combination scattering. Raman scattering of light
monochromatic light incident on the sample can be reflected, absorbed, or scattered. The process of light scattering must be elastic (that is, it can occur without energy exchange between light and matter) and inelastic (that is, redistribution of energy can occur between light and matter).
Elastic scattering of light is called Rayleigh. It is predominant: on average, only one photon in ten million is scattered inelastically. In Rayleigh scattering, the frequency of the scattered light is exactly equal to the frequency of the incident light.
Inelastic light scattering is called Raman scattering (RS), or Raman. In Raman scattering, light and matter exchange energy. As a result, the frequency of the scattered light can either decrease (while the energy passes from light to matter - this is Stokes scattering) or increase (while the energy passes from matter to light - this is Anti-Stokes scattering).
Scattering can be viewed as a very fast process of absorption and emission of a photon. With such absorption of a photon, the molecule does not go into a stable excited electronic state if the photon energy is insufficient for this process. It goes into an unstable excited state, from which it emits a photon after a very short time.
In Rayleigh scattering, a molecule absorbs a photon from the zero vibrational level, and passes to it after emission. In Stokes scattering, a molecule absorbs a photon from the zero vibrational level, but after emission it passes to the first one, absorbing part of the photon's energy. On the contrary, during Anti-Stokes scattering, the molecule absorbs a photon from the first vibrational level, and after emission it passes to zero, giving part of its energy to the emitted photon.
Under conditions of thermal equilibrium, the population of vibrational levels obeys the Boltzmann distribution, that is, the population is more than high levels decreases exponentially. Accordingly, the first level is populated to a much lesser extent than the zero one, which leads to a much lower intensity of the Anti-Stokes lines in the Raman spectrum compared to the intensity of the Stokes lines.
As a rule, the Raman spectrum is understood precisely as its more intense, Stokes part. The frequency of Rayleigh scattering (that is, the frequency of the radiation source) is taken as "zero", and the frequency of the line in the spectrum is calculated by subtracting the frequency of the Stokes line from the frequency of the Rayleigh radiation.
In the general case, the process of light scattering competes with the process of its absorption. When radiation is absorbed, the molecule is transferred to the lowest excited electronic state. The reverse transition to the ground state can either be completely non-radiative or be accompanied by the emission of light of a lower frequency. This radiation is called photoluminescence. Depending on the spin configuration of the excited electronic state, photoluminescence is divided into fluorescence and phosphorescence.
The photoluminescence lines are much more intense than the Raman lines. Thus, with a Raman spectrometer equipped with a suitable detector, it is possible to simultaneously obtain the Raman spectrum and the photoluminescence spectrum at the same point without any problems.
However, in some cases, the photoluminescence spectrum can be superimposed on the Raman spectrum, which is an undesirable effect. Below is a conditional Raman / photoluminescence spectrum of a colored polymer, which strongly fluoresces in the visible and near-IR regions when irradiated with light in the UV and visible ranges. In such cases, as a rule, one tries to select the frequency of the exciting radiation in such a way as to avoid the appearance of intense fluorescence. One of the options is the use of radiation sources in the near-IR range for excitation of Raman radiation.
The Raman spectrum of most organic molecules consists of lines corresponding to bending and stretching vibrations chemical bonds carbon (C) with other elements, usually hydrogen (H), oxygen (O) and nitrogen (N), as well as characteristic vibrations of various functional groups (hydroxyl -OH, amino group -NH2, etc.). These lines appear in the range from 600 cm-1 (stretching vibrations of single C-C ties) up to 3600 cm-1 (vibrations of the hydroxyl -OH group). In addition, in the spectra of organic molecules in the range of 250–400 cm–1, bending vibrations of the aliphatic chain appear.
In contrast to the IR spectrum, in which lines appear that correspond to vibrational transitions with a change in the dipole moment, lines appear in the Raman spectrum that correspond to vibrational transitions with a change in the polarizability of the molecule. Thus, IC and CR are not exclusive, but complementary. spectrometric methods. There are Raman spectrometers that make it possible to simultaneously obtain Raman and IR spectra at one point (LAbRAM ARAMIS IR2).
The Raman spectra of crystal lattices contain lines corresponding to the scattering of radiation by collective excited states of the lattice, which in solid state physics are considered as quasiparticles. The most common are Raman-active transitions involving optical and acoustic phonons, plasmons, and magnons.
Quasiparticle - a quantum of collective oscillation or perturbation of a many-particle system, which has a certain energy and, as a rule, momentum. There are a number of similarities and differences between quasiparticles and ordinary particles. In many field theories, such as conformal field theory, no distinction is made at all between particles and quasiparticles. (Wikipedia)
A phonon is a quasiparticle introduced by the Russian scientist I. Tamm. A phonon is a quantum oscillatory motion crystal atoms. The concept of the phonon turned out to be very fruitful in solid state physics. In crystalline materials, atoms actively interact with each other, and it is difficult to consider such thermodynamic phenomena as vibrations of individual atoms in them - huge systems are obtained from trillions of interconnected linear differential equations, which cannot be solved analytically. Vibrations of crystal atoms are replaced by propagation in the substance of the system sound waves, whose quanta are phonons. The phonon spin is zero (in units of h). The phonon is one of the bosons and is described by the Bose-Einstein statistics. Phonons and their interaction with electrons play a fundamental role in modern ideas about the physics of superconductors. (Wikipedia)
An acoustic phonon is characterized for small wave vectors by a linear dispersion law and a parallel displacement of all atoms in the unit cell. Such a dispersion law describes the acoustic oscillations of the lattice (that is why the phonon is called acoustic). (Wikipedia) The energy of acoustic phonons is usually low (on the order of 1 cm-1 to 30 cm-1).
