All formulas in physics and mathematics
On fig. 3.9.1 conditionally depicts the energy flows between the selected thermodynamic system and the surrounding bodies. Value Q> 0 if the heat flux is directed towards the thermodynamic system. Value A> 0 if the system performs positive work on the surrounding bodies.
If the system exchanges heat with surrounding bodies and does work (positive or negative), then the state of the system changes, i.e., its macroscopic parameters (temperature, pressure, volume) change. Because internal energy U is uniquely determined by macroscopic parameters characterizing the state of the system, it follows that the processes of heat transfer and work are accompanied by a change in Δ U internal energy of the system.
First law of thermodynamics is a generalization of the law of conservation and transformation of energy for a thermodynamic system. It is formulated as follows:
Change Δ U internal energy of a non-isolated thermodynamic system is equal to the difference between the amount of heat Q transferred to the system, and work A, a perfect system over external bodies.
|
Δ U = Q - A. |
The relation expressing the first law of thermodynamics is often written in a different form:
|
Q = Δ U + A. |
The amount of heat received by the system is used to change its internal energy and perform work on external bodies.
The first law of thermodynamics is a generalization of experimental facts. According to this law, energy cannot be created or destroyed; it is transferred from one system to another and is transformed from one form into another. An important consequence of the first law of thermodynamics is the assertion that it is impossible to create a machine capable of doing useful work without consuming energy from outside and without any changes inside the machine itself. Such a hypothetical machine is called perpetual motion machine (perpetuum mobile) of the first kind . Numerous attempts to create such a machine invariably ended in failure. Any machine can do positive work A over external bodies only by obtaining a certain amount of heat Q from surrounding bodies or a decrease in Δ U its internal energy.
Let us apply the first law of thermodynamics to isoprocesses in gases.
1. In isochoric process (V= const) the gas does no work, A= 0. Therefore,
The first law of thermodynamics for an isobaric process gives:
|
Q = U (T 2) - U (T 1) + p (V 2 - V 1) = ∆ U + p Δ |
With isobaric expansion Q> 0 - heat is absorbed by the gas, and the gas does positive work. With isobaric compression Q < 0 - тепло отдается внешним телам. В этом случае A < 0. Температура газа при изобарном сжатии уменьшается, T 2 < T one ; internal energy decreases, Δ U < 0.
3. In isothermal process the gas temperature does not change, therefore, the internal energy of the gas, Δ U = 0.
3. The first law of thermodynamics for an isothermal process is expressed by the relation
|
Q = A. |
Quantity of heat Q, obtained by the gas in the process of isothermal expansion, turns into work on external bodies. Under isothermal compression, work external forces produced over the gas is converted into heat, which is transferred to the surrounding bodies.
Along with isochoric, isobaric and isothermal processes Thermodynamics often considers processes occurring in the absence of heat exchange with surrounding bodies. Vessels with insulating walls are called adiabatic shells, and the processes of gas expansion or compression in such vessels are called adiabatic.
AT adiabatic processQ= 0; so the first law of thermodynamics takes the form
|
A = -Δ U, |
i.e., the gas does work due to the loss of its internal energy.
On surface ( p, V) the process of adiabatic expansion (or compression) of a gas is represented by a curve called adiabatic. During adiabatic expansion, the gas does positive work ( A> 0); therefore its internal energy decreases (Δ U < 0). Это приводит к понижению температуры газа. Вследствие этого давление газа при адиабатическом расширении убывает быстрее, чем при изотермическом (рис. 3.9.2).
In thermodynamics, an equation for an adiabatic process is derived for ideal gas. In coordinates ( p, V) this equation has the form
|
pVγ = const. |
This ratio is called Poisson equation . Here γ = Cp / CV- adiabatic index, Cp and CV- heat capacity of gas in processes with constant pressure and constant volume. For a monatomic gas
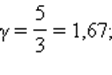
for diatomic
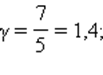
for polyatomic
The work done by a gas in an adiabatic process is simply expressed in terms of temperatures T 1 and T 2 initial and final states:
|
A = CV (T 2 - T 1). |
An adiabatic process can also be classified as an isoprocess. In thermodynamics, a physical quantity plays an important role, which is called entropy . The change in entropy in any quasi-static process is equal to reduced heat Δ Q / T received by the system. Since in any part of the adiabatic process Δ Q= 0, the entropy in this process remains unchanged.
