The liquid state of matter is the property of liquids. Terms, definitions and parameters. If the capillaries have a circular cross section, then
In the liquid state, the distance between the particles is much smaller than in the gaseous state. Particles occupy the bulk of the volume, constantly in contact with each other and are attracted to each other. Some ordering of particles (short range order) is observed. The particles are moving relative to each other.
In liquids, van der Waals interactions arise between particles: dispersion, orientation, and induction. Small groups of particles united by certain forces are called clusters. In the case of identical particles, clusters in a liquid are called associates
When immersed in rock, a volume of water equal to the volume of rock is displaced. In comparison, a block of wood acts in a more subtle way, sinking into water under the force of gravity until it displaces a volume of water equal to its own weight.
Evaporation and condensation of liquids
When all forces stabilize, the tree stops descending. This is then called the Archimedes Principle as it was conceived by the ancient Greek mathematician, physicist, astronomer and philosopher Archimedes. Archimedes. The principle further states that the forces supporting the wooden block will focus upward and inward on it, culminating at a point called the center of buoyancy. Always above the center of mass of a floating object, the location of the center of buoyancy is capable of indicating the object's stability.
In liquids, the formation of hydrogen bonds increases the ordering of particles. However, hydrogen bonds and van der Waals forces are fragile - molecules in the liquid state are in continuous chaotic motion, which is called brownian motion.
For the liquid state, the distribution of molecules according to the velocities and energies of Maxwell-Boltzmann is valid.
The greater the distance between the center of buoyancy and the center of mass, the more stable the object is, that is, the less likely it is to capsize. Another characteristic feature liquids is that if a denser liquid is poured into a less dense liquid and the two do not mix, they will separate, leaving the less dense liquid on top and the denser liquid on the bottom. The classic example of this is the separation that occurs with oil and water.
Submarines are a great example of buoyancy at work. While traveling on the surface of the ocean, a submarine has an average density slightly less than that of water. However, when he goes to dive, the submarine needs to take on water. This then increases its density beyond the water, resulting in its ability to flow.
The theory of liquids is much less well developed than that of gases, since the properties of liquids depend on the geometry and polarity of closely spaced molecules. In addition, the lack of a definite structure of liquids makes it difficult to formalize their description - in most textbooks, liquids are given much less space than gases and crystalline solids.
In order to rise to the surface again, the submarine must blow through the water tanks. It accomplishes this task by blowing air into them, which in turn displaces water and reduces the density of the submarine. Liquid pressure and surface tension.
While not necessarily an obvious fact, regardless of the depth from which it falls, the actual buoyancy of the submarine is not altered in any way. This is because water is essentially incompressible and its density is virtually the same at any depth. But even though density cannot change, there is an endpoint imposed by water pressure, the mass of water above an object that increases linearly with added depth.
There is no sharp boundary between liquids and gases - it completely disappears in critical points . For each gas, the temperature is known, above which it cannot be liquid at any pressure; with this critical temperature, the boundary (meniscus) between the liquid and its saturated steam. The existence of a critical temperature ("absolute boiling point") was established by D.I. Mendeleev in 1860
So if you double the depth of a submarine, you also double the pressure. While it's not such a hard concept to understand, what's not entirely obvious is that water pressure is the same regardless of direction. For example, if you were to press down on the top of a tank of water by submerging it, but ignored the idea that as the tank went deeper and deeper, the pressure acting on all sides and on the bottom of the tank was the same as pressing on the top part of the tank, you would be surprised how heavy the tank becomes.
However, if you were to open a valve at the bottom of the tank, water would flow out in the stream, reducing the overall pressure both inside and outside the tank. Plus, since the water is now moving, it has kinetic energy. And, as in accordance with Torricelli's principle, than more pressure on the valve, the more kinetic energy flow and the greater its speed.