Optical phonons exist only in crystals whose unit cell contains two or more atoms. These phonons are characterized at small wave vectors by vibrations of atoms such that the center of gravity of the unit cell remains immobile. The energy of optical phonons is usually quite high (on the order of 500 cm-1) and weakly depends on the wave vector. (Wikipedia)
Plasmon is a quasi-particle corresponding to the quantization of plasma oscillations, which are collective oscillations of a free electron gas. Plasmons play an important role in the optical properties of metals. Light below the plasma frequency is reflected because the electrons in the metal shield the electric field in the light's electromagnetic wave. Light above the plasma frequency gets through because the electrons can't respond fast enough to shield it. In most metals, the plasma frequency is in the ultraviolet region of the spectrum, making them shiny in the visible range. In doped semiconductors, the plasma frequency is usually in the ultraviolet region. (Wikipedia)
Surface plasmons (plasmons confined to surfaces) interact strongly with light, resulting in the formation of polaritons. They play a role in the surface enhancement of RS, Raman scattering light (SERS) and in explaining anomalies in the diffraction of metals. Surface plasmon resonance is used in biochemistry to detect the presence of molecules on a surface. (Wikipedia)
A magnon is a quasiparticle corresponding to an elementary excitation of a system of interacting spins. In crystals with several magnetic sublattices (for example, antiferromagnets), there can be several types of magnons with different energy spectra. Magnons obey Bose-Einstein statistics. Magnons interact with each other and with other quasiparticles. The existence of magnons is confirmed by experiments on the scattering of neutrons, electrons and light, accompanied by the birth or annihilation of a magnon.
The magnon concept was introduced in 1930 by Felix Bloch to explain quantitatively the phenomenon of the decrease in spontaneous magnetization in ferromagnets. At a temperature absolute zero a ferromagnet reaches a state of lowest energy, in which the atomic spins (as well as the magnetic moments) align in the same direction. As the temperature rises, the spins begin to deviate from the general direction, thereby increasing internal energy and reducing the total magnetization. If we imagine an ideally magnetized ferromagnet as a vacuum state, then the state at low temperatures, in which the ideal order is violated by a small number of inverted spins, can be represented as a gas of quasiparticles - magnons. Each magnon reduces the number of correctly aligned spins by h and the total magnetic moment along the quantization axis by gh, where g is the gyromagnetic ratio. (Wikipedia)
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
Ministry of Education of the Republic of Belarus
Belarusian State Pedagogical University named after Maxim Tank
COURSE WORK
On the topic: "Application of Raman spectroscopy in medicine"
Head: Maskevich Sergey Alexandrovich
Introduction
2. Application of Raman spectroscopy for the control of drugs, narcotic and toxic drugs
Conclusion
Bibliography
Introduction
When waves propagate in a material medium, the simplest situation corresponds to the absence of any interaction between waves. In this case, the waves penetrate one through the other without any changes in the frequency, amplitude and phase of the corresponding oscillatory processes.
A more complex picture is realized for interacting waves. In particular, for such waves, the process of amplitude modulation of high-frequency oscillations by low-frequency ones can be carried out. As a result of modulation, along with the original waves with high (w0) and low (W) frequencies, combination waves arise with frequencies w0 - W and w0 + W. It is this property of the waves that was used by the creators of the radiotelegraph in late XIX centuries. In this case, a radio wave served as a carrier high-frequency wave, and low-frequency waves corresponded to the sound range and represented the information necessary for transmission.
As is known, for the first time a wireless telegraph based on the modulation of electromagnetic waves of the radio range (w0 ~ 1011 Hz) by low-frequency Morse code signals was implemented in 1895 in the experiments of A.S. Popov. Similar studies were carried out at the same time in the West by F. Brown and T. Marconi. In 1909, they were awarded the Nobel Prize for the creation of a wireless telegraph.
F. Braun was a professor at the Department of Physics at the University of Strasbourg, when in 1899 L.I. Mandelstam. The object of research L.I. Mandelstam were acoustic waves in solids. As it turned out, such waves exist in material media even in the absence of any external sound signals. In this regard, L.I. Mandelstam in 1926 published a paper in which he considered the question of the modulation of light waves in solids by thermal (acoustic) waves.
Experiments on the study of light scattering in condensed media were started in 1926 in Moscow by G.S. Landsberg and L.I. Mandelstam. One of the objects of research was crystalline quartz; as a source of exciting radiation, intense lines of a mercury lamp were used, which were isolated from the spectrum of a gas discharge using absorption filters. As a result of these experiments, it was found that indeed in the spectrum of scattered light there is a weak radiation, the frequency of which is shifted with respect to the frequency of the primary, exciting radiation. It turned out that the spectrum contains several satellites symmetrical with respect to the frequency w0 of the exciting radiation with frequencies w0 - Wj (Stokes satellite) and w0 + Wj (anti-Stokes satellite). It was also found that the observed shifts Wj of the frequency w0 of the exciting radiation are several orders of magnitude higher than the characteristic frequencies of acoustic waves, which were considered as the cause of light scattering in Mandelshtam's theory. Subsequently, it was found that, along with acoustic waves, along with an exciting radiation wave, there can be many other types of waves, in particular, optical oscillation waves characterized by a counter-movement of nonequivalent atoms of a primitive cell of a crystal. This was the reason for the frequency shift of the exciting radiation observed in the experiments of Landsberg and Mandelstam. Subsequently, this type of scattering was called by them Raman scattering of light.
At the same time (in 1928), Indian physicists Ch. Raman and K. Krishnan carried out similar experiments on the study of light scattering in liquids. In the first experiments, Indian scientists used a sunbeam as a source of exciting radiation. Using certain combinations of absorption filters, they came to the conclusion that light is scattered in liquids, accompanied by a frequency shift w" = w0 - W (w0 is the frequency of the exciting radiation, w" is the frequency of the scattered light), and the results of their experiments were interpreted as a manifestation of optical analogue of the Compton effect. This phenomenon was later called the Raman effect. For the discovery of this phenomenon in 1930, Ch. Raman was awarded the Nobel Prize.
It should be noted that the term Raman scattering (RSS) proposed by Mandelstam and Landsberg has an independent meaning and is widely used in the scientific literature.
The study of a new type of light scattering, starting from the first works, immediately attracted the attention of wide circles of the scientific community. Undoubtedly, the discovery of this phenomenon is one of the most striking scientific achievements XX century.
1. Theory of the Raman scattering method
Monochromatic light incident on a sample can be reflected, absorbed, or scattered. The process of light scattering can be elastic (that is, it can occur without energy exchange between light and matter) and inelastic (that is, redistribution of energy can occur between light and matter).
