A photon is a quantum of an electromagnetic field. Photon. The structure of a photon. Movement principle
Photon
Since ancient times, it has been known about the phenomena of reflection and refraction of light. The essence of these phenomena, their true nature is still not clear to official science, everything is built on the theory of probability.
In modern scientific literature a photon is called a quantum electromagnetic field, presumably an elementary particle, which in the light of modern theories is presented as a carrier electromagnetic interaction. Although, in fact, the modern name “photon” means only an observed process - the smallest “portions”, “beams” of light that make up waves of electromagnetic radiation, including visible light, radio waves, X-rays, laser pulses and so on.
The concept of a photon (from the ancient Greek word "φωτός" (photos) - "light") was introduced in 1926 by the American chemist Gilbert Newton Lewis. By the way, he considered photons "uncreated and indestructible" (this is similar to the history of the introduction of the concept of an atom by chemist John Dalton, who based his reasoning on ancient knowledge of indivisible particles).
Today, the photon is denoted in physics by the symbol of the Greek letter gamma - γ. This designation is associated with the discovery in 1900 of gamma radiation, consisting of high-energy photons. It was made by the French physicist Paul Villars in the process of studying the radiation of radium in a strong magnetic field. Subsequently, the English physicist Ernest Rutherford, who previously named two types of uranium radiation as alpha and beta rays, found that the new type of radiation discovered by Villar had a high penetrating power. He gave the name to this radiation "gamma rays".
“In a scientific article in 1926, Gilbert Lewis writes: “I express the hypothesis that here we are dealing with a new type of atom, an unidentified object, uncreated and indestructible, which acts as a carrier of radiation energy and, after absorption, remains as the main component of the absorbed its atom until it comes out again with a new amount of energy ... I take the liberty of suggesting for this hypothetical new atom, which is not light, but plays an important role in all radiation processes, the name "photon" ”. An interesting fact is that Gilbert Lewis considered the photon to be precisely the “radiation/radiation energy carrier”, and not this energy itself (now physicists consider the photon as the carrier of electromagnetic force). Since then, the word "photon" has quickly come into use.
References: Lewis, Gilbert N. The conservation of photons Nature 118, 1926, pp. 874–875; Lewis, Gilbert N. The nature of light. Proceedings of the National Academy of Science 12, 1926, pp. 22-29; Roychoudhuri, Chandra, Kracklauer, A.F., Creath, Kathy. The Nature of Light: What is a Photon? CRC Press, 2008.
The discovery of the photon significantly stimulated the development of theoretical and experimental physics, including physical chemistry(photochemistry), quantum mechanics etc. People began to roughly understand and use the manifestations of such physical phenomena, how electricity, a stream of photons. But knowledge about the smallest structure of these phenomena is approximate, because until now official science cannot explain what exactly the same electron or photon itself consists of (although this knowledge about the true nature of the microcosm was in ancient times).
The nature of the photon remains a mystery to scientists. But even relying on the results of research that were recorded in the process of observation, thanks to experiments, discoveries were made that were widely used in the life of society. A variety of technical devices have been invented, the principle of operation of which is associated with the use of photons. For example, computed tomography, quantum generator (maser), laser, and so on. The laser has found the widest practical application in industry, medicine, everyday life, ranging from the creation of high-precision physical instruments - seismographs, gravimeters, laser scalpels used in microsurgery, to the creation of technological processes for welding, cutting metals, household laser printers, and so on. Photons are also used in spectral analysis (atomic spectroscopy deals with the study of the spectra of electromagnetic radiation of atoms). Through the study of photons, scientists have found that the atoms of each chemical element have well-defined resonant frequencies. It is at these frequencies that they emit and absorb light (photons). That is, just as each person has individual fingerprints, so each chemical element has its own unique emission and absorption spectrum. And all this is just the beginning of the study of such a unique structure as a photon, which takes an active part in various power processes and interactions in nature.
All theoretical physics of elementary particles is built on the theory of probability. However, the analysis of the objective foundations of the theory of probability was actively discussed only during the creation of quantum mechanics. Now the nature of probability is not discussed so vividly by physicists. On the one hand, everyone admits that it is part of the foundations of microprocesses, and on the other hand, in the course of the research itself, little is said about it, as if it plays a secondary role. This is especially true for elementary particle physics, where, with the characteristics of internal states and properties of elementary particles, the concept of probability is mostly hushed up. As one of the founders of quantum electrodynamics, American scientist Richard Feynman said: “No matter how hard we try to invent a reasonable theory explaining how a photon “decides” whether to pass through glass or bounce back, it is impossible to predict how a given photon will move. Here is a condition that leads to different results: identical photons fly in the same direction towards the same piece of glass. We cannot predict whether a given photon will hit A or B. All we can predict is that out of 100 photons emitted, an average of 4 will bounce off the surface. Does this mean that physics, a science of great precision, has been reduced to calculating the probability of an event, and not predicting exactly what will happen? Yes. The way it is". By the way, the mentioned problem about photons still remains an unresolved issue, except for official science. But for ALLATRA SCIENCE scientists, it has long been resolved.
Literature: Philosophical problems of elementary particle physics (thirty years later). Rep. Ed. Yu.B. Molchanov. M., 1994; Feynman R. QED is a strange theory of light and matter. M., 1988.
But what do photons and electrons actually represent, what exactly do these structures consist of? Due to what component is the photon stable and participates in force interactions? Why does this so-called "massless elementary particle" in modern physics not have electric charge? Why is the photon one of the smallest and most common elementary particles in the universe? Now official science cannot answer these questions, since the photon still, despite the rich accumulated experimental material, remains a mysterious elementary particle for it. But this situation is easy to fix. Knowing the basics of PRIMORDIAL ALLATRA PHYSICS, even a schoolboy can find answers to these questions.
IN REALITY, the PHOTON, if considered as a true elementary particle, consists of phantom Po particles. A photon can exist in two states: PHOTON-3 (γ3) and PHOTON-4 (γ4). Most photons consist of 3 phantom Po particles (photon-3). However, each of these photons under certain conditions can be converted into a photon consisting of 4 phantom Po particles (photon-4), and photon-4 can be converted into photon-3. According to its state, a photon can perform either the functions of a power particle (photon-3) or an "information" particle (photon-4), that is, in the latter case, act as a carrier of information about the elementary particle with which it interacts. It is noteworthy that for a photon moving along the ezoosmic grid, the spiral rotation of its phantom Po particles is more accelerated than that of the phantom Po particles of many other elementary particles. Thanks to such accelerated “swirls” of the photon structure, its speed of movement is greater compared to the speed of movement of many other elementary particles.