An adiabatic process (as well as other isoprocesses) is a quasi-static process. All intermediate states of the gas in this process are close to the states of thermodynamic equilibrium. Any point on the adiabat describes an equilibrium state.
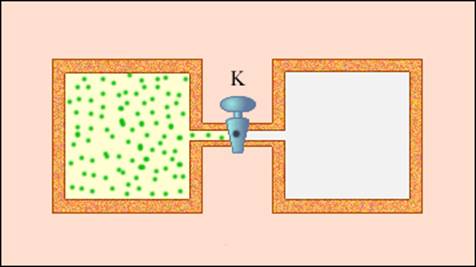
Not every process carried out in an adiabatic shell, i.e., without heat exchange with surrounding bodies, satisfies this condition. An example of a non-quasistatic process in which intermediate states are nonequilibrium is expansion of gas into a vacuum. On fig. 3.9.3 shows a rigid adiabatic shell, consisting of two communicating vessels separated by a valve K. In the initial state, the gas fills one of the vessels, and in the other vessel - a vacuum. After opening the valve, the gas expands, fills both vessels, and a new equilibrium state is established. In this process Q= 0, because no heat exchange with surrounding bodies, and A= 0, because shell is not deformable. It follows from the first law of thermodynamics: Δ U= 0, i.e., the internal energy of the gas remained unchanged. Since the internal energy of an ideal gas depends only on temperature, the temperature of the gas in the initial and final states is the same - points on the plane ( p, V) representing these states lie on one isotherm. All intermediate states of a gas are non-equilibrium and cannot be depicted on a diagram.
Expansion of a gas into a void - an example irreversible process. It cannot be carried out in the opposite direction.
|
|
All human activity is based on the consumption of energy. Without energy, it is difficult to imagine the activity of the organism, the functioning of all the benefits for people. It is important both within a person and for his external existence. Various branches of production cannot work by themselves, because they require energy. Transportation of goods, heating of premises, synthesis of new substances in the cells of the body - all this occurs due to the ability to produce work.
The place of energy in thermodynamics
In order to determine what place energy occupies in this branch of science, one should find out what exactly the term "thermodynamics" means. This is an area of physics in which the processes of energy conversion are studied in depth. Everyone knows the fact that energy is presented in several forms: it can be chemical, electrical, thermal, mechanical and light. This branch of science even has its own laws and principles, such as the first law of thermodynamics, as well as some others. Based on this, we can say that this field of knowledge would not exist if the properties of the above physical quantity were not known.
How does energy conversion take place?

Many do not understand the fact of the transition of energy from one state to another. But we are in Everyday life we use transitory energy so often that we no longer realize where it comes from. So, for example, getting into a car every day, we do not think about the fact that at the moment of driving, chemical energy is converted into electrical energy. In an electric motor, the initial Electric Energy goes into mechanical, and in the steam turbine heat is converted into mechanical physical quantity. With all this, there are certain quantitative ratios of different forms of energy. But during the transformation, processes occur with some quantitative losses: consumption useful energy always higher than its production. This phenomenon is easily explained: the transformation is not complete without friction, during which part of the energy becomes heat and dissipates in space. It turns out that this part can no longer be used as a useful value. Thus, any transformation is not lossless.
First law of thermodynamics
By studying the process of energy conversion, scientists discovered two fundamental laws. This article will focus on the first of them. This law of thermodynamics says that with any change - chemical or physical - the amount of energy remains constant. In another way, it is also called the law of conservation of energy. From this definition it follows that energy does not suddenly appear and does not just disappear. If in the course of any activity it becomes different forms, from one to another, then its total number does not change. In other words, energy is neither created nor destroyed, which reflects the first law of thermodynamics. The definition in different sources may change, but the essence remains the same.

The history of the discovery of this law
The beginning of the law takes from the middle of the XIX century. The German doctor Mayer, observing how the color of human blood changes in the tropics, thought about the relationship between such physical concepts like heat and energy. In turn, the famous scientist Joule drew attention to Mayer's developments and added something on his part. All the data on this law were combined by another German doctor, Helmholtz, when he studied physical foundations organs of perception - hearing and vision of a person. It was he who formulated the law in the form in which the whole world knows it today.