Elastic scattering of light is called Rayleigh. It is predominant: on average, only one photon in ten million is scattered inelastically. In Rayleigh scattering, the frequency of the scattered light is exactly equal to the frequency of the incident light.
Inelastic light scattering is called Raman scattering (RS), or Raman. In Raman scattering, light and matter exchange energy. As a result, the frequency of the scattered light can either decrease (in this case, the energy passes from light to matter - this is Stokes scattering), or increase (in this case, the energy passes from matter to light - this is Anti-Stokes scattering).
Scattering can be viewed as a very fast process of absorption and emission of a photon. With such absorption of a photon, the molecule does not go into a stable excited electronic state if the photon energy is insufficient for this process. It goes into an unstable excited state, from which it emits a photon after a very short time.
In Rayleigh scattering, a molecule absorbs a photon from the zero vibrational level, and passes to it after emission. In Stokes scattering, a molecule absorbs a photon from the zero vibrational level, but after emission it passes to the first one, absorbing part of the photon's energy. On the contrary, during Anti-Stokes scattering, the molecule absorbs a photon from the first vibrational level, and after emission it passes to zero, giving part of its energy to the emitted photon.
Under conditions of thermal equilibrium, the population of vibrational levels obeys the Boltzmann distribution, that is, the population of higher levels decreases exponentially. Accordingly, the first level is populated to a much lesser extent than the zero one, which leads to a much lower intensity of the Anti-Stokes lines in the Raman spectrum compared to the intensity of the Stokes lines.
As a rule, the Raman spectrum is understood precisely as its more intense, Stokes part. The frequency of Rayleigh scattering (that is, the frequency of the radiation source) is taken as "zero", and the frequency of the line in the spectrum is calculated by subtracting the frequency of the Stokes line from the frequency of the Rayleigh radiation.
In the general case, the process of light scattering competes with the process of its absorption. When radiation is absorbed, the molecule is transferred to the lowest excited electronic state. The reverse transition to the ground state can either be completely non-radiative or be accompanied by the emission of light of a lower frequency. This radiation is called photoluminescence. Depending on the spin configuration of the excited electronic state, photoluminescence is divided into fluorescence and phosphorescence.
The photoluminescence lines are much more intense than the Raman lines. Thus, with a Raman spectrometer equipped with a suitable detector, it is possible to simultaneously obtain the Raman spectrum and the photoluminescence spectrum at the same point without any problems.
However, in some cases, the photoluminescence spectrum can be superimposed on the Raman spectrum, which is an undesirable effect. Below is a conditional Raman/photoluminescence spectrum of a colored polymer that strongly fluoresces in the visible and near-IR region when irradiated with light in the UV (ultraviolet) and visible ranges. In such cases, as a rule, one tries to select the frequency of the exciting radiation in such a way as to avoid the appearance of intense fluorescence. One of the options is the use of radiation sources in the near-IR range for excitation of Raman radiation.
Conditional Raman/photoluminescence spectra of a colored polymer that strongly fluoresces in the visible and near-IR region when irradiated with light in the UV and visible ranges. The abscissa shows the radiation frequency
Conditional Raman/photoluminescence spectra of a colored polymer that strongly fluoresces in the visible and near-IR region when irradiated with light in the UV and visible ranges. The abscissa shows the difference between the Raman frequency and the excitation frequency (in cm-1)
The Raman spectrum of most organic molecules consists of lines corresponding to bending and stretching vibrations of the chemical bonds of carbon (C) with other elements, usually hydrogen (H), oxygen (O), and nitrogen (N), as well as characteristic vibrations of various functional groups ( hydroxyl -OH, amino group -NH2, etc.). These lines appear in the range from 600 cm-1 (stretching vibrations of single C-C bonds) to 3600 cm-1 (vibrations of the hydroxyl -OH group). In addition, in the spectra of organic molecules in the range of 250–400 cm–1, bending vibrations of the aliphatic chain appear.
In contrast to the IR spectrum, in which lines appear that correspond to vibrational transitions with a change in the dipole moment, lines appear in the Raman spectrum that correspond to vibrational transitions with a change in the polarizability of the molecule. Thus, IR and Raman are not exclusive, but complementary spectrometric methods. There are Raman spectrometers that make it possible to simultaneously obtain Raman and IR spectra at one point.
The Raman spectra of crystal lattices contain lines corresponding to the scattering of radiation by collective excited states of the lattice, which in solid state physics are considered as quasiparticles. The most common are Raman-active transitions involving optical and acoustic phonons, plasmons, and magnons.
Quasiparticle - a quantum of collective oscillation or perturbation of a many-particle system, which has a certain energy and, as a rule, momentum. There are a number of similarities and differences between quasiparticles and ordinary particles. In many field theories, such as conformal field theory, no distinction is made at all between particles and quasiparticles.
A phonon is a quasiparticle introduced by the Russian scientist I. Tamm. A phonon is a quantum of vibrational motion of crystal atoms. The concept of the phonon turned out to be very fruitful in solid state physics. In crystalline materials, atoms actively interact with each other, and it is difficult to consider such thermodynamic phenomena as vibrations of individual atoms in them - huge systems of trillions of interconnected linear differential equations are obtained, the analytical solution of which is impossible. Oscillations of crystal atoms are replaced by the propagation in the substance of a system of sound waves, the quanta of which are phonons. The phonon spin is zero (in units of h). The phonon is one of the bosons and is described by the Bose-Einstein statistics. Phonons and their interaction with electrons play a fundamental role in modern ideas about the physics of superconductors.
An acoustic phonon is characterized for small wave vectors by a linear dispersion law and a parallel displacement of all atoms in the unit cell. Such a dispersion law describes the acoustic oscillations of the lattice (that is why the phonon is called acoustic). The energy of acoustic phonons is usually low (on the order of 1 cm-1 to 30 cm-1).
Optical phonons exist only in crystals whose unit cell contains two or more atoms. These phonons are characterized at small wave vectors by vibrations of atoms such that the center of gravity of the unit cell remains immobile. The energy of optical phonons is usually quite high (on the order of 500 cm-1) and weakly depends on the wave vector.