Photon-3 and photon-4 move, as a rule, in the same energy flow, and there are always many more photons-3 in it than photons-4. For example, a stream of photons comes from the sun, where most of them are force photons (photons-3) responsible for energy, force interactions, but among them there are also information photons (photons-4) that carry information about the sun. Streams of photons-3 do not carry heat, they create it when the particles they collide with are destroyed. The greater the flux of photons-3 directed at right angles to the material object, the more heat is generated. Thanks to information photons (photons-4), a person, for example, sees light from the sun and the sun itself with his eyes, and thanks to power photons (photons-3), he feels the heat from the sun on himself, and so on. That is, thanks to photons-3, an energy flow is provided (as well as various force interactions in the material world), and thanks to photons-4, information is delivered in this energy flow (that is, participation in processes that allow, for example, a person to see the world around him).
PHOTON-3 comprises three phantom Po particles, or rather, from two phantom Po particles interconnected by one Allat phantom Po particle. It is the inclusion in the composition of the Allat phantom Po particle that makes the photon unique, stable, and also an active participant force interactions. By the way, a The Llat phantom Po particle will never be in the place of the first head phantom Po particle in any elementary particle that has it in its composition. It will always be located inside the elementary particle between the phantom Po particles, as the force basis of this particle.
Photon-3 can transform into photon-4, and photon-4 can transform into the state of photon-3. How does this process take place? A photon (meaning both photon-3 and photon-4) has a unique structure that distinguishes it from any other elementary particle. In particular, it has an unusual first (head) phantom Po particle. If appropriate conditions arise in the ezoosmic cell, under which it simultaneously enters from different sides two head phantom Po particles (one of which belongs to a photon, and the second to another elementary particle) and their closest approach occurs, then the following process occurs.
The head phantom particle Po of a photon due to its greater speed relative to the speed of movement of the head phantom particle Po of another elementary particle quickly rotates. Thus, it allows the force particle of the photon following it (the Allat phantom Po particle) to capture its head phantom Po particle from the counter elementary particle, which is the carrier of all information about this elementary particle.
Photon-3, capturing the head phantom Po particle of another elementary particle, attaches this informational particle to its structure. As a result, photon-3 is transformed into photon-4, consisting of four phantom Po particles. In this case, the elementary particle from which the head phantom Po particle was removed undergoes destruction, as a result of which energy is released. In general, such a process of capturing information by a photon occurs only if the head phantom Po particle of the elementary particle passes through this ezoosmic cell, and not other phantom Po particles that are part of the elementary particle.
When photon-3 knocks out the head phantom Po particle from an elementary particle, it turns from a “capturer” into a “transporter”, that is, an information carrier (photon-4). Returning to the associative example with a train and wagons, this is similar to how a train of three wagons, moving at full speed, grabs a locomotive from an oncoming train. Thus, it becomes a train with two locomotives, one diplomatic car and one simple car, until conditions arise under which it can free itself from the locomotive captured in its composition. The remaining cars of the oncoming train, having lost the locomotive, are disbanded in the depot (in the ezoosmic membrane).
PHOTON-4 consists of four phantom Po particles: a unique head phantom Po particle, an “alien” head phantom Po particle (information particle), an Allatian phantom Po particle, and a final phantom Po particle. It is the entry of this “alien” head phantom Po particle into the composition of photon-4 that makes it information-filled, that is, carrying information about a given ("foreign") elementary particle. But in general, when there are many such photons, they carry information about a particular subject, object, phenomenon, and so on. The photon exists in this state (photon-4) until similar conditions arise again in the ezoosmic cell, under which it is released from the “foreign” head phantom Po particle, that is, the process of “information reset” occurs. At the same time, the head phantom Po particle of the photon rotates again, and due to the participation of the Allat power Po particle in this process, the “alien” head phantom Po particle is pushed out into the limits of its own septon field of the counter head phantom Po particle of the elementary particle. The photon itself, transforming into the state of photon-3, leaves the ezoosmic cell. The released head phantom Po particle dumps information into its own septon field of the real Po particle and the passing head phantom Po particle of the elementary particle (thereby enriching their internal potential with new information) and irrevocably goes to the ezoosmic membrane.
After resetting (transferring) the informational “foreign” head phantom Po particle, photon-4 again turns into photon-3, that is, it goes into its original state, in which it is characterized by the multivariability of various actions. For example, photon-3 can participate in other interactions, be part of elementary particles, and so on. It can disappear (due to the ezoosmic membrane) in one place and appear in another place, that is, it can make an almost instantaneous transition in the ezoosmic grid over large ("cosmic") distances. Of course, this is just short information about a photon, intended for primary acquaintance. In addition, there is a lot of unique information obtained in the course of research regarding the patterns and paradoxes of the photon's behavior in various environments, its features wave properties, interactions with other elementary particles, photon behavior control algorithms and much more.
In general, summarizing the above information, we can say that the main function of photon-3 is energy interactions, which are mainly associated with the process of destruction of matter and the release of energy, and photon-4 is information interactions associated with the transfer of information. Knowing the functions and features of a photon, the principles of its interaction with other elementary particles and especially the septon field, one can understand many processes of the macro- and microworld in which it is directly involved. Thanks to this knowledge, answers to many questions can be found. For example, how does a person actually perceive visual information? What is actually a shadow, heat or cold, if we consider these processes at the level of the ezoosmic grid? Due to what root causes does the destruction of a substance that is under prolonged exposure to sunlight occur? What are the features of the connection of a photon with a gravitational and electromagnetic field? And much more. Knowledge about the photon helps to understand the root causes of an action performed due to the participation of a photon in it, and to perform more accurate calculations of photon interactions without the use of expensive equipment and technology.
In one of the key philosophical treatises of Taoism called "Le-tzu" (I-III centuries AD), there are such lines about the absolute, about how the world that received the name comes from an unnamed absolute whole.
“In the beginning was the Great Simplicity,
then appeared Great Beginning,
then came the Great Foundation,
after which the Great Substantiality appeared.
There was no breath in the Great Simplicity yet.
The Great Beginning was the beginning of breathing,
The Great Ground was the beginning of all forms,
The Great Materiality is the beginning of all things.
Breath, form and thing have not yet separated, which is called Chaos. Look closely and you won't see, listen to it and you won't hear. The name of this is "Simplicity". The simple has neither form nor boundaries. Having undergone a transformation, it became One, and from One - Seven, Seven turned into Nine. On this, the transformations are exhausted and again come to the One. And this One is the beginning of transformations of all forms. The clean and light went up and formed the Sky, the dirty and heavy went down and formed the Earth, and the breath that penetrated both gave birth to man. This is how Heaven and Earth contained the seed of all living things, and all things came to life.”
In the ancient Chinese treatise "Tao Te Ching" (chapter 42) there are such lines: "The Tao produced one. One - two. Two three. And three are all things. Every thing bears yin and contains yang.