Types of energy exchange
The heat that occurs during the transition of one type of energy to another affects the change in the internal energy of an object. When it changes, there is a counteraction to the work of external forces that affect this subject. Internal energy can be kinetic (this is the movement of the atoms of an object), potential (it is stored in chemical bonds between atoms), the gravitational energy of the entire system (i.e., the influence on the subject of gravitational forces that also appear inside). Based on these types, scientists have identified certain types of energy exchange.
- The first of them is usually understood as pre-isolated systems - there is no exchange of energy and other elements of a certain system.
- The second type - closed systems, is characterized by the absence of the exchange of elements of systems, but to a small extent there is an exchange of energy.
- Open systems (the third type of exchange) are characterized by the exchange of both energy and their elements.
The concept of internal energy in thermodynamics
The internal energy of an object or system in thermodynamics plays almost key role. In fact, this is a complex combination of several streams of activity of molecules and atoms. It consists of such types as the energy of the rotational and translational motion of molecules, the energy of the motions of atoms and groups of atoms, which is inside the molecules and is emitted when these particles vibrate. Another term for the internal energy of an object is nuclear energy atoms and the energy of interaction between molecules. U ext depends on the initial state of the object and the final indicators of its state. This energy is continuous, as indicated by its first law of thermodynamics. It can be easily calculated using the following formula: the heat added to the system minus the work done by this system. Here we are talking not about the nature of the process of changing internal energy, not even about the final state of equilibrium, since this already determines the second law of thermodynamics.
Relationship between heat and internal energy
These concepts are very closely related. It is assumed that heat is part of the internal energy of the system. Let's figure out what heat is. The object itself does not possess such in the usual sense of the word. We can say that heat is the energy that passes from an object that has high temperature, to an object with a temperature lower than that of the first.

In other words, this is part of the internal energy of the object, which is transferred from it, since it has higher temperatures. Such transfer in physics and chemistry is called heating.
First law of thermodynamics, chemistry
This law is of fundamental importance not only for physical experiments, the study of phenomena and the discovery of new processes, but also for another science - chemistry - and its related branches. When studying the existence and transition of energy into different forms from the perspective of the chemical reactions of an organism or an object, a horizon of interesting studies and patterns opens up in the plane of bioenergetics.

Many may feel that the first law of thermodynamics is far from reality. ordinary person that molecules and atoms for understanding are subject only to a small number of dedicated people. But this is far from true. The life of every living organism is based on metabolism, which cannot occur without the conversion of energy. Therefore, of particular interest not only for physicists, but also for nutritionists, sports instructors are chemical processes energy conversion in the human body. After all, knowing all the features of this process, you can successfully help people get rid of extra pounds, as well as take care of themselves with the help of a properly composed menu consisting of healthy food.
First law of thermodynamics is the law of conservation of energy applied to thermal phenomena. It is formulated as follows: the amount of heat Q received by the system goes to changeits internal energy and doing workover external bodies:
The first law of thermodynamics is a generalization of experimental facts. According to this law, energy cannot be created or destroyed; it is transferred from one system to another and is transformed from one form into another. An important consequence of the first law of thermodynamics is the assertion that it is impossible to create a machine capable of doing useful work without consuming energy from outside and without any changes inside the machine itself. Such a hypothetical machine was called a perpetual motion machine (perpetuum mobile) of the first kind. Numerous attempts to create such a machine invariably ended in failure. Any machine can perform positive work A on external bodies only by receiving some amount of heat Q from surrounding bodies or by reducing ΔU of its internal energy.
Let us apply the first law of thermodynamics to isoprocesses in ideal monatomic gases.
1. In an isochoric process (V = const), the gas does no work, A = 0. Therefore:
Here U 1 and U 2 are the internal energies of the gas in the initial and final states. During isochoric heating, heat is absorbed by the gas (Q > 0), and its internal energy increases. During cooling, heat is transferred to external bodies (Q< 0).
Please note that the amount of heat can be calculated either through a change in temperature, or through the values of pressure and volume in the initial and final state of the gas.