Plasmon is a quasi-particle corresponding to the quantization of plasma oscillations, which are collective oscillations of a free electron gas. Plasmons play an important role in the optical properties of metals. Light below the plasma frequency is reflected because the electrons in the metal shield the electric field in the light's electromagnetic wave. Light above the plasma frequency gets through because the electrons can't respond fast enough to shield it. In most metals, the plasma frequency is in the ultraviolet region of the spectrum, making them shiny in the visible range. In doped semiconductors, the plasma frequency is usually in the ultraviolet region.
Surface plasmons (plasmons confined to surfaces) interact strongly with light, resulting in the formation of polaritons. They play a role in surface Raman enhancement, Raman scattering (SERS) and in explaining anomalies in metal diffraction. Surface plasmon resonance is used in biochemistry to detect the presence of molecules on a surface.
A magnon is a quasiparticle corresponding to an elementary excitation of a system of interacting spins. In crystals with several magnetic sublattices (for example, antiferromagnets), there can be several types of magnons with different energy spectra. Magnons obey Bose-Einstein statistics. Magnons interact with each other and with other quasiparticles. The existence of magnons is confirmed by experiments on the scattering of neutrons, electrons and light, accompanied by the birth or annihilation of a magnon.
The magnon concept was introduced in 1930 by Felix Bloch to explain quantitatively the phenomenon of the decrease in spontaneous magnetization in ferromagnets. At a temperature of absolute zero, a ferromagnet reaches its lowest energy state, in which the atomic spins (as well as the magnetic moments) align in the same direction. As the temperature rises, the spins begin to deviate from the general direction, thereby increasing the internal energy and decreasing the total magnetization. If we imagine an ideally magnetized ferromagnet as a vacuum state, then the state at low temperatures, in which the ideal order is violated by a small number of inverted spins, can be represented as a gas of quasiparticles - magnons. Each magnon reduces the number of correctly aligned spins by h and the total magnetic moment along the quantization axis by gh, where g is the gyromagnetic ratio.
2. Application of Raman spectroscopy for control
medicinal, narcotic and toxic drugs
Method of spectroscopy of Raman scattering of light (Raman spectroscopy).
Raman spectroscopy makes it possible to identify the gases of inhaled and exhaled air and measure their concentration by analyzing the light emission of gas molecules as they return to their original (unexcited) energy state after exposure to a laser beam. Patient gas samples enter the measuring chamber, where they are irradiated with an argon laser. As a result of laser irradiation, gas molecules pass into an excited state, but upon the reverse transition to the initial (unexcited) state, gas molecules already emit light of lower energy and longer wavelength. This phenomenon is known in physics as the "Raman shift".
The amount of "Raman" wave shift for each gas is specific, which allows identification of gases in the sample (for example, which inhalation anesthetic is used). The gas concentration is determined from the intensity of the secondary radiation. As well as mass spectrometry, the Raman spectroscopy method allows you to determine the concentration of all components of the gas mixture. The results of mass spectrometry and Raman spectroscopy are equally accurate, despite the presence of fundamental differences in technology. The advantages of Raman spectroscopy are faster results and the possibility of self-calibration.
Main advantages
Raman scattering technology refers to vibrational molecular spectroscopy. Vibrations arise in molecules due to the displacement of the nuclei from the equilibrium position. Vibrational spectra are recorded in the form of infrared spectra and Raman spectra (Raman spectra).
The Raman spectrum occurs when a substance is irradiated with monochromatic light in the ultraviolet or visible range. Under the influence of light, the molecules of a substance are polarized and scatter light. In this case, the scattered light differs from the frequency of the initial radiation by an amount corresponding to the frequency of normal vibrations of the molecule. The individuality of this characteristic determines the high selectivity of the method.
The appearance of the Raman spectrum can be represented as follows: a quantum of incident radiation interacts with a molecule that is in the ground or excited vibrational state. If the interaction is elastic, then the energy state of the molecule does not change, and the frequency of the scattered radiation will be the same as that of the incident radiation (the Rayleigh band of the Raman spectrum). In the case of an inelastic interaction, energy is exchanged between the radiation quantum and the molecule, due to which scattered radiation arises, which can be of a higher or lower frequency (anti-Stokes and Stokes bands, respectively). Thus, the Raman spectrum is formed.
The Raman spectroscopy method makes it possible to obtain an individual spectral imprint, unique in relation to the considered molecule or the whole molecular structure.
Compared to FT-IR spectroscopy, Raman spectroscopy has significant key advantages:
Raman spectrum of sulfur
* The Raman spectroscopy method can be used for the analysis of aqueous solutions, since the high absorption effect of water, in contrast to the IR Fourier method, does not significantly affect it.
* Intensity spectral lines in solution is directly proportional to the concentration of specific compounds;
* Raman spectrum does not depend on changes in solution temperature;
* The Raman spectroscopy method practically does not require sample preparation, the use of reagents, and is not affected by the cell material, such as glass;
* The use of Raman spectrometers allows you to achieve high resolution and sensitivity, unattainable for IR-Fourier spectrometry.
These advantages, due to the specific nature of the method, make Raman spectrometry a powerful tool for analyzing and monitoring chemical composition. Each compound has its own unique Raman spectrum. Depending on the instrumentation used, this technique can be used to analyze solids, solutions, as well as provide information about the physical characteristics of the medium under study, such as crystal lattice, orientation, polymorphic forms, etc.
An image of a cell obtained using a Raman spectrometer.
3. Raman scattering of light as a method for studying matter
Raman spectra can be used to measure the natural vibration frequencies of molecules and crystals. This opens up wide possibilities for the identification of substances and the study of the transformations occurring in them under the influence of external influences. Let's give some examples. One and the same substance can have several modifications, say, carbon is in the form of graphite, diamond, an amorphous phase. Chemical or spectral analyzes do not make it possible to distinguish between these phases, but the Raman spectra for them will differ, since not only chemical composition substance, but also its structure. With the help of RRS, one can study the processes of crystal melting and crystallization of liquids, explore chemical reactions in solutions, detect the appearance of thin films on the surface of solids and characterize their structure, etc. Changes in temperature, pressure, and other external factors lead to a change in the symmetry of the lattice of some crystals (structural phase transformations). The rearrangement of the crystal lattice naturally leads to a change in its vibrational spectrum, and Raman is a subtle tool for analyzing these transformations.