Literature: Chuang Tzu. Le Tzu. Translation Malyavin VV Philosophical heritage. In 3 volumes. - M: Thought, 1995; Tao Te Ching: The Book of the Way of Life / comp. and translation by V. V. Malyavin. – M.: Feoriya, 2010; Werner, Edward T.C. Myths and Legends of China. George G. Harrap & Co. Ltd. London Bombay Sydney, 1922.
Without taking into account quantization, quantum properties were assigned to objects emitting and absorbing light (see, for example, Bohr's theory). Despite the fact that semiclassical models influenced the development of quantum mechanics (which is evidenced in particular by the fact that some of their provisions and even consequences are explicitly included in modern quantum theories), experiments confirmed Einstein's correctness about the quantum nature of light (see, for example, , photoelectric effect). It should be noted that the quantization of the energy of electromagnetic radiation is no exception. AT quantum theory many physical quantities are discrete (quantized). Examples of such quantities are: angular momentum, spin, and energy of bound systems.
The introduction of the concept of a photon contributed to the creation of new theories and physical instruments, and also stimulated the development of the experimental and theoretical basis of quantum mechanics. For example, the maser, the laser, the phenomenon of Bose-Einstein condensation were invented, the quantum field theory and the probabilistic interpretation of quantum mechanics were formulated. In the modern Standard Model of particle physics, the existence of photons is a consequence of the fact that physical laws are invariant under local gauge symmetry at any point in spacetime (see section below for more details). This same symmetry determines the photon's intrinsic properties, such as electric charge, mass, and spin.
History of the name and designation
The photon was originally called "light quantum" by Albert Einstein. das Lichtquant). Modern name, which the photon derives from the Greek word φῶς, "phōs" ("light"), was introduced by the chemist Gilbert N. Lewis, who published his theory, in which photons were considered "uncreated and indestructible". Although Lewis's theory did not find its confirmation, being in conflict with experimental data, the new name for electromagnetic field quanta began to be used by many physicists.
History of the development of the photon concept
In most theories developed before the 18th century, light was viewed as a stream of particles. One of the first such theories was presented in the Book of Optics by Ibn al-Haytham in 1021. In it, the scientist represented a light beam in the form of a stream of tiny particles, which "lack all noticeable qualities, except for energy." Since such models could not explain such phenomena as refraction, diffraction and birefringence, a wave theory of light was proposed, the founders of which were Rene Descartes (1637), Robert Hooke (1665), and Christian Huygens (1678). However, models based on the idea of the discrete structure of light remained dominant, largely due to the influence of the authority of Isaac Newton, who held these theories. AT early XIX centuries, Thomas Young and Augustin Fresnel clearly demonstrated the phenomena of interference and diffraction of light in their experiments, after which, by about 1850, wave models became generally accepted. In 1865, James Maxwell proposed as part of his theory that light is an electromagnetic wave. In 1888, this hypothesis was confirmed experimentally by Heinrich Hertz, who discovered radio waves.
Studies of the properties of blackbody radiation, which took place for almost forty years (1860-1900), ended with the advancement of Max Planck's hypothesis that the energy of any system, when emitting or absorbing electromagnetic frequency radiation, can change only by an amount that is a multiple of the quantum energy (that is, discretely), where is Planck's constant. It was shown by Albert Einstein that such a concept of energy quantization must be accepted in order to explain the observed thermal equilibrium between matter and electromagnetic radiation. On the same basis, he theoretically described the photoelectric effect, for this work Einstein received the Nobel Prize in Physics in 1921. On the contrary, Maxwell's theory admits that electromagnetic radiation can have any energy (that is, it is not quantized).
Many physicists initially assumed that the quantization of energy is the result of some unknown property of matter that absorbs and emits electromagnetic waves. In 1905, Einstein suggested that the quantization of energy is a property of electromagnetic radiation itself. Recognizing the validity of Maxwell's theory, Einstein pointed out that many of the then anomalous results of experiments could be explained if the energy of a light wave was localized into particle-like quanta that moved independently of each other, even if the wave propagated continuously in space. In and 1916, Einstein showed, based on the validity of the law of radiation of a completely black body, that a quantum of energy must also have momentum. The momentum of a photon was discovered experimentally by Arthur Compton, for this work he received the Nobel Prize in Physics in 1927. However, the issue of agreement wave theory Maxwell with the experimental substantiation of the discrete nature of light remained open. A number of authors argued that the emission and absorption of electromagnetic waves occurs in portions, quanta, but the processes of wave propagation are continuous. The quantum nature of the phenomena of radiation and absorption proves the presence of individual energy levels in microsystems, including the electromagnetic field, and the impossibility of a microsystem to have an arbitrary amount of energy. Corpuscular representations are in good agreement with the experimentally observed patterns of emission and absorption of electromagnetic waves, in particular, with the patterns thermal radiation and photoelectric effect. However, in their opinion, experimental data indicate that the quantum properties of an electromagnetic wave do not manifest themselves during the propagation, scattering, and diffraction of electromagnetic waves, if they are not accompanied by a loss of energy. In propagation processes, an electromagnetic wave is not localized at a certain point in space, it behaves as a single whole and is described by Maxwell's equations. The solution was found within the framework of quantum electrodynamics (see the wave-particle duality section below) and its successor, the Standard Model.
In accordance with quantum electrodynamics, the electromagnetic field in the volume of a cube with an edge length d can be represented as plane standing waves, spherical waves, or plane traveling waves. In this case, the volume is considered to be filled with photons with the energy distribution , where n is an integer. The interaction of photons with matter leads to a change in the number of photons n on (radiation or absorption).
Attempts to preserve Maxwell's theory
As mentioned in Robert Milliken's Nobel Lecture, Einstein's 1905 predictions were tested experimentally in several independent ways in the first two decades of the 20th century. However, before the famous Compton experiment, the idea of the quantum nature of electromagnetic radiation was not generally accepted among physicists (see, for example, the Nobel lectures of Wilhelm Wien, Max Planck and Robert Milliken), which was associated with the success of Maxwell's wave theory of light. Some physicists believed that the quantization of energy in the processes of emission and absorption of light was a consequence of certain properties of the substance that emits or absorbs light. Niels Bohr, Arnold Sommerfeld and others developed models of the atom with discrete energy levels, which explained the presence of emission and absorption spectra of atoms and, moreover, were in excellent agreement with the observed spectrum of hydrogen (however, it was not possible to obtain the spectra of other atoms in these models) . Only the scattering of a photon by a free electron, which (according to the ideas of the time) did not have an internal structure and, accordingly, energy levels, forced many physicists to admit quantum nature Sveta.