Table 7.2 - Critical parameters (t k, p k, V k) of some substances
| Substance | t to, about C | p k, atm | V to, cm 3 / mol | t melt o C | t bale about C |
| He | -267,9 | 2,26 | 57,8 | -271,4 | -268,94 |
| H2 | -239,9 | 12,8 | 65,0 | -259,2 | -252,77 |
| N 2 2 | -147,0 | 33,54 | 90,1 | -210,01 | -195,82 |
| O 2 2 | -118,4 | 50,1 | -218,76 | -182,97 | |
| CH 4 | -82,1 | 45,8 | 99,0 | -182,49 | -161,58 |
| CO2 | +31,0 | 72,9 | 94,0 | -56,16 | -78.48(subl) |
| NH3 | 132,3 | 111,3 | 72,5 | -77,76 | -33,43 |
| Cl2 | 144,0 | 76,1 | -101,0 | -34,06 | |
| SO2 | 157,5 | 77,8 | -75,48 | -10,02 | |
| H2O | 374,2 | 218,1 | 0,0 | 100,0 |
Pressure saturated vapors – partial pressure, at which the rates of evaporation and condensation of steam are equal:
Recall that the main characteristics of the liquid are as follows. They are liquid-like substances that have the ability to flow and do not have the ability to reconfigure their former shape after deformation. They can take the form of any container into which they are poured.
They have similar properties to solids in terms of color consistency and density. They have a tendency to look for a common level. What we see around us, living or non-living, is made up of matter. "Matter" can be defined as "any substance that has and occupies space". Matter has different forms. There are three different forms of matter; solids, liquids and gases. They differ from each other depending on the specific feature characteristic of each form, which depends on the molecules in different forms matter.
where A and B are constants.
Boiling temperature is the temperature at which the saturated vapor pressure of a liquid is equal to atmospheric pressure.
Liquids have fluidity- the ability to move under the action of small shear forces; the liquid occupies the volume in which it is placed.
The resistance of a fluid to flow is called viscosity[Pa. With].
Characteristics of each substance; solid, liquid, or depends on the different attraction between these molecules. In this article, we will discuss the differences between the two forms of matter, liquids and gases. There are four main points of distinction by which we can distinguish between liquids and gases at the most basic level; shape, volume, hardness and ability to flow.
Liquids The molecules of liquids have a moderate force of attraction; the force between molecules is less solids and more gases. This results in easier and freer movement of molecules within liquids. Molecular motion results in liquids having a defined and fixed volume. Liquids take on the shape of the container they are stored in as molecules move around to fill the space. They do not have a definite shape and have the ability to flow. Liquids may flow; thus, they are also referred to as "liquid".
Surface tension [J / m 2] - the work required to create a unit of surface.
liquid crystal state- substances in the liquid state, with a high degree of order, occupy an intermediate position between crystals and liquid. They have fluidity, but at the same time they have a long-range order. For example - derivatives of brown acid, azoliths, steroids.
Liquids are not hard. For example, when frozen below 0 degrees Celsius, it solidifies on ice. Some examples of liquids are water, oils, milk, juices, etc. Gases Molecules in gases have a very weak attractive force between them and are very loosely packed. Thus, they do not have a specific shape, and they take the form of a container. Due to their molecular structure, gases also do not have a specific volume and become the volume of the container in which they are stored. Gases can flow easily; they can be shown simply by lighting incense.
Clearance temperature- the temperature at which liquid crystals (LC) pass into the usual liquid state.
7.5 Solids
AT solid state particles are so close to each other that strong bonds arise between them, there is no forward movement and fluctuations persist around its position. Solids can be in an amorphous and crystalline state.
Properties of liquid films
The smell of incense moves from one part of the room to another. They can be easily compressed as they have a lot of space between molecules. Liquids have less attractive force between molecules than solids and more gases; gases have a very weak force of attraction between molecules, which is the least of the three states of matter. Liquids have a certain volume; gases do not have a definite volume. Liquids cannot be easily compressed; gases can be easily compressed.
Everything on Earth is made of matter, but this matter is not always the same. Matter can exist in four different phases, and kinetic theory matter helps us understand the differences between these phases. Solids, liquids, gases and plasmas: these words should be familiar to you because they are four phases of matter that can be different, that can deal with different forms. What exists is that many substances can exist as more than one phase. Take water for example: water can exist as a solid, liquid and gas.
7.5.1 Substances in the amorphous state
In the amorphous state, substances do not have an ordered structure.
vitreous state - a solid amorphous state of a substance, which is obtained as a result of deep supercooling of a liquid. This state is nonequilibrium, but glasses can exist for a long time. Glass softening occurs in a certain temperature range - the glass transition range, the boundaries of which depend on the cooling rate. With an increase in the rate of cooling of a liquid or vapor, the probability of obtaining a given substance in a glassy state increases.