2. In an isobaric process (p = const), the gas does work, its temperature changes, and hence the internal energy. Consequently:
With isobaric expansion, the temperature increases, the internal energy increases, ΔU > 0; the gas does positive work, A > 0; Q > 0 – heat is absorbed by the gas During isobaric compression, the temperature decreases, the internal energy decreases, ΔU > 0; the gas does negative work, A< 0; Q < 0 – тепло отдается внешним телам.
Please note that the amount of heat can be calculated either through a change in temperature, or through a change in volume at a known gas pressure.
3. In an isothermal process, the gas temperature does not change, which means that the internal energy of the gas does not change, ΔU = 0. Therefore:
![]() . (8.12)
. (8.12)
Here T is the temperature of the isothermal process; V 1 p 1 and V2p 2 - volume and pressure in the initial and final state of the gas. Formula (8.12) is obtained by calculating the work as the area of a curvilinear trapezoid in the diagram ( p V) for an isothermal process.
Along with isochoric, isobaric, and isothermal processes, thermodynamics often considers processes that occur in the absence of heat exchange with surrounding bodies. Such processes are called adiabatic.
4. In an adiabatic process Q = 0; so the first law of thermodynamics becomes:
or ![]() . (8.13)
. (8.13)
 At adiabatic expansion
gas work is positive A > 0, gas temperature decreases T 2 < Т
1, the internal energy of the gas decreases, i.e. i.e. the gas does work due to the loss of its internal energy.
At adiabatic expansion
gas work is positive A > 0, gas temperature decreases T 2 < Т
1, the internal energy of the gas decreases, i.e. i.e. the gas does work due to the loss of its internal energy.
At adiabatic compression gas work is negative A< 0, температура газа увеличивается T 2 >T 1, the internal energy of the gas increases, i.e. e. the positive work of external forces acting on the gas goes to increase the internal energy of the gas.
On the diagram (p, V), the process of adiabatic expansion (or compression) of a gas is depicted by a curve called adiabatic. During adiabatic expansion, the temperature of the gas decreases. As a result, the gas pressure decreases faster during adiabatic expansion than during isothermal expansion (Fig. 8.4).
>>Physics: The First Law of Thermodynamics
Law of energy conservation
The first law of thermodynamics is the law of conservation of energy, extended to thermal phenomena. It shows on what causes the change in internal energy depends.
By the middle of the XIX century. Numerous experiments have proven that mechanical energy never disappears without a trace. For example, a hammer falls on a piece of lead, and the lead heats up in a very definite way. The forces of friction slow down the bodies, which in this case are heated.
Based on many similar observations and generalization of experimental facts, the law of conservation of energy was formulated:
Energy in nature does not arise from nothing and does not disappear: the amount of energy is unchanged, it only changes from one form to another.
The law of conservation of energy governs all natural phenomena and binds them together. It is always carried out absolutely precisely; there is not a single case known when this great law was not carried out.
This law was discovered in the middle of the XIX century. German scientist, doctor by education R. Mayer (1814-1878), English scientist J. Joule (1818-1889) and received the most accurate formulation in the works of the German scientist G. Helmholtz (1821-1894).
The law of conservation and transformation of energy, extended to thermal phenomena, is called the first law of thermodynamics.
In thermodynamics, bodies are considered, the position of the center of gravity of which practically does not change. mechanical energy of such bodies remains constant, only the internal energy of each body can change.
So far, we have considered processes in which the internal energy of the system is changed either due to the performance of work or due to heat exchange with surrounding bodies.
AT general case when a system passes from one state to another, the internal energy changes simultaneously both due to the performance of work and due to the transfer of heat.
The first law of thermodynamics is formulated precisely for such general cases:
The change in the internal energy of the system during its transition from one state to another is equal to the sum of the work of external forces and the amount of heat transferred to the system:
If the system is isolated, then the work of external forces is zero ( BUT= 0) and the system does not exchange heat with surrounding bodies ( Q= 0). In this case, according to the first law of thermodynamics or U 1 \u003d U 2. Internal energy isolated system remains unchanged (preserved).