However, even after Compton's experiments, Bohr, Hendrik Kramers and John Slater made one last attempt to save the classical Maxwellian wave model of light, without taking into account its quantization, by publishing the so-called BCS theory. To explain the experimental data, they proposed two hypotheses:
- Energy and momentum are conserved only statistically (on average) in interactions between matter and radiation. In separate elementary processes, such as radiation and absorption, the laws of conservation of energy and momentum are not fulfilled.
This assumption made it possible to reconcile the stepwise change in the energy of the atom (transitions between energy levels) with the continuity of the change in the energy of the radiation itself. - The radiation mechanism is specific. In particular, spontaneous radiation was considered as radiation stimulated by a "virtual" electromagnetic field.
However, Compton's experiments showed that energy and momentum are exactly conserved in elementary processes, and that his calculations of the change in the frequency of an incident photon in Compton scattering are accurate to 11 decimal places. After that, Bohr and his co-authors gave their model "the noblest funeral possible, as far as possible." Nevertheless, the collapse of the BCS model inspired Werner Heisenberg to create matrix mechanics.
One of the experiments confirming the quantization of light absorption was the experiment of Walter Bothe, conducted by him in 1925. In this experiment, a thin metal foil was irradiated with low-intensity X-rays. In this case, the foil itself became a source of weak secondary radiation. Based on classical wave concepts, this radiation should be distributed uniformly in space in all directions. In this case, two counters located to the left and to the right of the foil should have recorded it simultaneously. However, the result of the experiment turned out to be exactly the opposite: the radiation was recorded either by the right or left counter, and never by both at the same time. Consequently, absorption occurs in separate quanta. Experience thus confirmed the original position photon theory radiation, and thus became another experimental proof of the quantum properties of electromagnetic radiation.
In a vacuum, the energy and momentum of a photon depend only on its frequency (or, equivalently, its wavelength):
, ,and, therefore, the magnitude of the momentum is:
,where - Planck's constant, equal to; - wave vector and - its value (wave number); - angular frequency . The wave vector indicates the direction of the photon's motion. The spin of a photon does not depend on frequency.
Classical formulas for the energy and momentum of electromagnetic radiation can be obtained from the concept of photons. For example, radiation pressure is carried out due to the transfer of momentum of photons to the body during their absorption. Indeed, pressure is a force acting per unit surface area, and the force is equal to the change in momentum divided by the time of this change.
Wave-particle duality and the uncertainty principle
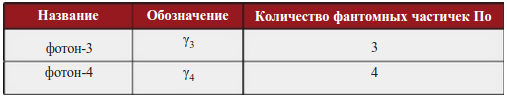
 Heisenberg's thought experiment to locate an electron (shaded in blue) using a high-resolution gamma-ray microscope. Incident gamma rays (shown in green) are scattered by an electron and enter the aperture angle of the microscope θ. Scattered gamma rays are shown in red in the figure. Classical optics shows that the position of an electron can only be determined up to a certain value Δ x, which depends on the angle θ and on the wavelength λ of the incident rays.
Heisenberg's thought experiment to locate an electron (shaded in blue) using a high-resolution gamma-ray microscope. Incident gamma rays (shown in green) are scattered by an electron and enter the aperture angle of the microscope θ. Scattered gamma rays are shown in red in the figure. Classical optics shows that the position of an electron can only be determined up to a certain value Δ x, which depends on the angle θ and on the wavelength λ of the incident rays.
It is important to note that the quantization of light and the dependence of energy and momentum on frequency is necessary to fulfill the uncertainty principle applied to a charged massive particle. This can be illustrated by the famous thought experiment with an ideal microscope that determines the coordinate of an electron by irradiating it with light and recording the scattered light (Heisenberg's gamma microscope). The position of an electron can be determined with an accuracy equal to the resolution of a microscope. Based on the concepts of classical optics:
where is the aperture angle of the microscope. Thus, the uncertainty of the coordinate can be made arbitrarily small by reducing the wavelength of the incident rays. However, after scattering, the electron acquires some additional momentum, the uncertainty of which is equal to . If the incident radiation were not quantized, this uncertainty could be made arbitrarily small by reducing the radiation intensity. The wavelength and intensity of the incident light can be changed independently of each other. As a result, in the absence of light quantization, it would be possible to simultaneously determine with high accuracy the position of an electron in space and its momentum, which contradicts the uncertainty principle.
Similarly, the uncertainty principle for photons forbids simultaneous accurate measurement of the number of photons (see the Fock state and the second quantization section below) in an electromagnetic wave and the phase of that wave (see the coherent state and squeezed coherent state):
Bose-Einstein photon gas model
Quantum statistics, applied to systems of particles with integer spin, was proposed in 1924 by the Indian physicist S. Bose for light quanta and developed by A. Einstein for all bosons. Electromagnetic radiation inside a certain volume can be considered as an ideal gas, consisting of a set of photons that practically do not interact with each other. The thermodynamic equilibrium of this photon gas is achieved by interaction with the walls of the cavity. It occurs when the walls emit as many photons per unit time as they absorb. In this case, a certain energy distribution of particles is established inside the volume. Bose obtained Planck's law of blackbody radiation without using electrodynamics at all, but simply by modifying the calculation of the quantum states of a system of photons in phase space. In particular, it was found that the number of photons in an absolutely black cavity, whose energy falls on the interval from to is equal to:
where is the volume of the cavity, is the Dirac constant , is the temperature of the equilibrium photon gas (coincides with the temperature of the walls).
In a state of equilibrium, electromagnetic radiation in an absolutely black cavity (the so-called thermal equilibrium radiation, or blackbody radiation) is described by the same thermodynamic parameters as an ordinary gas: volume, temperature, energy, entropy, etc. Radiation exerts pressure on the walls, since photons have momentum. The relationship of this pressure with temperature is reflected in the equation of state for a photon gas:
where is the Stefan-Boltzmann constant.
Einstein showed that this modification is equivalent to recognizing that photons are strictly identical to each other, and between them the presence of a “mysterious nonlocal interaction” is implied, now understood as a requirement that quantum mechanical states be symmetrical with respect to particle permutation. This work eventually led to the concept of coherent states and contributed to the invention of the laser. In the same articles, Einstein extended Bose's ideas to elementary particles with integer spin (bosons) and predicted the phenomenon of a mass transition of particles of a degenerate bosonic gas to a state with a minimum energy when the temperature drops to a certain critical value (Bose-Einstein condensation). This effect was observed experimentally in 1995, and in 2001 the authors of the experiment were awarded the Nobel Prize. In the modern sense, bosons, including photons, obey Bose-Einstein statistics, and fermions, for example, electrons, obey Fermi-Dirac statistics.