The difference between these states is the amount of energy. Solid particles have the least amount of energy, which is part of why their particles are so closely related. Liquids have more energy than solids, so they will take on the shape of their container, but only up to the surface.
Mechanical properties of the liquid
Gases have even more energy than liquids. Especially since their particles spread out to fill the entire space of their container. Gas particles have so much energy that they simply cannot stay still. They fly in all directions, placing as much distance as possible between themselves and the rest of the gas particles.
At the end of the 60s of the XX century, amorphous metals (metallic glasses) were obtained - for this it was necessary to cool the molten metal at a speed of 10 6 - 10 8 deg / s. Most amorphous metals and alloys crystallize when heated above 300 ° C. One of the most important applications is microelectronics (diffusion barriers at the metal-semiconductor interface) and magnetic storage devices (FMD heads). The latter is due to the unique magnetic softness (the magnetic anisotropy is two orders of magnitude less than in conventional alloys).
Plasmas are ionized gases and are uncommon in their natural form on Earth. You have seen them as artificial things like neon signs and fluorescent light bulbs. But in the rest of the universe, plasma is actually the most common phase of matter! Most stars are plasma, like the aurora borealis you see around the polar regions. Plasma only exists under certain conditions, so we'll end our discussion of that here for this tutorial.
The kinetic theory of matter states that all matter is made up of small particles that are in random motion and have space between them. This means that no matter what the phase material is, it is composed of individual moving particles.
Amorphous substances isotropic, i.e. have the same properties in all directions.
7.5.2 Substances in the crystalline state
Solid crystalline substances have an ordered structure with repeating elements, which allows them to be studied by diffraction x-rays(method of X-ray diffraction analysis, used since 1912.
This theory sounds pretty simple, but it actually explains a lot about physical properties matter and its actions. You may be surprised to learn that the particles of a solid are actually moving, it just isn't enough for you. This type of vibratory motion is why the solid didn't change shape no matter what container you put it in.
Remember how liquid particles have more energy than solid particles? The extra energy in this state allows the particles to move more freely, and they spread out more than solid particles, which creates more space between these particles. This is why the liquid will take the shape of its container up to its surface.
Single crystals (single compounds) are characterized by anisotropy - the dependence of properties on the direction in space.
The regular arrangement of particles in solid body shown as a crystal lattice. Crystalline substances melt at a certain temperature called melting point.
And since gases have even more energy than liquids, their particles move much more. This is why the gas will expand to fill the entire container, not just its surface like a liquid. Not only do the particles of a solid body not move very much, but they also touch each other very strongly by strong attractive forces. These forces hold particles in place and are what give a solid a fixed size and shape.
On the other hand, gas particles are so far apart that the forces of attraction between them are considered to be negligible. The gas particles are considered independent of each other, which means that the gas is opposite solid matter and has no fixed size or shape.
Crystals are characterized by energy, crystal lattice constant and coordination number.
Permanent lattice characterizes the distance between the centers of particles occupying nodes in the crystal in the direction of the characteristic axes.
coordination number usually called the number of particles directly adjacent to a given particle in a crystal (see Figure 7.2 - coordination number eight for both cesium and chlorine)
Since the movement of liquid particles is between a solid and a gas, the forces of attraction between its particles are also in the middle range of the other two phases. Liquid particles have more freedom than solid particles, so the liquid can flow freely. This means that, like a gas, a liquid does not have a fixed shape. But since the particles are not as free as the particles of a gas, the liquid has a fixed volume.
The kinetic theory of matter is also useful in explaining why substances can change phase under certain conditions. You know that water can be solid, liquid or gaseous, but how does it work? A phase change occurs when energy is added to or removed from a substance, usually in the form of heat.
The energy of the crystal lattice called the energy required to destroy one mole of a crystal and remove particles beyond the limits of their interaction.
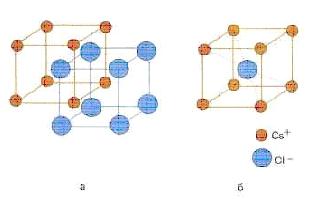
Figure 7.2 - The structure of a cesium chloride CsCl crystal (a) and the body-centered cubic unit cell of this crystal (b)
7.5.3 Crystal structures
The smallest structural unit of a crystal, which expresses all the properties of its symmetry, is elementary cell. With repeated repetition of the cell in three dimensions, a crystal lattice is obtained.