Often instead of work A external bodies on the system consider the work A´ systems over external bodies. Given that ![]() , the first law of thermodynamics (13.10) can be written as follows:
, the first law of thermodynamics (13.10) can be written as follows:
The amount of heat transferred to the system is used to change its internal energy and to perform work on external bodies by the system.
The impossibility of creating a perpetual motion machine
From the first law of thermodynamics follows the impossibility of creating a perpetual motion machine - a device capable of performing an unlimited amount of work without fuel or any other materials. If no heat is supplied to the system ( Q=0), then work A´ according to equation (13.11) can be done only due to the loss of internal energy: ![]() . After the energy supply is exhausted, the engine will stop working.
. After the energy supply is exhausted, the engine will stop working.
Work and amount of heat - characteristics of the process of changing internal energy
In this state, the system always has a certain internal energy.
But we cannot say that the system contains a certain amount of heat or work. Both work and the amount of heat are quantities that characterize change in internal energy systems as a result of a particular process.
The internal energy of the system can change in the same way both due to the performance of work by the system and due to the transfer of any amount of heat to the surrounding bodies. For example, a heated gas in a cylinder can reduce its energy by cooling down without doing work ( fig.13.6). But he can lose exactly the same amount of energy by moving the piston, without giving off heat to the surrounding bodies. To do this, the walls of the cylinder and the piston must be heat-tight ( fig.13.7).
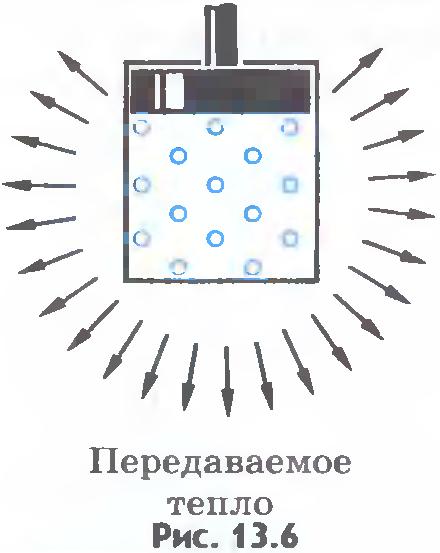
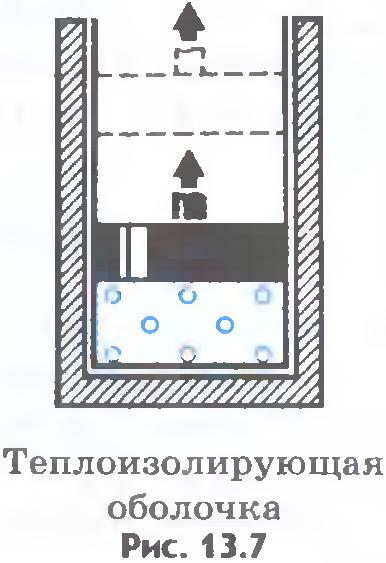
In the future, throughout the course of physics, we will get acquainted with other forms of energy, ways of their transformation and transfer.
The internal energy of a system of bodies changes when work is done and when the amount of heat is transferred. In each state, the system has a certain internal energy. Work and the amount of heat are not contained in the body, but characterize the process of changing its internal energy.
The history of the emergence of thermodynamics
Each of us has a way to subconsciously feel heat and cold. This happens on an intuitive level. Man had an idea about the temperature regime long before the scientific theories about the heating of bodies appeared.
But modern man accustomed to the benefits of civilization and many useful things seem familiar to him. Now every home has modern thermometers or thermometers to measure the temperature. But such devices appeared not so long ago. In ancient times, naturally, no thermometers existed at all. For a very long time, people used their sensations to determine body temperature. The concepts of cold, warm and hot people were guided until the thermoscope was invented.
Knowledge of heat on a scientific basis began to develop with the invention of this device. According to historical data, the inventor of the first thermoscope was the scientist and astronomer Galileo Galilei. Thanks to this new invention, in those days, a person could determine the degree of "warmth" of the body. This is how we can say that from that moment the history of the development of thermodynamics began.
However, Galileo's device had a significant drawback, since the thermoscope readings depended on atmospheric pressure, and therefore it became necessary to improve it. In 1714, another device was invented that vaguely resembled the current thermometer. It was a flask, which was first filled with water, and then mercury or alcohol was added. Such a mercury thermometer was invented by the German physicist Gabriel Fahrenheit.