Spontaneous and stimulated emission
Einstein began by postulating simple relationships between the rates of absorption and emission reactions. In his model, the rate of absorption of photons of frequency and the transition of atoms from an energy level to a higher level with energy is proportional to the number of atoms with energy and the spectral density of radiation for surrounding photons of the same frequency:
.Here, is the absorption reaction rate constant (absorption coefficient). To implement the reverse process, there are two possibilities: the spontaneous emission of photons and the return of an electron to a lower level through interaction with a random photon. According to the approach described above, the corresponding reaction rate , which characterizes the emission of frequency photons by the system and the transition of atoms from the higher energy level to the lower one with energy , is equal to:
.Here is the coefficient of spontaneous emission , is the coefficient responsible for stimulated emission under the action of random photons. At thermodynamic equilibrium, the number of atoms in the energy state and on average should be constant in time, therefore, the values and should be equal. In addition, by analogy with the conclusions of the Boltzmann statistics, the relation holds:
,where is the multiplicity of degeneracy of the energy levels and , is the energy of these levels, is the Boltzmann constant , is the temperature of the system. From the above it follows that:
.The coefficients and are called the Einstein coefficients.
Einstein did not succeed in fully explaining all these equations, but he believed that in the future it would be possible to calculate the coefficients , and , when "mechanics and electrodynamics are changed so as to correspond to the quantum hypothesis." And it really happened. In 1926, Paul Dirac obtained the constant using a semiclassical approach, and successfully found all these constants based on the fundamental principles of quantum theory. This work became the foundation of quantum electrodynamics, i.e. the theory of electromagnetic field quantization. Dirac's approach, called the method of second quantization, has become one of the main methods of quantum field theory. It should be noted once again that in early quantum mechanics, only the particles of matter, and not the electromagnetic field, were treated as quantum mechanical.
Einstein was concerned that his theory seemed incomplete because it did not describe the direction spontaneous emission photon. The probabilistic nature of the motion of light particles was first considered by Isaac Newton in his explanation of the phenomenon of birefringence (the effect of splitting a beam of light into two components in anisotropic media) and, generally speaking, the phenomenon of splitting of light beams by the boundary of two media into reflected and refracted beams. Newton suggested that the "hidden variables" that characterize light particles determine which of the two split beams a given particle will go. Similarly, Einstein, starting to distance himself from quantum mechanics, hoped for the emergence of a more general theory of the microworld, in which there would be no place for chance. Notably, Max Born's introduction of the probabilistic interpretation of the wave function was stimulated by the later work of Einstein, who was looking for a more general theory.
Second quantization
Mathematically, the second quantization method is that a quantum system consisting of a large number identical particles , is described using wave functions, in which the role of independent variables is played by occupation numbers. The second quantization is carried out by introducing operators that increase and decrease the number of particles in a given state (occupancy numbers) by one. These operators are sometimes called birth and annihilation operators. Mathematically, the properties of the filling and annihilation operators are given by permutation relations, the form of which is determined by the particle spin. With such a description, the wave function itself becomes an operator.
In modern physical notation quantum state electromagnetic field is written as a Fock state, the tensor product of the states of each electromagnetic mode:
where represents the state with the number of photons in the mode. The creation of a new photon (for example, emitted in an atomic transition) in the mode is written as follows:
Photon as a gauge boson
Main article: Gauge theory
Maxwell's equations describing the electromagnetic field can be obtained from the representations of the gauge theory as a consequence of fulfilling the requirement of the gauge invariance of the electron with respect to the transformation of space-time coordinates. For an electromagnetic field, this gauge symmetry reflects the ability of complex numbers to change the imaginary part without affecting the real part, as is the case with energy or the Lagrangian.
In the Standard Model, the photon is one of the four gauge bosons involved in the electroweak interaction. The remaining three (W + , W − and Z 0) are called vector bosons and are responsible only for the weak interaction. Unlike the photon, vector bosons have a mass , they must be massive due to the fact that the weak interaction manifests itself only at very small distances,<10 −15 см. Однако кванты калибровочных полей должны быть безмассовыми, появление у них массы нарушает калибровочную инвариантность уравнений движения. Выход из этого затруднения был предложен Питером Хиггсом , теоретически описавшим явление спонтанного нарушение электрослабой симметрии . Оно позволяет сделать векторные бозоны тяжёлыми без нарушения калибровочной симметрии в самих уравнениях движения. Объединение фотона с W и Z калибровочными бозонами в электрослабом взаимодействии осуществили Шелдон Ли Глэшоу , Абдус Салам и Стивен Вайнберг , за что были удостоены Нобелевской премии по физике в 1979 году . Важной проблемой квантовой теории поля является включение в единую калибровочную схему и сильного взаимодействия (так называемое «великое объединение »). Однако ключевые следствия посвящённых этому теорий, такие как распад протона , до сих пор не были обнаружены экспериментально.
Contribution of photons to the mass of the system
Photons in matter
Light travels in a transparent medium at a speed less than the speed of light in a vacuum. For example, photons that experience many collisions on their way from the radiating solar core can take about a million years to reach the surface of the Sun. However, moving in outer space, the same photons reach the Earth in just 8.3 minutes. The value characterizing the decrease in the speed of light is called the refractive index of a substance.
From a classical point of view, the slowdown can be explained as follows. Under the influence of the electric field strength of the light wave, the valence electrons of the atoms of the medium begin to make forced harmonic oscillations. The oscillating electrons begin, with a certain delay, to radiate secondary waves of the same frequency and intensity as the incident light, which interfere with the original wave, slowing it down. In the corpuscular model, deceleration can instead be described by mixing photons with quantum perturbations in matter (quasiparticles, like phonons and excitons) to form a polariton. Such a polariton has a non-zero effective mass , which is why it is no longer able to move at a speed . The effect of interaction of photons with other quasiparticles can be observed directly in the Raman effect and in the Mandelstam-Brillouin scattering.
Similarly, photons can be viewed as particles always moving at the speed of light, even in matter, but experiencing a phase shift (lag or advance) due to interactions with atoms that change their wavelength and momentum, but not their speed. Wave packets consisting of these photons move at a speed less than . From this point of view, photons are, as it were, "naked", which is why they are scattered by atoms, and their phase changes. Whereas, from the point of view described in the previous paragraph, photons are “dressed” through interaction with matter and move without scattering and phase shift, but at a lower speed.
Depending on the frequency, light propagates through matter at different speeds. This phenomenon in optics is called dispersion. When certain conditions are created, it is possible to achieve that the speed of propagation of light in a substance becomes extremely small (the so-called "slow light"). The essence of the method is that using the effect of electromagnetically induced transparency, it is possible to obtain a medium with a very narrow dip in its absorption spectrum. In this case, an extremely steep change in the refractive index is observed in the region of this dip. That is, in this area, a huge dispersion of the medium (with a normal spectral dependence - an increase in the refractive index towards an increase in frequency) and its transparency for radiation are combined. This provides a significant reduction in the group velocity of light (up to 0.091 mm / under certain conditions).