There are seven basic cells: cubic, tetrahedral, hexagonal, rhombohedral, orthorhombohedral, monoclinic, and triclinic. There are seven derivatives of the basic unit cells, for example, body-centered, cubic, face-centered.
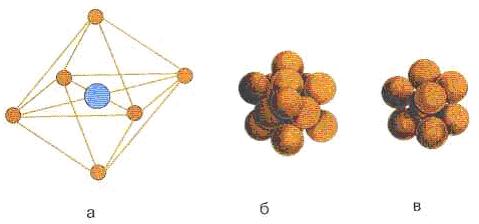
a - unit cell of NaCl crystal; b - dense face-centered cubic packing of NaCl; c - body-centered cubic packing of CsCl crystal Figure Figure 7.3 - Unit cell
Isomorphic substances- substances of similar chemical nature, forming the same crystal structures: CaSiO 4 and MgSiO 4
Polymorphism– compounds that exist in two or more crystal structures, such as SiO 2 (as hexagonal quartz, rhombic tridymite and cubic cristoballite.)
Allotropic modifications- polymorphic modifications simple substances, for example, carbon: diamond, graphite, carbine, fullerene.
By the nature of the particles in the nodes of the crystal lattice and chemical bonds between them, the crystals are subdivided into:
1) molecular- at the nodes there are molecules, between which van der Waals forces act, which have low energy: ice crystals;
2) atomically- covalent crystals- at the nodes of the crystals are atoms that form strong with each other covalent bonds, have a high lattice energy, for example, diamond (carbon);
3) ionic crystals- the structural units of crystals of this type are positively and negatively charged ions, between which electrical interaction, characterized by a sufficiently high energy, for example NaCL, KCL;
4) metal crystals- substances that have high electrical conductivity, thermal conductivity, malleability, plasticity, metallic glare and high reflectivity with respect to light; the bond in crystals is metallic, the energy of the metallic bond is intermediate between the energies of covalent and molecular crystals;
5) mixed bond crystals– there are complex interactions between particles that can be described by superimposing two or more types of bonds on top of each other, for example, clathrates (compounds are included) – formed by the inclusion of molecules (guests) in the cavity of a crystal framework consisting of particles of a different type (hosts): gas clathrates CH four . 6H 2 O, urea clathrates.
Liquid, occupying an intermediate position between gases and crystals, combines the properties of both types of these bodies..
1. Like a solid, a liquid slightly compressible due to the dense arrangement of molecules. (However, if water could be completely released from compression, then the water level in the world ocean would rise by 35 m and water would flood 5,000,000 km 2 of land.)
2. Like a solid, a liquid saves volume but like a gas takes the form of a vessel .
3. For crystals typical long range order in the arrangement of atoms (crystal lattice), for gases- full chaos. For liquid there is an intermediate state short range order , i.e. the arrangement of only the nearest molecules is ordered. When moving away from this molecule at a distance of 3–4 effective molecular diameters, the order is blurred. Therefore, liquids are close to polycrystalline bodies, consisting of very small crystals (about 10 9 m), arbitrarily oriented relative to each other. Due to this, the properties of most liquids are the same in all directions (and there is no anisotropy, as in crystals).
4. Most liquids, like solids, with increasing temperature increase their volume , while reducing its density (at a critical temperature, the density of a liquid is equal to the density of its vapor). Water is different famous anomaly , consisting in the fact that at +4 С water has a maximum density. This anomaly is explained by the fact that water molecules are partially assembled into groups of several molecules (clusters), forming peculiar large molecules. H 2 O, (H 2 O) 2 , (H 2 O) 3 … with different density. At different temperatures, the ratio of the concentrations of these groups of molecules is different.
Exist amorphous bodies (glass, amber, resins, bitumen...), which are usually considered as supercooled liquids with a very high viscosity. They have the same properties in all directions (isotropic), short-range order in the arrangement of particles, they do not have a melting point (when heated, the substance gradually softens and passes into a liquid state).