But measuring temperature in Fahrenheit was not very convenient, so the French scientist Réaumur proposed the Celsius scale, which is successfully popular to this day.

Thermodynamics is the science of the basic ways in which the internal energy of bodies is converted to perform mechanical work.
Where is thermodynamics used?
Since all the laws of thermodynamics are of a general nature, and, moreover, do not depend on the specific details of the structure of matter at the atomic level, they are widely used in various scientific and technical issues. Thermodynamics has found wide application in the field of energy, thermal engineering, in chemical reactions, phase transitions and even black holes.
Thermodynamics is indispensable in the physical sciences, chemical, it is used in mechanical engineering, the aerospace industry. It is of great importance in cell biology, medicine and even in economics.
Exercise
1. How is the first law of thermodynamics formulated?
2. In what case is the change in internal energy negative?
3. Why can we say that the system has internal energy, but it cannot be said that it has a reserve of a certain amount of heat or work?
G.Ya.Myakishev, B.B.Bukhovtsev, N.N.Sotsky, Physics Grade 10
First law of thermodynamics - The change in internal energy ΔU of a non-isolated thermodynamic system is equal to the difference between the amount of heat Q transferred to the system and the work A of external forces
Instead of the work A performed by external forces on a thermodynamic system, it is often more convenient to consider the work A' performed by a thermodynamic system on external bodies. Since these works are equal in absolute value, but opposite in sign:
Then after such a transformation first law of thermodynamics will look like:
![]()
First law of thermodynamics - In a non-isolated thermodynamic system, the change in internal energy is equal to the difference between the amount of heat Q received and the work A’ performed by this system
talking plain language first law of thermodynamics speaks of energy that cannot itself be created and disappear into nowhere, it is transferred from one system to another and turns from one form to another (mechanical to thermal).
An important consequence first law of thermodynamics is that it is impossible to create a machine (engine) that is capable of doing useful work without consuming energy from outside. Such a hypothetical machine was called a perpetual motion machine of the first kind.
First law of thermodynamics in isoprocesses
1. In an isochoric process(V=const). In an isochoric process, the volume of the gas remains constant, so the gas does no work. The change in the internal energy of the gas occurs due to heat exchange with the surrounding bodies:
Here U1 and U2 are the internal energies of the gas in the initial and final states. The internal energy of an ideal gas depends only on temperature. During isochoric heating, heat is absorbed by the gas Q > 0, and its internal energy increases. During cooling, heat is transferred to external bodies Q< 0
2. In an isobaric process(P=const). With the isobaric expansion of a gas, the amount of heat supplied to it is spent both on increasing its internal energy and on doing work by the gas:
With isobaric expansion, Q > 0, heat is absorbed by the gas, and the gas does positive work. Under isobaric compression Q< 0 – тепло отдается внешним телам. В этом случае A < 0. Температура газа при изобарном сжатии уменьшается, T2 < T1; внутренняя энергия убывает, ΔU < 0.
3. In an isothermal process(T=const). In an isothermal process, the gas temperature does not change, therefore, the internal energy of the gas does not change, ΔU = 0.
The amount of heat Q received by the gas in the process of isothermal expansion is converted into work on external bodies. Under isothermal compression, the work of external forces produced on the gas is converted into heat, which is transferred to the surrounding bodies. Along with isochoric, isobaric, and isothermal processes, thermodynamics often considers processes that occur in the absence of heat exchange with surrounding bodies.
4. AT adiabatic process (Q=0). For an adiabatic process, the first law of thermodynamics is:
That is, the gas does work due to the loss of its internal energy. On the plane (p, V), the process of adiabatic expansion (or compression) of a gas is represented by a curve called an adiabat. During adiabatic expansion, the gas does positive work (A > 0); therefore its internal energy decreases (ΔU< 0). Это приводит к понижению температуры газа. Вследствие этого давление газа при адиабатическом расширении убывает быстрее, чем при изотермическом расширении.
In the formula we used:
Change in internal energy
Quantity of heat
The work of external forces
Work done by the system
Gas volume