Photons can also be absorbed by nuclei, atoms or molecules, thus causing a transition between their energy states. A classic example is indicative of the absorption of photons by the visual pigment of the retinal rods rhodopsin, which contains retinal, a derivative of retinol (vitamin A), responsible for human vision, as was established in 1958 by the American biochemist Nobel laureate George Wald and his collaborators. Absorption of a photon by a molecule of rhodopsin causes the reaction of trans-isomerization of retinal, which leads to the decomposition of rhodopsin. Thus, in combination with other physiological processes, the energy of a photon is converted into the energy of a nerve impulse. Absorption of a photon can even cause chemical bonds to break, as in the photodissociation of chlorine; such processes are the object of study in photochemistry.
Technical application
There are many technical devices that somehow use photons in their work. Below are just a few of them for illustration purposes.
An important technical device using photons is the laser. His work is based on the phenomenon of stimulated emission discussed above. Lasers are used in many areas of technology. Technological processes (welding, cutting and melting of metals) are carried out mainly by gas lasers with a high average power. In metallurgy, they make it possible to obtain superpure metals. Ultrastable lasers are the basis of optical frequency standards, laser seismographs, gravimeters, and other precision physical instruments. Frequency-tunable lasers (for example, the dye laser) have revolutionized spectroscopy, significantly increasing the resolution and sensitivity of the method up to the observation of the spectra of individual atoms. Lasers are also used in medicine as bloodless scalpels, in the treatment of eye and skin diseases. Laser ranging contributed to the refinement of space navigation systems, expanded knowledge about the atmospheres and the structure of the surface of the planets, made it possible to measure the rotation speed of Venus and Mercury, significantly refined the characteristics of the motion of the Moon and the planet Venus compared to astronomical data. With the use of lasers, they are trying to solve the problem of controlled thermonuclear fusion. Lasers are widely used in everyday life (laser printers, DVDs, laser pointers, etc.).
The emission and absorption of photons by matter is used in spectral analysis. The atoms of each chemical element have strictly defined resonant frequencies, as a result of which it is at these frequencies that they emit or absorb light. This leads to the fact that the emission and absorption spectra of atoms and molecules consisting of them are individual, like human fingerprints.
According to the methods used, several types of spectral analysis are distinguished:
- Emissive, using the emission spectra of atoms, less often - molecules. This type of analysis involves burning some sample in a gas burner flame, DC or AC electric arc, or high-voltage electric spark. A special case of emission analysis is luminescent analysis.
- absorption, which uses the absorption spectrum, mainly of molecules, but can also be applied to atoms. Here, the sample is completely converted into a gaseous state and light from a source of continuous radiation is passed through it. At the exit, against the background of a continuous spectrum, an absorption spectrum of the evaporated substance is observed.
- x-ray, which uses the X-ray spectra of atoms, as well as the diffraction of X-rays as they pass through the object under study to study its structure. The main advantage of the method is that the X-ray spectra contain few lines, which greatly facilitates the study of the composition of the sample. Among the shortcomings are the low sensitivity and complexity of the equipment.
In a qualitative spectral analysis, only the composition of the sample is determined without indicating the quantitative ratio of the components. The latter problem is solved in quantitative spectral analysis, based on the fact that the intensity of the lines in the spectrum depends on the content of the corresponding substance in the sample under study. Thus, by the spectrum of a substance, its chemical composition can be determined. Spectral analysis is a sensitive method and is widely used in analytical chemistry, astrophysics, metallurgy, mechanical engineering, geological exploration and other branches of science.
Latest Research
It is now believed that the properties of photons are well understood in terms of theory. The Standard Model treats photons as spin-1 gauge bosons with zero rest mass and zero electric charge (the latter follows, in particular, from the local unitary symmetry U(1) and from experiments on electromagnetic interaction). However, physicists continue to look for inconsistencies between experiment and the provisions of the Standard Model. The accuracy of ongoing experiments to determine the mass and charge of photons is constantly increasing. The discovery of even the slightest amount of charge or mass in photons would deal a serious blow to the Standard Model. All experiments carried out so far show that photons have neither charge nor rest mass. The highest accuracy with which it was possible to measure the charge of a photon is 5 10 −52 C(or 3 10 −33 ); for mass - 1.1 10 −52 kg (6 10 −17 eV / 2 or 1 10 −22 ).
Much modern research is devoted to the application of photons in the field of quantum optics. Photons seem to be suitable particles for creating super-efficient quantum computers based on them. The study of quantum entanglement and related quantum teleportation is also a priority area of modern research. In addition, there is a study of nonlinear optical processes and systems, in particular, the phenomenon of two-photon absorption, in-phase modulation and optical parametric oscillators. However, such phenomena and systems mostly do not require the use of photons in them. They can often be modeled by considering atoms as non-linear oscillators. The non-linear optical process of spontaneous parametric scattering is often used to create entangled photon states. Finally, photons are used in optical communication, including
Photon. The structure of a photon. The principle of movement.
Part 1. Initial data.
Part 1. Initial data.
1.1. A photon is an elementary particle, a quantum of electromagnetic radiation.
1.2. A photon cannot be divided into several parts and does not spontaneously decay in a vacuum.
1.3. A photon is a truly electrically neutral particle. The speed of movement (movement) of a photon in vacuum is equal to "c".
1.4. Light is a stream of localized particles - photons.
1.5 . Photons are emitted in many natural processes, for example: when charged particles move with acceleration (bremsstrahlung, synchrotron, cyclotron radiation) or when an electron passes from an excited state to a state with a lower energy. This occurs as a result of the main fundamental transformation in Nature - the transformation of the kinetic energy of a charged particle into electromagnetic (and vice versa).
1.6. The photon is characterized by corpuscular-wave dualism:
On the one hand, photons demonstrate the properties of a wave in the phenomena of diffraction and interference at scales comparable to the wavelength of a photon;
On the other hand, a photon behaves like a particle that is emitted or absorbed entirely by objects whose dimensions are much smaller than its wavelength (for example, atomic nuclei) or are considered pointlike (electron).
1.7. Considering the fact that single photons demonstrate the properties of a wave, it can be quite reliably argued that a photon is a “miniwave” (a separate, compact “piece” of a wave). In this case, the following properties of waves should be taken into account:
a) uh electromagnetic waves (and photon) are transverse waves in which the vectors of electric (E) and magnetic (H) fields oscillate perpendicular to the direction of wave propagation. Electromagnetic waves (photon) can be transferred from the source to the receiver, including through a vacuum. They do not require a medium for their distribution.
b) half of the energy of electromagnetic waves (and photons) is magnetic.
c) to characterize the intensity of the wave process, three parameters are used: the amplitude of the wave process, the energy densitywave process and energy flux density.