Used in technology magnetic fluids - these are ordinary liquids (water, kerosene, various oils), into which (up to 50%) are introduced the smallest particles (several microns in size) of a solid ferromagnetic material (for example, Fe 2 O 3). The movement of the magnetic fluid and its viscosity can be controlled by a magnetic field. In the strong magnetic fields magnetic fluid hardens instantly.
Some organic substances, the molecules of which have a filamentous form or the form of flat plates, can be in a special state, possessing both the properties of anisotropy and fluidity. They're called liquid crystals . To change the orientation of the molecules of a liquid crystal (in this case, its transparency changes), a voltage of about 1 V and a power of the order of microwatts are required, which can be provided by direct supply of signals from integrated circuits without additional amplification. Therefore, liquid crystals are widely used in electronic clock indicators, calculators, and displays.
When freezing, water increases in volume by 11%, and if water freezes in a closed space, a pressure of 2500 atmospheres can be reached (water pipes, rocks are destroyed ...).
withdrawals one of the biggest: 1) the dielectric constant(therefore, water is a good solvent, especially salts with ionic bonds - the entire periodic table is contained in the World Ocean); 2) heat of fusion(slow melting of snow in spring); 3) heat vaporization; 4) surface tension; 5) heat capacity(mild coastal climate).
Exists light (1 g / cm 3) and heavy (1.106 g/cm3) water . Light water ("living") - biologically active - it is protium oxide H 2 O. Heavy water ("dead") - suppresses the vital activity of organisms - it is deuterium oxide D 2 O. Protium (1 amu), deuterium (2 amu) and tritium (3 amu) are isotopes of hydrogen. There are also 6 isotopes of oxygen: from 14 O up to 19 O that can be found in a water molecule.
In water treatment magnetic field its properties change: wettability changes solids, their dissolution is accelerated, the concentration of dissolved gases changes, the formation of scale in steam boilers is prevented, the hardening of concrete is accelerated by 4 times and its strength increases by 45%, there is a biological effect on humans (magnetic bracelets and earrings, magnetolights, etc.) and plants (germination and crop yields increase).
silver water can be stored for a long time (about six months), since water is neutralized from microbes and bacteria by silver ions (it is used in astronautics, for canning food, disinfecting water in pools, for medicinal purposes to prevent and combat gastrointestinal diseases and inflammatory processes).
Drinking water disinfection in city water pipes carried out by chlorination and ozonation of water. There are also physical methods of disinfection using ultraviolet radiation and ultrasound.
Solubility of gases in water depends on temperature, pressure, salinity, presence of other gases in the aqueous solution. In 1 liter of water at 0 С, the following can be dissolved: helium - 10 ml, carbon dioxide - 1713 ml, hydrogen sulfide - 4630 ml, ammonia - 1300000 ml (ammonia). When diving to great depths, scuba divers use special breathing mixtures so that when they ascend, they do not get "carbonated blood" due to the dissolution of nitrogen in it.
All living organisms 60-80% water. The blood of humans and animals is similar in salt composition to ocean water. Man and animals can synthesize water in their bodies, form it during the combustion of food products and the tissues themselves. In a camel, for example, the fat contained in the hump can, as a result of oxidation, give 40 liters of water.
At electrolysis two types of water can be obtained: 1) acidic water (“dead”), which acts as an antiseptic (similar to how many pathogenic microbes die in acidic gastric juice); 2) alkaline water (“live”), which activates biological processes (increases productivity, heals wounds faster, etc.).
You can learn about other features of water (structured, energy-informational, etc.) from the Internet.
TRIZ task 27. Water worker
Most often, various mechanisms have "solid-state" working bodies. Give examples of technical devices in which the working body is water (liquid). What laws of development of technical systems does such a working body correspond to?
TRIZ task 28. Water in a sieve
In the famous problem How to carry water in a sieve? there is an explicit physical contradiction: there should be holes in the sieve so that bulk solids can be sieved through it, and there should be no holes so that water does not pour out. One of the possible solutions to this problem can be found in Ya.I. Perelman in "Entertaining Physics", where it is proposed to lower the sieve into molten paraffin so that the sieve mesh is not wetted with water. Based techniques for eliminating technical and physical contradictions suggest 10-20 other ways to solve this problem.






