The arrangement of electrons by energy levels is a table. Arrangement of electrons in energy levels
|
l= 0, 1, ... to n-1 |
m = - l; 0;+ l | |||
|
l= 0(s) | ||||
|
l= 0(s), l= 1(p) |
m = -1, 0, +1 |
| ||
|
l= 0(s), l= 1(p) l= 1(d) |
m = -1, 0, +1 m = -2, -1, 0, +1,+2 |
|
Note: The table shows the maximum possible number of electrons in the energy level.
Electronic formula
Electrons fill the orbitals of an atom in order of increasing energy. The closer the orbital is to the nucleus (energy level with a lower number), the stronger the electron is attracted to the nucleus, the more favorable such an arrangement is for it.
For example, the atomic number of a carbon atom is six, which means that the nuclear charge is +6, the number of electrons is also six. These electrons are located on two energy levels (period number). The first energy level of the carbon atom is completely filled: there are two electrons with opposite spins (1s 2) in a single orbital (see Table 7). The remaining four electrons occupy the second energy level, with two in their preferred s orbitals and two in p orbitals (2s 2 2p 2).
Distribution of electrons over energy levels and sublevels obey:
1. Pauli principle
2. Klechkovsky's rule
3. Hund's rule.
Pauli principle. In 1925, P. Pauli postulated the principle that an atom cannot have two electrons that have exactly the same set quantum numbers: n l, m, s. It follows that each orbital cannot have more than two electrons, and they must have opposite spins, i.e. padding is allowed and padding and is not allowed.
V. Klechkovsky's rule. The increase in energy and, accordingly, the filling of the orbital occurs in the order of increasing the sum of quantum numbers n + l, and when equal to the sum n+ l in ascending order of n.
For example, Ca +20)))) 1s 2s 2p 3s 3p 3d 4s 4p 4d
sequence of energy sublevels
if s = 0, p = 1, d = 2
Consequently, the filling of levels and sublevels with electrons occurs in the order of increasing sum of n and l: 1s 2 2s 2 2p 6 3s 2 3p 6 3 d 0 4s 2
The exception is some d and f elements, in which the so-called dip is observed (the dip of an electron from the s sublevel to the preexternal d or f). For example, Cu, Ag, Cr, Pd, Pt.
Hund's rule.The filling of orbitals p, d, f sublevels in the normal state of the atom begins with single electrons with the same spins. After single electrons occupy all the orbitals of the sublevel, the orbitals are filled with second electrons with opposite spins. For example, electronic structure nitrogen atom has the following structure 1s2s2p
The properties of the elements depend on the electronic configuration of the valence electrons. Valence electrons, as a rule, are in the outer energy level (period number), the number of valence electrons can be determined by the group number (with the exception of d elements of group VIII). For example, a nitrogen atom has five valence electrons (V group), which are located on the second energy level (the element is in the second period).
All elements of the PSE according to the structure of valence electrons can be divided into s-, p-, d-, f- elements, otherwise they are called families:
s-elements are in groups I, II (main subgroups)
p-elements are in groups III-VIII (main subgroups)
d-elements are in groups I-VIII (side subgroups)
f-elements are classified as lanthanides and actinides.
All elements of the family have a similar structure:
s-elements in the unexcited state have valence electrons only at the s-sublevel.
p-elements in the unexcited state have valence electrons in the outer s and p-sublevels.
d-elements in the unexcited state have valence electrons in the outer s and pre-outer d-sublevels.
The similar electronic configuration of the elements of the family ensures the similarity of the chemical properties of the elements of this family.
DEFINITION
Scandium is located in the fourth period of group III of the secondary (B) subgroup of the Periodic Table.
Refers to elements of the d-family. Metal. Designation - Sc. Ordinal number - 21. Relative atomic mass- 44.956 amu
The electronic structure of the scandium atom
The scandium atom consists of a positively charged nucleus (+21), inside which there are 21 protons and 24 neutrons, and 21 electrons move around in four orbits.
Fig.1. Schematic structure of the scandium atom.
The distribution of electrons in orbitals is as follows:
1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2 .