1.8. In addition, when considering the scheme of the photon structure and the principle of its movement, the following data were taken into account:
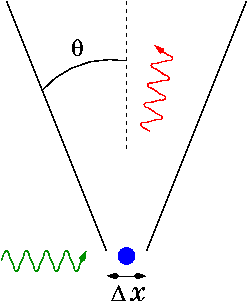
a) the emission of a photon practically passes over a period of time of the order of 10 -7 sec - 10 -15 sec. During this period, the electromagnetic field of the photon increases from zero to a maximum and again falls to zero. See fig.1.
b) the graph of the change in the photon field cannot in any way be a piece of a truncated sinusoid, since in the places of cutting, infinite forces would arise;
in) since the frequency of an electromagnetic wave is a quantity that is observed in experiments, the same frequency (and wavelength) can also be attributed to an individual photon. Therefore, photon parameters, like waves, are described by the formula E = h* f , where h is Planck's constant, which relates the magnitude of the photon's energy to its frequency ( f).
Rice. 1. A photon is a material particle and is a compact (having a beginning and an end), indivisible "piece" of a wave, in which the electromagnetic field increases from zero to a certain maximum and again falls to zero. Magnetic fields are conventionally not shown.
Part 2. Basic principles of photon structure.
2.1. In almost all articles on electromagnetic waves (photons), the figures describe and graphically show a wave consisting of two fields - electric and magnetic, for example, the quote: "An electromagnetic field is a combination of electric magnetic fields ...". However, the existence of a “two-component” electromagnetic wave (and photon) is impossible for one simple reason: a one-component electric and one-component magnetic field in an electromagnetic wave (photon) does not exist and cannot exist. Explanation:
a) there are theoretical models-formulas-laws that are used to calculate or determine parameters under ideal conditions (for example, a theoretical model of an ideal gas). This is perfectly acceptable. However, for calculations in real conditions, correction factors are introduced into these formulas, which reflect the real parameters of the medium.
b) there is also a theoretical model called "electric field". This is acceptable for solving theoretical problems. However, in reality there are only two electric fields: the electric field-plus (#1) and the electric field-minus (#2). Substances called "chargeless? electrically neutral? electric field No. 3 does not exist in reality, and cannot exist. Therefore, when modeling real conditions in a theoretical model called "electric field", it is always necessary to take into account two "correction factors" - the real electric field-plus and the real electric field-minus.
c) there is a theoretical model called "magnetic field". This is quite acceptable for some tasks. However, in reality, a magnetic field always has two magnetic poles: pole #1 (N) and pole #2 (S). Substances called "non-polar? magnetic field No. 3 does not exist and cannot exist in reality. Therefore, when modeling real conditions in a theoretical model called “magnetic field”, it is always necessary to take into account two “correction factors” - pole-N and pole-S.
2.2. Thus, taking into account the above, we can make a quite unambiguous conclusion: a photon is a compact (having a beginning and an end), material particle, in which matter is a combination of two electric (plus or minus) and two magnetic (N-S) fields that can propagate from their sources without attenuation (in vacuum) over arbitrarily long distances. See fig.2.
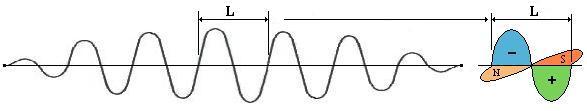
Fig.2. A photon is a combination of two electric fields (plus and minus) and two magnetic fields (N and S). In this case, the overall electroneutrality of the photon is fully observed. In this paper, it is assumed that the minus electric field is joined to the magnetic field-N, and the plus electric field is joined to the magnetic field-S.
Part 3. Quantum of energy and quantum of mass.
3.1. On the one hand, a photon is a compact, indivisible particle, in which the electromagnetic field increases from zero to a certain maximum and again falls to zero. That is, the photon has a very real linear size (beginning and end).
3.2. However, on the other hand, photon parameters, like waves, are described by the formula E = h* f , where h - Planck's constant (eV * sec), the elementary quantum of action (the fundamental world constant), which relates the value of the photon energy to its frequency ( f).
3.3. This allows us to assume that all photons consist of a well-defined number (n) of "independent" electrically neutral "averaged" elementary energy quanta (eV) with absolutely the same wavelength ( L ). In this case, the energy of any photon is: E = e 1 *n, where (e 1 ) is the energy of an elementary quantum, (n) is their number in a photon. See fig.3.
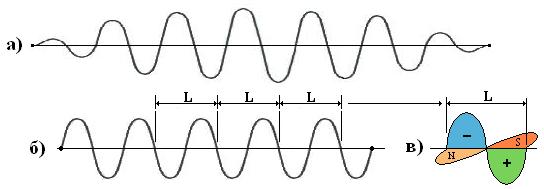
Fig.3.
a) "normal" photon (electromagnetic field increases from zero to a certain maximum and again falls to zero);
b) the same photon from "averaged" quanta. It can be assumed that any photon consists of a well-defined number of absolutely identical "averaged" elementary energy quanta;
c) elementary "averaged" photon energy quantum. The elementary quantum of energy (dimension - eV) is absolutely the same for all electromagnetic waves of all ranges and is similar to the elementary quantum of Planck's action, (dimension - eV * sec). In this case: E (eV) = h* f = e 1 *n.
3.4. The matter of a photon. Photons are emitted as a result of the main fundamental transformation in Nature - the transformation of the kinetic energy of a charged particle into electromagnetic energy and vice versa - the transformation of the electromagnetic energy of photons into the kinetic energy of a charged particle. However, the kinetic energy is non-material, and the electromagnetic energy of a photon has all the properties of matter. Thus: as a result of the main fundamental transformation in Nature, the non-material kinetic energy of a charged particle is converted into the energy of electric and magnetic fields of a photon, which has quite real properties of matter: momentum, speed, mass, and other characteristics. Since the photon is material, all its constituent parts are also material. That is: an elementary quantum of energy is automatically an elementary quantum of mass.
3.5. Any photon consists of a well-defined number of "independent" electrically neutral elementary energy quanta. And reviewing the schema structure of the elementary quantum shows that:
a) an elementary quantum cannot be divided into two equal parts, since this will automatically be a violation of the charge conservation law;
b) it is also impossible to “cut off” a smaller part from an elementary quantum, since this will automatically lead to a change in the value of the Planck constant (fundamental constant) for this quantum.
3.6. Consequently:
First. The transformation of the electromagnetic energy of photons into the kinetic energy of a charged particle cannot be a continuous function - electromagnetic energy can be converted into the kinetic energy of particles (and vice versa) only at energy values that are multiples of one elementary quantum of energy.
Second. Since the shells of quarks, protons, neutrons, and other particles arecompacted electrically neutral matter of photons, then the masses of these shells also matter , multiples of the elementary mass quantum.