Figure 1. shows the scheme of transitions, the result of which is the presence of red and violet satellites in the Raman spectrum (RS). Find the natural vibration frequency of the nuclei in the sulfur molecule? 0 and the quasi-elastic force coefficient, if it is known that in the vibrational spectrum of Raman scattering of light, the red and violet satellites closest to the unshifted line correspond to wavelengths of 346.6 and 330.0 nm. What is the excitation wavelength of the RC? spectrum? What is the ratio of the intensities of the red and violet satellites at room temperature? Ignore the anharmonicity of vibrations.
Posted on http://www.allbest.ru/
Figure 1. Scheme of the emergence of Stokes (a) and anti-Stokes (b) satellites in Raman scattering
Analyzing the schemes presented in Figure 1, we see that the frequencies of the red and purple satellites are related to the natural oscillation frequency by the ratios:
where? the frequency of the light that excites the RR. Similar relationships will take place for the wave numbers corresponding to the lines - satellites:
From formulas (2) one can obtain:
Taking into account that, from (3) we obtain the wave number corresponding to the natural vibrations of the molecule:
After calculations, we find:
In this case, the frequency is rad / s.
The quasi-elastic force coefficient is related to the natural oscillation frequency by the relation:
where is the mass of the sulfur atom.
As a result of calculations, we get:
The wave number of the exciting line can be found by formula (2):
Based on this
After calculations, we get:
Figure 1. shows that the red satellite is the result of light scattering by molecules that were in the ground vibrational state, and the purple satellite is the result of light scattering by excited molecules. The difference in the intensity of the satellites is mainly due to the difference in the population of the states. If we do not take into account the influence of other factors, then the ratio of the intensities of the violet and red satellites will be equal to the ratio of the populations. Therefore, taking into account the Boltzmann formula, we obtain:
Assuming 300 K, after calculations we find:
Answer: 338.1; 0.031.
Raman scattering light spectroscopy medicinal
Conclusion
AT last years the features of the RRS phenomenon itself are intensively studied. Researchers have approached essentially a number of new phenomena. These include: 1) resonant Raman scattering, which consists in a sharp increase in the effective scattering cross section as the frequency of the exciting radiation approaches the electron absorption band of the substance; 2) stimulated Raman, consisting in a sharp decrease in the width and an increase in the intensity of one or more Raman lines to values comparable to the intensity of the exciting radiation; 3) hyper-Raman scattering of light, which consists in the appearance of Raman satellites in the frequency region of the second optical harmonic of the exciting radiation; 4) giant RS, which consists in increasing up to 105-106 times the effective scattering cross section for a number of molecules adsorbed on the rough surface of some metals; 5) coherent anti-Stokes scattering, which consists in a sharp increase in the intensity and angular directivity of the scattering signal when the substance is simultaneously excited by two laser light sources. All these phenomena open up new possibilities for solving scientific and practical problems and will undoubtedly be used in the future.
As one of the very promising directions Let us note the so-called Raman microscopy, which has been developed in recent years. Here, work is proceeding along the path of creating new types of microscopes that make it possible to obtain an image of microobjects "in the light" of various Raman lines. In this case, it is possible to distinguish such details of microobjects that are either completely indistinguishable or poorly distinguishable in a conventional microscope. In particular, with the help of differences in the Raman spectra, it is possible to distinguish "healthy" cells from "sick" ones and to establish the microscopic nature of the disease; opportunities open up for obtaining data on the isotopic composition of substances and microdefects, as well as on stresses in solids. Of great interest is the direction associated with selective heating in the process of forced Raman vibrational degrees of freedom of solids for the catalysis of chemical, biological and even nuclear processes.
Thus, Raman scattering studies, which began in the 20th century, have come a long way from unique experiments in academic laboratories to large-scale experiments of great practical importance.
Bibliography
1. Landsberg G.S., Mandelstam L.I. A new phenomenon in light scattering
2. V.L. Ginzburg, I.L. Fabelinsky, "On the history of the discovery of Raman Scattering of Light"
3. Guseva E.V., Orlov R.Yu. Spectroscopy of Raman scattering of light (Raman spectroscopy). Application in mineralogy and materials science
4. Guseva E.V., Melnikov F.P., Orlov R.Yu., Uspenskaya M.E. Raman spectroscopy of light for the analysis of gas-liquid inclusions
5. M. M. Sushchinskii, Raman scattering spectra of molecules and crystals
6. Brandmuller I., Moser G., Introduction to Raman spectroscopy
7. Kohlrausch K. "Raman Spectra"
Hosted on Allbest.ru
Similar Documents
Elastic and inelastic light scattering, the theory of the combination method. The use of Raman spectroscopy for the control of medicinal, narcotic and toxic drugs. Raman scattering of light as a method for studying matter, main advantages.
term paper, added 10/28/2011
The concept of Raman scattering of light. Variable field of a light wave. Quantum transitions in Raman scattering of light. The appearance of additional lines in the scattering spectrum. The device of the Raman microscope, the main areas of its application.
abstract, added 01/08/2014
Investigation of ultrananocrystalline diamond films by Raman scattering methods. Power influence laser radiation on the information content of the spectra. Prospects of UNCD films as a new nanomaterial for use in electronics.
term paper, added 01/30/2014
General information on the interaction of radiation with matter. Characteristics of the Raman spectrometer. Analysis of the low-frequency part of the spectrum of strontium-borate glass. Processing of the obtained experimental spectra to improve their quality.
term paper, added 12/03/2012
Physical mechanism of scattering by an individual particle. Mutual amplification or suppression of scattered waves. Multiple scattering of light. Total intensity of scattering by a cluster of particles. Polarization of light during scattering. The use of polarized light.
term paper, added 06/05/2015
Spectral measurements of light intensity. Investigation of light scattering in magnetic colloids of cobalt ferrite and magnetite in kerosene. Curves of the decrease in the intensity of scattered light with time after turning off the electric and magnetic fields.
