, sprouting throughout the volume with long crystals.
Why? The transformation of a liquid into a crystal occurs primarily on impurities and inhomogeneities - dust particles, air bubbles, irregularities on the walls of the vessel. In pure
water does not have crystallization centers, and it can supercool, staying fluid. In this way, it was possible to bring the water temperature to minus 70°C.
How does it happen in nature?
In late autumn, very clean rivers and streams begin to freeze from the bottom. Through a layer of clear water it is clearly visible that algae and driftwood at the bottom are overgrown with a loose ice coat. At some point, this bottom ice emerges, and the surface of the water instantly turns out to be bound by an ice crust.
The temperature of the upper layers of water is lower than the deep ones, and freezing seems to start from the surface. However, pure water freezes reluctantly, and ice first of all forms where there is a suspension of silt and a solid surface - near the bottom.
Spongy masses often appear downstream of waterfalls and dam spillways. inland ice, growing in foamy water. Rising to the surface, it sometimes clogs the entire channel, forming the so-called zazhory, which can even dam the river.
Why is ice lighter than water?
Inside the ice there are many pores and gaps filled with air, but this is not the reason,
which can explain the fact that ice is lighter than water. Ice and without microscopic pores
still has a density less than that of water. It's all about features internal structure ice. In an ice crystal, water molecules are located at the nodes of the crystal lattice so that each has four "neighbors".
Water doesn't have crystal structure, and the molecules in the liquid are closer together,
than in a crystal, i.e. water is denser than ice.
First, when ice melts, the released molecules still retain the structure of the crystal lattice, and the density of water remains low, but gradually the crystal lattice is destroyed, and the density of water increases.
At a temperature of + 4°C, the density of water reaches a maximum, and then, with an increase in temperature, it begins to decrease due to an increase in the rate of thermal motion of molecules.
How does a puddle freeze?
When cooled, the upper layers of water become denser and sink down. Their place is taken by denser water. This mixing continues until the temperature of the water drops.
up to +4 degrees Celsius. At this temperature, the density of water is maximum.
With a further decrease in temperature, the upper layers of water can already be more compressed,
and gradually cooling to 0 degrees, the water begins to freeze.

In autumn, the air temperature at night and day is very different, so the ice freezes in layers.
The lower surface of the ice on a freezing puddle is very similar to the transverse tree trunk cut:
visible concentric rings. The width of the ice rings can be used to judge the weather. Usually a puddle
starts to freeze from the edges, because there is less depth. The area of the formed rings decreases with approach to the center.
INTERESTING!
That in the pipes of the underground part of buildings, water often freezes not in frost, but in thaw!
This is due to the poor thermal conductivity of the soil. Heat travels through the earth so slowly
that the minimum temperature in the soil occurs later than on the surface of the earth. The deeper
the more late. Often, during frosts, the soil does not have time to cool,
and only when a thaw sets in on the earth, frosts reach the earth.
That, freezing in a corked bottle, water breaks it. What happens to a glass if you freeze water in it? Water, freezing, will expand not only upwards, but also to the sides, and the glass will shrink. This will still lead to the destruction of the glass!
DID YOU KNOW?
There is a known case when the contents of a bottle of narzan well chilled in the freezer, opened on a hot summer day, instantly turned into a piece of ice.
The metal "cast iron" behaves interestingly, which expands during crystallization. This allows it to be used as a material for artistic casting of thin lace lattices and small table sculptures. After all, when solidifying, expanding, cast iron fills everything, even the most subtle details of the form.
In the Kuban, strong drinks are prepared in winter - “freezes”. To do this, the wine is exposed to frost. First of all, water freezes, and a concentrated solution of alcohol remains. It is drained and the operation is repeated until the desired strength is achieved. The higher the concentration of alcohol, the lower the freezing point.
The most hailstone, fixed by people, fell in Kansas, USA.
Its weight was almost 700 grams.
Oxygen in gaseous state at a temperature of minus 183 degrees C turns into a liquid,
and at a temperature of minus 218.6 degrees C, solid oxygen is obtained from liquid oxygen.

In the old days, people used ice to store food. Carl von Linde created the first home refrigerator powered by a steam engine that pumped freon gas through pipes. Behind the refrigerator, the gas in the pipes condensed and turned into a liquid. Inside the refrigerator, liquid freon evaporated and its temperature dropped sharply, cooling the refrigerator compartment. Only in 1923, Swedish inventors Balzen von Platen and Carl Muntens created the first electric refrigerator, in which freon turns from a liquid into a gas and takes heat from the air in the refrigerator.
THIS IS YES!
Several pieces dry ice, abandoned into burning gasoline, put out the fire.
There is ice that would burn fingers if it could be touched. It is obtained under very high pressure, at which water passes into solid state at temperatures well above 0 degrees Celsius.
As the temperature decreases, a substance can change from a liquid state to a solid state.
This process is called solidification or crystallization.
During the solidification of a substance, the same amount of heat is released, which is absorbed during its melting.

The calculation formulas for the amount of heat during melting and crystallization are the same.

The melting and solidification temperatures of the same substance, if the pressure does not change, are the same.
Throughout the process of crystallization, the temperature of a substance does not change, and it can simultaneously exist in both liquid and solid states.
LOOK AT THE BOOKSHELF
INTERESTING ABOUT CRYSTALLIZATION
Colored ice?
If you add a little paint or tea leaves to a plastic glass with water, stir it and, having received a colored solution, wrap the glass on top and expose it to frost, then a layer of ice will begin to form from the bottom to the surface. However, don't expect to get colored ice!

