Relative atomic mass of chemical elements
Lesson 4. Chemical elements. Signs of chemical elements. Relative atomic mass.
Chemical element- a collection of atoms of the same kind.
Why were identical atoms named that way?The word “element” (lat. elementum) was used in antiquity (by Cicero, Ovid, Horace) as part of something (element of speech, element of education, etc.). In ancient times, the saying was widespread: “As words are made up of letters, so bodies are made up of elements.” Hence the probable origin of this word: by the name of a number of consonant letters in the Latin alphabet: l, m, n, t ("el" - "em" - "en" - "tum").
CHEMICAL LANGUAGE
Mankind uses many different languages. In addition to natural languages (Japanese, English, Russian - more than 2.5 thousand in total), there are also artificial languages, for example, Esperanto. Among artificial languages, the languages of various sciences stand out. So, chemistry uses its own, chemical language. chemical language- a system of symbols and concepts designed for concise, concise and visual recording and transmission of chemical information. A message written in most natural languages is divided into sentences, sentences into words, and words into letters.
We will speak with you in a special, chemical language. In it, as in our native Russian, we will first learn letters - chemical symbols, then we will learn to write words - formulas based on them, and then, with the help of the latter, - sentences - equations of chemical reactions:
The Bulgarian educators Cyril and Methodius are the authors of the Slavic script-alphabet. But the father of chemical writing is the Swedish scientist J. Ya. Berzelius, who proposed as letters - symbols chemical elements use the initial letters of their Latin names, or, if the names of several elements begin with this letter, then add one more of the subsequent letters of the name to the initial letter.
Chemical signs (chemical symbols) - letter designations of chemical elements. They consist of the first or the first and one of the following letters of the Latin name of the element, for example, carbon - C (Carboeum), calcium - Ca (Calcium), cadmium - Cd ...
Chemical element symbolis a symbol for a chemical element.
History reference: Chemists ancient world and the Middle Ages, symbolic images, letter abbreviations, as well as combinations of both, were used to designate substances, chemical operations and devices. The seven metals of antiquity were depicted by astronomical signs of the seven heavenly bodies: the Sun ( ☉ , gold), Moons (☽ , silver), Jupiter (♃ , tin), Venus (♀, copper), Saturn (♄ , lead), Mercury (☿ , mercury), Mars (♁, iron).
The metals discovered in the 15th-18th centuries - bismuth, zinc, cobalt - were designated by the first letters of their names. The sign of wine spirit (lat. spiritus vini) is made up of the letters S and V. The signs of strong vodka (lat. aqua fortis, nitric acid) and golden vodka (lat. aqua regis, aqua regia, a mixture of hydrochloric and nitric acids) are made up of the sign of waterÑ and uppercase letters F and R, respectively. The sign of glass (lat. vitrum) is formed from two letters V - straight and inverted.
Attempts to streamline the ancient chemical signs continued until the end of the 18th century. AT early XIX century, the English chemist J. Dalton proposed to designate the atoms of chemical elements by circles, inside which were placed dots, dashes, initial letters of the English names of metals, etc.
Dalton's chemical signs gained some distribution in Great Britain and Western Europe, but were soon supplanted by purely alphabetic signs, which the Swedish chemist J. J. Berzelius proposed in 1814. The principles he expressed for compiling chemical signs have retained their force to this day. In Russia, the first printed report on the chemical signs of Berzelius was made in 1824 by the Moscow physician I. Ya. Zatsepin.
RELATIVE ATOMIC MASS
History reference: The English scientist John Dalton (1766–1844) in his lectures showed students models of atoms carved from wood, showing how they can combine to form various substances. When one of the students was asked what atoms were, he replied: "Atoms are wooden cubes painted in different colors, which Mr. Dalton invented."
Of course, Dalton became famous not for his "cubes" and not even for the fact that at the age of twelve he became a school teacher. The emergence of modern atomistic theory is associated with the name of Dalton. For the first time in the history of science, he thought about the possibility of measuring the masses of atoms and proposed specific methods for this. It is clear that it is impossible to directly weigh the atoms. Dalton talked only about "the ratio of the weights of the smallest particles of gaseous and other bodies", that is, about their relative masses. Even today, although the mass of any atom is known exactly, it is never expressed in grams, as this is extremely inconvenient. For example, the mass of an atom of uranium - the heaviest of the elements existing on Earth - is only 3.952 10 –22 d. Therefore, the mass of atoms is expressed in relative units, showing how many times the mass of atoms of a given element is greater than the mass of atoms of another element, taken as a standard. In fact, this is the “weight ratio” according to Dalton, i.e. relative atomic mass. The masses of atoms are very small.