article, added 03/19/2007
Determining the structure of matter as one of the central tasks of physics. Using the method of molecular light scattering in liquids. Lifetime of fluctuations in liquids. The mechanism that cuts the wing of the dispersion contour in real physical systems.
abstract, added 06/22/2015
The history of finding out the cause of the blue color of the sky: the theory of the ancient Greeks; Goethe's and Newton's hypotheses. The erroneousness of the Rayleigh theory of light scattering by thermal vibrations of the gaseous shell of the planet. Molecular scattering of light: Smoluchowski's theory of opalescence.
abstract, added 09/23/2012
Investigation of collision processes and development of scattering theory. Elastic scattering, in which the molecule remains in its original state after a collision. Calculation of the integral over the coordinates of the incident electron using the Fourier relation.
dissertation, added 05/19/2014
Quantum theory of Compton scattering. The direction of motion of the recoil electron. Light pressure. Serial regularities in the spectra of the hydrogen atom. Thomson, Rutherford model. Bohr's postulates. De Broglie's hypothesis. Elements of quantum mechanical theory.
Raman (Raman) scattering of light. Physical mechanism of scattering. Basic equations and parameters.
Raman (Raman) scattering of light.
In this lecture, we turn to the analysis of the effects of stimulated Raman scattering (SRS), perhaps one of the most important nonlinear wave processes. The essence of spontaneous Raman (Raman) scattering (RS) is as follows. When quasi-monochromatic radiation passes through a homogeneous medium, spectral components appear in the scattered radiation spectrum at frequencies ω s , which are com-
bination of the pump radiation frequency ω n and the frequency of natural oscillations of the medium ω 0 :
Here n is an integer. In this case, the scattered radiation is practically isotropic and its intensity is on the order of 10–6 –10–8 of the pump intensity. At present, this phenomenon is widely used for the qualitative and quantitative analysis of mixtures of liquids and gases, since it allows one to judge the composition by the ratio of intensities at frequencies ω s , i
a mixture that contains molecular components i having the corresponding frequencies ω 0, i .
ωn + 2 ω0 |
||||||||||
ωn + ω0 |
||||||||||
ωн , ωн − n ω0 |
||||||||||
ωn + ω0 |
||||||||||
ωn + 2 ω0 |
||||||||||
(Stokes components), and the intensity of these components was comparable with the pump intensity. In addition, when observing at a pump beam perpendicular to the axis, a number of rings were observed on the screen. In this case, the rings contained spectral components satisfying the condition ω a = ω n + n ω 0
(anti-Stokes components). This phenomenon is called stimulated Raman scattering (SRS).
Physical mechanism of scattering.
The physics of this phenomenon can be explained using the simple classical model of the interaction of light with molecules proposed by Placzek. The effect of Raman scattering is associated with the dependence of the electronic polarizability of the molecule α on the nuclear configuration, given by the coordinates of the nuclei in the molecule q:
α (q)= α 0 | + α 0 "q + ......α 0 " = | ∂ α . | ||
∂qq = 0 |
The term with ∂ α ∂ q q = 0 describes the modulation of light by molecular vibrations;
in this case, new frequency components appear in the polarization spectrum of the molecule, shifted by the frequency ω 0 of nuclear vibrations:
p = α (q) E= α 0 E+ α 0 "qE. |
Under conditions when the displacement of the coordinates of the nuclei in the molecule q is determined by thermal motions in the medium, equation (15.3) describes spontaneous Raman scattering. If the incident light field has a frequency ω n, and can-
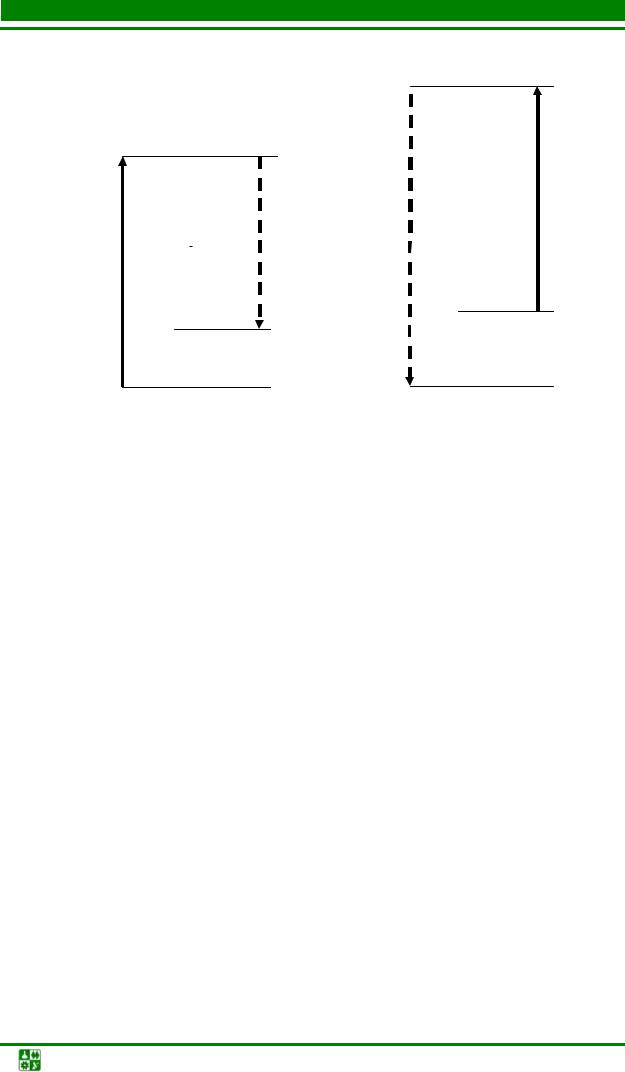
molecular vibrations occur with an average frequency ω 0 due to (15.1), then Stokes (ω c = ω n − ω 0 ) and anti-Stokes (ω a = ω n + ω 0 ) components appear in the light field scattered by the molecule.
Spontaneous Raman scattering has a fairly simple quantum interpretation (Fig. 15.2). When light radiation passes through a medium, a two-photon process is possible, in which a light quantum with a pumping frequency ω n is absorbed with simultaneous emission of a light quantum
at the frequency of the Stokes component ω s , and the molecule goes into an excited
condition. In the case when light interacts with a molecule in an excited state, then a two-photon process also occurs, in which a quantum at a frequency ω n disappears, a quantum at a frequency ω a is emitted, and the molecule does not
goes to the ground state.