The outer energy level of the scandium atom contains 3 electrons, which are valence. The oxidation state of scandium is +3. The energy diagram of the ground state takes the following form:
Examples of problem solving
EXAMPLE 1
EXAMPLE 2
| Exercise | The element gallium has two natural isotopes 69 Ga and 71 Ga. Calculate the mass fractions of these isotopes in natural gallium if the relative atomic mass of the element is 69.72. |
| Solution | Isotopes are atoms of the same chemical element that have different mass numbers(the same number of protons, but different - neutrons). Let us take as x the number of atoms of the gallium isotope 69 Ga in every hundred atoms of natural chlorine, then the number of atoms of the isotope 71 Ga will be equal to (100-x). The mass of atoms of the isotope 69 Ga will be equal to 69x, and 71 Ga - 71 × (100-x). Let's make an equation: 69x + 74x(100x) = 69.72x100%. Let's find x: 69x + 7400 - 74x = 6972; |
| Answer | The content of the 69 Ga isotope in natural chlorine is 85.6%, and that of the 71 Ga isotope is 14.4%. |
I. Electronic formulas of atoms chemical elements are made up in the following order:
· First, by the number of the element in the table of D. I. Mendeleev, the total number of electrons in the atom is determined;
· Then, according to the number of the period in which the element is located, the number of energy levels is determined;
· The levels are divided into sublevels and orbitals, and filled with electrons in accordance with The principle of least energy
· For convenience, electrons can be divided into energy levels using the formula N=2n2 and considering that:
1. at the elements main subgroups ( s-; p -elements), the number of electrons in the outer level is equal to the group number.
2. at the elements side subgroups usually on the outside two electron (with the exception of atomsCu, Ag, Au, Cr, Nb, Mo, Ru, Rh, whose outer level one electron, atPdat the outer level zero electrons);
3. the number of electrons in the penultimate level is total number electrons in an atom minus the number of electrons in all other levels.
II. Electron filling order atomic orbitals determined :
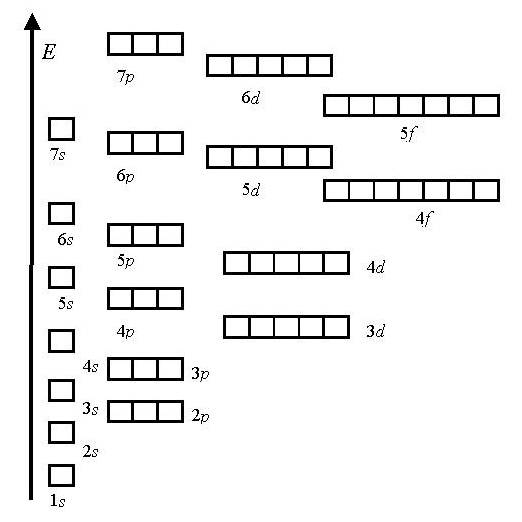
1.Principle of least energy
Energy scale:
1s<2s<2p<3s<3p<4s<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s…
2. The state of an atom with a completely or half-filled sublevel (ie, when there is one unpaired electron in each orbital) is more stable.
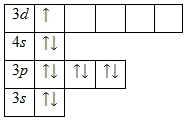
This explains the "failure" of the electron. Thus, the following distribution of electrons corresponds to the stable state of the chromium atom:
Cr: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 , but not 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4 ,
i.e. there is a "failure" of the electron from 4 s-sublevel to 3 d-sublevel.
CHECK YOURSELF: Electronic formulas of elements.
Download the module : training apparatus“Filling the electron shells of atoms of elements” contains a periodic table of chemical elements and a diagram depicting the distribution of electrons in an atom.
You are invited to determine the chemical element by the electronic structure of the atom.
1. Determine in the main subgroups of which groups of the periodic system there are chemical elements whose electronic schemes of atoms are shown in the first column of the table.
Electronic circuits Groups 1 2 3 4 5 6 7 2nd 4th SCH AND E X BUT B At 2nd 8th 2nd AT And To M H O P 2nd 1st M FROM S F YU BUT I 2nd 8th 5th R B T AT And E G 2nd 8th 1st H D AND And To Z M 2nd 8th 7th L O AT H At P E 2nd 8th 3rd R T FROM X C H F 2nd 3rd SCH S To E M H To 2nd 8th 6th O I BUT AT FROM And O 2nd 5th YU P R M Y AT X 2. Determine in the main subgroups of which groups of the periodic system are chemical elements, the number of protons and neutrons of which are given in the first column of the table.