Absolute masses of some atoms:
m(C) \u003d 1.99268 ∙ 10 -23 g
m(H) \u003d 1.67375 ∙ 10 -24 g
m(O) \u003d 2.656812 ∙ 10 -23 g
At present, a unified measurement system has been adopted in physics and chemistry. Introduced atomic mass unit (a.m.u.)
m (a.m.u.) \u003d 1/12 m (12C) \u003d 1.66057 ∙ 10 -24 g.
Ar(H) = m(atom) / m (a.m.u.) = 1.67375 ∙ 10 -24 g / 1.66057 ∙ 10 -24 g = 1.0079 amu
Ar - shows how many times a given atom is heavier than 1/12 of a 12C atom, this is a dimensionless quantity.
Relative atomic mass
is 1/12 of the mass of a carbon atom whose mass is 12 a.m.u.
Relative atomic mass is a dimensionless quantity!!!
For example, the relative atomic mass of an oxygen atom is 15.994. It is not always necessary to consider the relative atomic mass values themselves. You can use the values \u200b\u200bgiven in the periodic system of chemical elements by D. I. Mendeleev. It should be written like this:
Ar(O) = 16 .
We always use the rounded value.
Exception represents the relative atomic mass of the chlorine atom: Ar(Cl) = 35.5.
The relationship between the absolute and relative masses of an atom is represented by the formula:
Distribution of elements in nature. The main mass of cosmic matter is H and He (99.9%).
Of the 107 chemical elements, only 89 are found in nature; nuclear reactions(negligible amounts of Te, Pm, Np, Fr are formed during spontaneous fission of uranium and are present in uranium ores). In the accessible part of the Earth, 10 elements with atomic numbers ranging from 8 to 26 are most common. They are contained in the earth's crust in the following relative quantities:
The listed 10 elements make up 99.92% of the mass of the earth's crust.
Element |
atomic number |
|
47,00 |
||
29,50 |
||
8,05 |
||
4,65 |
||
In this article, we will consider different possibilities for expressing the mass of chemical elements.
Atoms differ among themselves: in mass, size and structure.
Mass and sizes of atoms.
Today's science has the ability to determine the mass and size of an atom. The smallest atom is He (helium), approximately its size is 0.00000000098m. (98 * 10 -10). And the lightest atom is the H (hydrogen) atom and its mass is 0.0000000000000000000000000016735kg. (1.6735 * 10 −27).
Most of the atoms of chemical elements are usually much larger than a helium atom.
And the largest atom is the Fr (francium) atom and its size is approximately 0.00000000686m. Which is 7 times larger than the He (helium) atom.
Even more atoms of different chemical elements are more different in mass, the mass of an atom is denoted by the letter m a and is expressed in SI.
And the volume unit of mass
During the formation of atomic - molecular teachings in the 19th century. people did not yet know about the exact sizes and masses of atoms, so scientists began to use their relative values. Relative values were calculated from mass ratios simple substances in reactions with each other. Scientists chemists have suggested that they are proportional to the masses of atoms. John Dalton adopted the unit of comparison of the lightest atom - H (hydrogen)
As a comparison, 1/12 of the mass of carbon a.e.m (atomic mass unit) is used. International designation - u (unit - unit).
Atomic mass unit- this is 1/12 of the mass of a carbon atom and it is equal to 1.66 10 −27 kg.
About the relative atomic mass.
Comparing the average masses of various elements with a.e.m (u), one can obtain the values of the relative atomic masses of the chemical. elements. Relative atomic mass is physical quantity, showing how many times the mass of an atom of a chemical element is greater than 1/12 of a carbon atom.
Notation: A r Formula.
From the lesson materials, you will learn that the atoms of some chemical elements differ from the atoms of other chemical elements in mass. The teacher will tell you how chemists measured the mass of atoms, which are so small that you can't even see them with an electron microscope.
Topic: Initial chemical ideas
Lesson: Relative atomic mass of chemical elements
At the beginning of the 19th century (150 years after the work of Robert Boyle), the English scientist John Dalton proposed a method for determining the mass of atoms of chemical elements. Let's consider the essence of this method.