LECTURE No. 15. STIMULATED RAMAN SCATTERING
ωс | ωn |
ωa |
ωn
Therefore, at α 0 "≠ 0, a force arises in the light field that acts on molecular vibrations:
f = − | ∂W | ∂α | E 2 \u003d α 0 "E 2. | |||
∂q | ∂q | q=0 |
This force can lead to their resonant buildup if the field contains two spectral components with frequencies ω 1 and ω 2 , the difference between which
ω 1 − ω 2 ≈ ω 0 is approximately equal to the frequency of molecular vibrations. In these
conditions, chaotic intramolecular oscillations, which have a fluctuation character, are superimposed by regular forced oscillations, the phases of which are determined by the phases of the light fields.
Stimulated Raman scattering is a process that occurs due to optical excitation of intramolecular vibrations; the resonant doublet arises due to the Stokes scattering of a powerful laser wave

LECTURE No. 15. STIMULATED RAMAN SCATTERING
(pump waves). If intramolecular vibrations are excited by light, the scattering pattern also changes qualitatively. The weak growth of scattered light components in spontaneous scattering is replaced by an unstable exponential growth of scattered components in stimulated scattering. In nonlinear optics, this circumstance is used for efficient frequency conversion.
Basic equations and parameters.
Let us present a simple theoretical model describing the SRS process. Using relation (15.5 ), we write the equation for molecular vibrations (we neglect the effects of changes in the population difference) in the form
q + ω 0 2 q= | α "E 2, | |||||
where m is the effective mass of the molecule; T | - relaxation time. |
|||||
Let us consider the interaction of the pump wave E n and the Stokes wave E c |
||||||
in the medium described by equation (15.6). In this case | ||||||
E \u003d En + Es, | ||||||
Åí | = А н (t ,z )expi (ω í t − k í z )+ k c . |
|||||
= А c (t ,z )expi (ω c t − k c z )+ k c , | ||||||
where p n ip s are the refractive indices at frequencies ω n and ω s | = ω n − ω 0 . The equation |
|||||
(15.6 ) describes the action of the field on molecular vibrations. In turn, the oscillations q create a nonlinear polarization in the medium P nl = N α "q E and, thus
thus, have an inverse effect on the field, which is expressed by the equation
∂2E | ∂2E | 4 π∂ 2 P | ||||
∂z 2 | ∂t2 | nl. |
||||
c2∂t2 | ||||||
According to expression (15.6), the resonance curve of the molecular oscil- |
||||||
lator is characterized by a width | δω0 | 2 T . This quantity is also called |
||||
line width of spontaneous Raman scattering and is usually expressed
are in cm–1 δν 0 = δω 0 2 π c = 1 π cT .
Considering the interaction of waves close to monochromatic, we write q in a form similar to (15.7 )–(15.8 ):
q = Q(t, z)exp i(ω 0 t − k0 z) + kc . |
Then in the stationary case, the solution of equation (15.6), taking into account (15.7) - (15.8), for ω 0 = ω n − ω s has the form (15.11)
2 ωmc
We note here that the wave vector k 0 of molecular excitations is determined by the wave vectors of pumping k n and Stokes radiation k s: k 0 = k n – k s and is a “fictitious” wave vector, since the vibrations of each molecule occur independently, and the phase of these vibrations is determined by the phase difference pump and Stokes components, which, in turn, depend on the dispersion of the medium. Considering the amplitudes And with And n, and Q changing relatively slowly in space, from equation (15.9) we obtain for a slowly changing amplitude a system of truncated equations of the following form:
∂À ñ | 1 gA А, |
|||
∂z | ||||
∂À í | 1ω i |
|||
gA А . |
||||
∂z | ||||
The parameters of these equations are: the relaxation time T, the frequencies of the interacting waves, and the constant that determines the optical nonlinearity of the medium
g"= | 4 πTN α" ωc | |||
2mnn | ||||
Considering A n strong and given, from equation (15.12) for A with we obtain a solution in the form
Ac = A0 c exp gz. |
Here A 0 s is the amplitude of the Stokes component at the entrance to the medium, which is a thermal and very weak radiation; g \u003d g" A n 2.
Hence, we see that the amplitude of the Stokes component grows exponentially along the propagation of the pump beam. The amplitude of molecular vibrations also grows exponentially with distance, which in quantum language means an increase in the population of the excited state of molecules. The latter should also lead to an increase in the anti-Stokes component. Characteristics this process will be discussed below.
Thus, using the classical Placzek model, it is shown that the field of the Stokes component can grow exponentially along the direction of the pump beam.
Raman scattering of light, scattering of light waves, in which the frequencies of incident and re-emitted (scattered) waves differ from each other by the frequencies of natural vibrations in matter (frequencies of quantum transitions between electronic, vibrational and rotational energy levels in atoms and molecules, frequencies of optical phonons in crystals etc.).
The effect of Raman scattering of light was predicted by the Austrian physicist A. Smekal in 1923; discovered by G. S. Landsberg and L. I. Mandelstam in 1928 during the scattering of light in crystals. At the same time, Raman scattering of light in liquids was registered by C. V. Raman and the Indian physicist K. S. Krishnan.
The classical picture of Raman scattering of light can be considered using the example of molecular vibrations. fluctuations atomic nuclei in a molecule with a frequency Ω modulate the polarizability of the molecule, which leads to a corresponding change in the permittivity of the medium. The amplitude of a light wave with a frequency ω 0 passing through a medium with a modulated permittivity, is modulated with frequency Ω. This means that in the wave spectrum, in addition to the main component, "side" lines appear with frequencies ω s = ω 0 -Ω and ω a = ω 0 + Ω. The low-frequency line (ω s) is usually called the Stokes component of Raman scattering, and the high-frequency line (ω a) is called the anti-Stokes component.
A similar interpretation of Raman scattering of light is also valid for the case of elastic vibrations in solids, for the rotational motion of molecules, for the intraatomic (intramolecular) motion of electrons, etc.