3.7. Note: nevertheless, the division of elementary quanta into two absolutely equal parts (positive and negative) is quite possible (and occurs) during the formation of electron-positron pairs. In this case, the mass of the electron and positronmatter , multiples of half the elementary quantum of mass (see " Electron. Formation and structure of the electron. Magnetic Monopole of the Electron).
Part 4. Basic principles of photon movement.
4.1. The movement of a material photon-particle can be carried out only in two ways:
Option-1: photon moves by inertia;
Option-2: the photon is a self-propelled particle.
4.2. For unknown reasons, it is the inertial motion of electromagnetic waves (and photons) that is either implied or mentioned and graphically shown in almost all articles on electromagnetic waves, for example: Wikipedia. electromagnetic radiation. English. See fig.4.
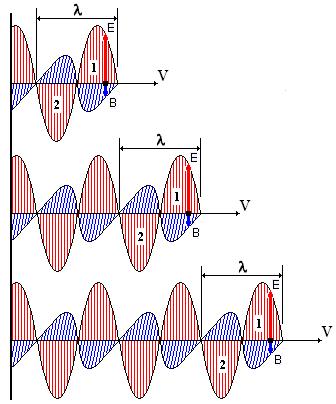
Fig.4. An example of the inertial movement of a photon (Wikipedia. Electromagnetic radiation). The photon moves past the observer from left to right with a speed V = "with". In this case, all the petals of the sinusoid do not change their parameters, that is: in the photon reference frame, they are absolutely motionless.
4.3. However, the inertial motion of a photon is impossible, for example, for the following reason: when a photon passes through an obstacle (glass), its speed decreases, but after passing through an obstacle (one or more), the photon again “instantly” and restores its speed to “c” = const. With inertial motion, such an independent recovery of speed is impossible.
4.4. An "instantaneous" increase in speed by a photon (up to "c" = const) after passing through an obstacle is possible only if the photon itself is a self-propelled particle. In this case, the mechanism of photon self-movement can only be the polarity reversal of the available electric (plus and minus) and magnetic (N and S) fields with simultaneous displacement of the photon by half a period, that is, with a doubled frequency (2 * f). See fig.5.
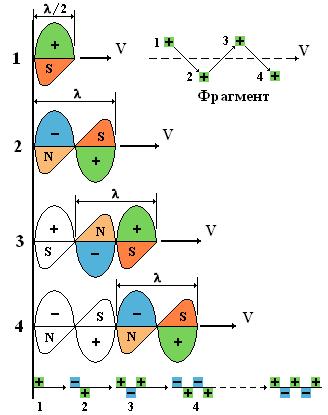
Fig.5. Scheme of photon movement due to polarity reversal of fields. "Fragment" - the sequence of polarity reversal of the field-plus.
4.5. The explanation of the photon movement mechanism was based on the following data:
a) the electromagnetic field of a photon is a combination of variable electric (plus or minus) and magnetic (N and S) fields;
b) the electric and magnetic fields of a photon cannot disappear - they can only turn into each other. The generation of a magnetic field by an alternating electric field is a fundamental natural phenomenon;
c) a magnetic field appears only in the presence of a time-varying electric field and vice versa (every change in the electric field excites a magnetic field and, in turn, a change in the magnetic field excites an electric field). Therefore, the magnetic fields of a photon can arise only if the photon has variable signs and time-varying electric fields (in the photon's frame of reference).
4.6. When explaining the mechanism of photon polarity reversal, the following options were considered:
a) the presence of free space ahead of the photon. A photon is a compact, indivisible “piece” of a wave in the form of a sinusoid, in which the electromagnetic fields increase from zero to a certain maximum and again fall to zero. That is: the "body" of a photon has a very real geometric length (beginning and end). The movement of a photon occurs due to the movement of a photon at a distance of one half-cycle (1/2L) for each act of polarity reversal. And this movement can always occur only in one direction (forward), where there is free space in front of the photon;
b) "The struggle of opposites." The electromagnetic field of a photon is a combination of alternating electric (plus or minus) and magnetic (N and S) fields. In this paper, it is assumed that the minus electric field is joined to the magnetic field-N, and the plus electric field is joined to the magnetic field-S. But in this case, there is a constant (and legitimate) desire of the magnetic fields N and S to dock with each other, that is, to create a full-fledged "bipolar magnet". To do this, one of the magnetic fields must shift by half a period. However, magnetic and electric fields are "tightly" interconnected, and any attempt of the magnetic field to "free itself" from the electric field "instantly" leads to a reaction of counteraction - it causes a polarity reversal (transfer) of all fields and their automatic shift for half a period.
4.7. Since there are no other options for explaining the mechanism of self-movement of a photon, the movement of a photon due to the reversal of the fields, apparently, is the only solution to the problem. For only the polarity reversal mode allows maintaining the photon self-motion mode and at the same time ensuring compliance with the fundamental law of Nature - the generation of a magnetic field in the presence of an electric field that changes in sign and changes in time (and vice versa). The proposed variants of the polarity reversal mechanism (causes and sequence) require additional studies, which cannot be presented in this paper. Nevertheless, the above explanations are an acceptable way out of the current situation in solving the problem of the constancy of the speed of light, since they allow one or another degree of certainty to explain the mechanism of photon self-motion.
4.8. photon speed. Speed (s) of electromagnetic waves (photons) in vacuum, their frequency ( f ) and wavelength (L ) are rigidly related by the formula: с = f*L . However, it should be borne in mind that the movement of a photon occurs due to the simultaneous reversal of its electric and magnetic fields, during which the photon is displaced by a distance of one half-cycle (L / 2) for each act of polarity reversal, that is, with a doubled frequency. With this in mind, the speed formula will look like c \u003d 2 f*L /2, which is absolutely identical to the main formula: c = f*L.
5. Way:
5.1. A photon is a localized (compact) material particle, in which matter is a combination of two electric (plus and minus) and two magnetic (N and S) fields, the values of which increase from zero to a certain maximum and again fall to zero. In this case, the overall electroneutrality of the photon is fully observed.
5.2. As a result of the main fundamental transformation in Nature, the non-material kinetic energy of a charged particle is transformed into the material energy of the electric and magnetic fields of a photon. A photon is material and consists of a well-defined number of absolutely identical "averaged" elementary energy quanta, which are automatically elementary mass quanta.
5.3. A photon is a self-propelled particle capable of moving from its source to arbitrarily large distances (in a vacuum). It does not require a medium for its movement. The movement of a photon occurs due to the polarity reversal of alternating electric (plus or minus) and magnetic (N and S) fields, during which the photon is displaced by a distance of one half-cycle for each polarity reversal event.
5.4. In this work, it is assumed that in each elementary quantum, the minus electric field joins with the magnetic field-N, and the plus electric field joins with the magnetic field-S. Other options for joining fields require additional elaboration and were not considered in this paper.