Dalton proposed a model according to which the molecule complex substance includes only one atom of different chemical elements. For example, he believed that a water molecule consists of 1 hydrogen atom and 1 oxygen atom. According to Dalton, the composition of simple substances also includes only one atom of a chemical element. Those. An oxygen molecule must consist of one oxygen atom.
And then, knowing the mass fractions of elements in a substance, it is easy to determine how many times the mass of an atom of one element differs from the mass of an atom of another element. Thus, Dalton believed that the mass fraction of an element in a substance is determined by the mass of its atom.
It is known that the mass fraction of magnesium in magnesium oxide is 60%, and the mass fraction of oxygen is 40%. Following the path of Dalton's reasoning, we can say that the mass of a magnesium atom is 1.5 times greater than the mass of an oxygen atom (60/40 = 1.5):
The scientist noticed that the mass of the hydrogen atom is the smallest, because. there is no complex substance in which the mass fraction of hydrogen would be greater than the mass fraction of another element. Therefore, he proposed to compare the masses of the atoms of the elements with the mass of the hydrogen atom. And in this way he calculated the first values of the relative (relative to the hydrogen atom) atomic masses of chemical elements.
The atomic mass of hydrogen was taken as a unit. And the meaning relative mass sulfur turned out to be 17. But all the obtained values \u200b\u200bare either approximate or incorrect, because. the technique of the experiment of that time was far from perfect, and Dalton's installation on the composition of matter was incorrect.
In 1807 - 1817. Swedish chemist Jöns Jakob Berzelius did a great deal of research to refine the relative atomic masses of elements. He managed to get results close to modern ones.
Much later than the work of Berzelius, the masses of atoms of chemical elements began to be compared with 1/12 of the mass of a carbon atom (Fig. 2).
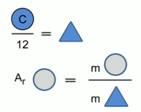
Rice. 1. Model for calculating the relative atomic mass of a chemical element
The relative atomic mass of a chemical element shows how many times the mass of an atom of a chemical element is greater than 1/12 of the mass of a carbon atom.
Relative atomic mass is denoted A r , it has no units of measurement, as it shows the ratio of the masses of atoms.
For example: A r (S) = 32, i.e. a sulfur atom is 32 times heavier than 1/12 the mass of a carbon atom.
The absolute mass of 1/12 of a carbon atom is a reference unit, the value of which is calculated with high accuracy and is 1.66 * 10 -24 g or 1.66 * 10 -27 kg. This reference mass is called atomic mass unit (a.u.m.).
The values of the relative atomic masses of chemical elements do not need to be memorized, they are given in any textbook or reference book on chemistry, as well as in the periodic table of D.I. Mendeleev.
When calculating the values of relative atomic masses, it is customary to round up to integers.
An exception is the relative atomic mass of chlorine - for chlorine, a value of 35.5 is used.
1. Collection of tasks and exercises in chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. "Chemistry, Grade 8" / P.A. Orzhekovsky, N.A. Titov, F.F. Hegel. – M.: AST: Astrel, 2006.
2. Ushakova O.V. Chemistry workbook: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. Grade 8” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; under. ed. prof. P.A. Orzhekovsky - M .: AST: Astrel: Profizdat, 2006. (p. 24-25)
3. Chemistry: 8th grade: textbook. for general institutions / P.A. Orzhekovsky, L.M. Meshcheryakova, L.S. Pontak. M.: AST: Astrel, 2005.(§10)
4. Chemistry: inorg. chemistry: textbook. for 8 cells. general institutions / G.E. Rudzitis, FuGyu Feldman. - M .: Education, JSC "Moscow textbooks", 2009. (§§8,9)
5. Encyclopedia for children. Volume 17. Chemistry / Chapter. edited by V.A. Volodin, leading. scientific ed. I. Leenson. – M.: Avanta+, 2003.
Additional web resources
1. A single collection of digital educational resources ().
2. Electronic version of the journal "Chemistry and Life" ().
Homework
p.24-25 Nos. 1-7 from Workbook in chemistry: 8th grade: to the textbook by P.A. Orzhekovsky and others. “Chemistry. Grade 8” / O.V. Ushakova, P.I. Bespalov, P.A. Orzhekovsky; under. ed. prof. P.A. Orzhekovsky - M.: AST: Astrel: Profizdat, 2006.