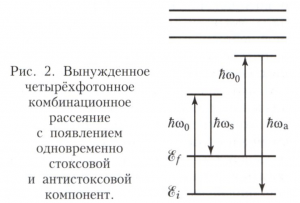 According to quantum theory, Raman scattering of light is a two-photon process (see Multiphoton processes), in which in one elementary act of interaction electromagnetic field with a quantum system (atom, molecule, etc.) one photon of incident radiation with frequency ω 0 is absorbed and a photon of scattered radiation with frequency ω is emitted. In this case, the quantum system passes from the initial state with the energy E i to the final state with the energy E f . In accordance with the law of conservation of energy, the frequency of scattered radiation is determined by the equality ћω = ћω 0 - ћω fi , where ω fi = (E f - E i)/ћ is the quantum transition frequency, ћ is Planck's constant. If the quantum system was originally in a state of lower energy, E i< E f (рис. 1 ,а), то рассеянное излучение смещено в сторону меньших частот на величину Ω = |ω fi |, то есть ω = ω s = ω 0 -Ω (стоксова компонента). Если же E i >E f (Fig. 1b), then the scattered light has a higher frequency: ω = ω a = ω 0 + Ω (anti-Stokes component). Thus, ω is a combination of the frequency of the incident radiation and the natural frequencies of the substance: hence the name of the effect. It is also widespread (especially in foreign literature) the name Raman scattering, or the Raman effect.
According to quantum theory, Raman scattering of light is a two-photon process (see Multiphoton processes), in which in one elementary act of interaction electromagnetic field with a quantum system (atom, molecule, etc.) one photon of incident radiation with frequency ω 0 is absorbed and a photon of scattered radiation with frequency ω is emitted. In this case, the quantum system passes from the initial state with the energy E i to the final state with the energy E f . In accordance with the law of conservation of energy, the frequency of scattered radiation is determined by the equality ћω = ћω 0 - ћω fi , where ω fi = (E f - E i)/ћ is the quantum transition frequency, ћ is Planck's constant. If the quantum system was originally in a state of lower energy, E i< E f (рис. 1 ,а), то рассеянное излучение смещено в сторону меньших частот на величину Ω = |ω fi |, то есть ω = ω s = ω 0 -Ω (стоксова компонента). Если же E i >E f (Fig. 1b), then the scattered light has a higher frequency: ω = ω a = ω 0 + Ω (anti-Stokes component). Thus, ω is a combination of the frequency of the incident radiation and the natural frequencies of the substance: hence the name of the effect. It is also widespread (especially in foreign literature) the name Raman scattering, or the Raman effect.
Photons of scattered radiation can be emitted as a result of either spontaneous quantum transitions or transitions induced by radiation at a frequency ω (see Stimulated Emission). In the first case, we are talking about the so-called spontaneous Raman scattering of light, in the second - about stimulated Raman scattering (see Stimulated light scattering). During spontaneous scattering, radiation occurs in all directions, and individual acts of photon emission are not correlated with each other. Therefore, the scattered radiation turns out to be incoherent. Its intensity is proportional to the intensity of the incident radiation and the density of the number of quantum systems (atoms, molecules) that are at the initial level. Since under normal conditions of population quantum levels decrease rapidly with increasing energy, the intensity of the anti-Stokes components, for which the starting level is excited, is, as a rule, significantly less than the intensity of the Stokes scattering lines.
To obtain spectra of spontaneous Raman scattering of light, it is necessary to use sources of intense monochromatic radiation. As such, mercury lamps were used for a long time. Since the 1960s, lasers have been the main sources of exciting radiation.
Spontaneous Raman scattering of light is usually recorded in the direction perpendicular to the beam of exciting light. In this case, the spectrum contains a central line with a frequency ω 0 due to Rayleigh scattering of the incident radiation (see Light Scattering) and a series of satellite lines in the Stokes (low frequency) and anti-Stokes regions. Measuring the frequency intervals between the lines provides information about the structure of the energy spectrum of a substance, and the width and shape of the spectral lines make it possible to judge the relaxation processes in the substance.
Since Raman scattering is a two-photon process, it obeys selection rules different from those for single-photon processes responsible for resonant light absorption and luminescence. Therefore, Raman spectra often exhibit quantum transitions that are absent in absorption and luminescence spectra. Raman scattering of light makes it possible to study, using optical radiation, elementary excitations whose frequencies lie in the far infrared region and even in the radio range.
Stimulated Raman scattering (SRS) manifests itself at a high intensity of the incident radiation (pump). In this case, the density of scattered photons becomes so significant that the predominant contribution to the production of new photons comes from stimulated emission processes. As a result, correlations arise between elementary scattering events in different points medium, and the scattered radiation becomes coherent.
During SRS, in addition to two-photon processes, a significant role is played by four-photon parametric processes with frequencies 2ω 0 - ω s - ω a = 0 (Fig. 2), which are responsible for the simultaneous appearance of the Stokes and anti-Stokes components. Since phase matching of waves occurs during parametric processes, the anti-Stokes component is observed during SRS only in the direction of pump propagation. At the same time, efficient generation of the Stokes component is possible both in direct and in reverse direction. SRS is widely used for frequency conversion of laser radiation.
Spectroscopy of spontaneous Raman scattering of light is an effective method for studying the structure of molecules and the processes of their interaction with environment. The methods of Raman scattering of light are also used to study quasiparticles in solids, the structure of nanostructured formations and processes in them. Raman scattering of light is used in spectral analysis, which is widely used in chemical research. Each chemical compound has its own specific Raman spectrum, on the basis of which it is possible to identify this compound and detect it in a mixture (see Spectral analysis). The parameters of some lines in the Raman spectra are preserved in different chemical compounds containing the same structural element, for example C=C, C-H, etc. This is used in structural analysis molecules with unknown structure.
Stimulated Raman scattering of light has become the basis for a number of methods of nonlinear spectroscopy, which make it possible to carry out studies in matter with high spatial and temporal resolution.
Lit.: Sushchinsky M. M. Raman scattering spectra of crystal molecules. M., 1969; he is. Raman scattering of light and the structure of matter. M., 1981; Shen IR Principles of nonlinear optics. M., 1989.