Where the freezing of water began, there will be an absolutely transparent layer of ice. Its upper part will be colored, and even stronger than the original solution. If the concentration of paint was very high, then a puddle of its solution may remain on the surface of the ice.
The fact is that transparent fresh ice is formed in solutions of paint and salts. growing crystals displace any foreign atoms and impurity molecules, trying to build a perfect lattice while it is possible. Only when the impurities have nowhere to go, does the ice begin to build them into its structure or leave them in the form of capsules with a concentrated liquid. Therefore, sea ice is fresh, and even the dirtiest puddles are covered with transparent and clean ice.
At what temperature does water freeze?
Is it always at zero degrees?
But if boiled water is poured into an absolutely clean and dry glass and placed outside the window in frost at a temperature of minus 2-5 degrees C, covered with clean glass and protected from direct sunlight, then in a few hours the contents of the glass will cool below zero, but remain liquid.
If you then open a glass and throw a piece of ice or snow or even just dust into the water, then literally before your eyes the water will instantly freeze, sprouting throughout the volume with long crystals.
Why?
The transformation of a liquid into a crystal occurs primarily on impurities and inhomogeneities - dust particles, air bubbles, irregularities on the walls of the vessel. Pure water has no centers of crystallization and can be supercooled while remaining liquid. In this way, it was possible to bring the water temperature to minus 70°C.
How does it happen in nature?
In late autumn, very clean rivers and streams begin to freeze from the bottom. Through a layer of clear water it is clearly visible that algae and driftwood at the bottom are overgrown with a loose ice coat. At some point, this bottom ice emerges, and the surface of the water instantly turns out to be bound by an ice crust.
The temperature of the upper layers of water is lower than the deep ones, and freezing seems to start from the surface. However, pure water freezes reluctantly, and ice first of all forms where there is a suspension of silt and a solid surface - near the bottom.
Downstream of waterfalls and dam spillways, there is often a spongy mass of in-water ice growing in churning water. Rising to the surface, it sometimes clogs the entire channel, forming the so-called zazhory, which can even dam the river.
Why is ice lighter than water?
Inside the ice there are many pores and gaps filled with air, but this is not the reason that can explain the fact that ice is lighter than water. Ice and without microscopic pores
still has a density less than that of water. It's all about the features of the internal structure of ice. In an ice crystal, water molecules are located at the nodes of the crystal lattice so that each has four "neighbors".
Water, on the other hand, does not have a crystalline structure, and molecules in a liquid are located closer than in a crystal, i.e. water is denser than ice.
First, when ice melts, the released molecules still retain the structure of the crystal lattice, and the density of water remains low, but gradually the crystal lattice is destroyed, and the density of water increases.
At a temperature of + 4°C, the density of water reaches a maximum, and then, with an increase in temperature, it begins to decrease due to an increase in the rate of thermal motion of molecules.
How does a puddle freeze?
When cooled, the upper layers of water become denser and sink down. Their place is taken by denser water. Such mixing occurs until the water temperature drops to +4 degrees Celsius. At this temperature, the density of water is maximum.
With a further decrease in temperature, the upper layers of water can already shrink more, and gradually cooling down to 0 degrees, the water begins to freeze.

In autumn, the air temperature at night and day is very different, so the ice freezes in layers.
The bottom surface of ice on a freezing puddle is very similar to a cross section of a tree trunk:
concentric rings are visible. The width of the ice rings can be used to judge the weather. Usually the puddle starts to freeze from the edges, because. there is less depth. The area of the formed rings decreases with approach to the center.
INTERESTING
That in the pipes of the underground part of buildings, water often freezes not in frost, but in thaw!
This is due to the poor thermal conductivity of the soil. Heat passes through the earth so slowly that the minimum temperature in the soil occurs later than on the surface of the earth. The deeper, the more late. Often, during frosts, the soil does not have time to cool, and only when a thaw sets in on the ground does frost reach the ground.
That, freezing in a corked bottle, water breaks it. What happens to a glass if you freeze water in it? Water, freezing, will expand not only upwards, but also to the sides, and the glass will shrink. This will still lead to the destruction of the glass!
DID YOU KNOW
There is a known case when the contents of a bottle of narzan well chilled in the freezer, opened on a hot summer day, instantly turned into a piece of ice.
The metal "cast iron" behaves interestingly, which expands during crystallization. This allows it to be used as a material for artistic casting of thin lace lattices and small table sculptures. After all, when solidifying, expanding, cast iron fills everything, even the most subtle details of the form.
In the Kuban, strong drinks are prepared in winter - “freezes”. To do this, the wine is exposed to frost. First of all, water freezes, and a concentrated solution of alcohol remains. It is drained and the operation is repeated until the desired strength is achieved. The higher the concentration of alcohol, the lower the freezing point.
The largest hailstone recorded by people fell in Kansas, USA. Its weight was almost 700 grams.
Oxygen in a gaseous state at a temperature of minus 183 degrees C turns into a liquid, and at a temperature of minus 218.6 degrees C, solid oxygen is obtained from liquid