Number of protons and neutrons Groups 1 2 3 4 5 6 7 13r 14n BUT G E G O FROM B 12p 12n AT L SCH To And P H 15r 16n B O P E E To At 19р 20n M C At BUT D S E 17p18n Y Z F L Z L E 6p 6n To M I H YU YU I 8p 8n P FROM T D H T AT
Download the module and run teats on the topic "Periodic law and the periodic system of chemical elements
D. I. Mendeleev and the structure of the atom"
Download the module and run t eats on the topic "Basic information about the structure of atoms." The module includes 10 test questions on the topic. In particular, you are invited to construct the electronic formula of the sulfur atom by transferring numerical and alphabetic designations to the appropriate zone.
Tasks for fixing
3. What is the composition of the nucleus of an atom?
5. Compare isotopes.
6. Write down the scheme for the formation of the Na +1 ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the scheme for the formation of the O -2 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes.
6. Write down the formation scheme of the S-2 ion
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the scheme for the formation of the Mg +2 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the formation scheme of the Si +4 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the scheme for the formation of the Al +3 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the scheme for the formation of the Cl -1 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
1. Write down the position of the element in the periodic system of the chemical element of D.I. Mendeleev.
2. Determine the charge of the nucleus, the total number of electrons.
3. What is the composition of the nucleus of an atom?
4. Draw a diagram of the distribution of electrons in layers (levels).
5. Compare isotopes ![]() .
.
6. Write down the scheme for the formation of the K +1 ion.
7. Draw a diagram of the distribution of electrons over layers (levels) in an ion.
The periodic system of chemical elements is a strictly ordered set of chemical elements, their natural classification, which is a tabular or other graphical expression of the periodic law.
A large number of different versions of the tables of the periodic system are known. However, only those of them that turned out to be close to the table compiled by D.I. Mendeleev. Currently, two forms of the periodic system are mainly used: long-period and short-period.
The vertical graphs of the periodic system are called groups, and the horizontal ones periods. The short period form consists of eight groups and seven periods. The families of lanthanides and actinides are located below the table.
Period - a successive series of elements in whose atoms the same number of energy levels are filled with electrons (the number of levels is equal to the number of the period). Periods contain 2, 8, 8, 18, 32 and 32 elements. The last period is not completed. The different number of elements in the periods is explained by the different sequence of filling the energy sublevels. Elements that are filled with electrons s- sublevel are called s- elements for which it is filled R- sublevel - R- elements for which it is filled d- and f- sublevels - respectively d- and f- elements. The periods begin with alkali metals, in the atoms of which, at a new electronic level, s- electron. Periods end with elements whose atoms are completely filled p- the sublevel of the outer level with six electrons (inert gases), except for the first period, ending with helium, in which the level is built up by 2 s- electrons. In the 4th and 5th periods between s- and p- elements placed 10 d- elements, and in the 6th and 7th periods - 10 d- elements and 14 f- elements.
The periodic system consists of 8 groups (or 32 groups in the long period version of the system). Groups are designated by Roman numerals I - VIII and consists of two subgroups: BUT and B.
Each group consists of elements whose atoms have a similar structure of electron shells. For example, potassium 4 s 1 (IA), and copper - 3d 10 4 s 1 (I B). These elements on the outer 4 s- Each sublevel has one electron. Elements that are part of the same group are called electronic analogues.
Elements that are part of one subgroup are full electronic analogues, since they have the same structure of electronic levels. For example, elements I A groups have the general formula ns 1 , and the elements I B -(n-1)d 10 ns 1 .
Since the publication of the Periodic Table of Elements, more than 40 new elements have appeared in it. Based on the periodic law, transuranic elements with atomic numbers from 93 to 105 (15 in total) were obtained artificially. Their production is based on the use of the heaviest element existing on Earth - uranium-238, the nuclei of which are “built on” by bombardment with neutrons. This is how elements up to fermium are obtained 100 Fm 257 in nuclear reactors. Transuranides are also formed in thermonuclear explosions.
All elements with atomic numbers over 100 are produced in accelerators. Heavy atomic nuclei (targets) are bombarded with heavy ions. After the emission of neutrons, both of them merge, forming new elements with the sum of the atomic numbers of the components. The periodic system evolves, new elements are discovered. According to the theory of probability, calculated using modern computer technology, it is predicted that the 8th period should contain 50 elements, among which there will be a new group of chemically similar elements, consisting of 18 elements, so far called octadecanoids (Z=121-138). The upper limit of possible stability, as it can be determined by the current level of knowledge, is close to atomic number 174. However, this is not the limit, since, for example, from the Seaborg stability diagram of chemical elements, the existence of elements with atomic numbers up to 500 is possible.