Mass spectrometric method of analysis is based on. Mass spectrometry
Mass spectrometer
mass spectrometer
Mass spectrometer
- a device for determining the masses of atoms (molecules) by the nature of the movement of their ions in electric and magnetic fields.
A neutral atom is not affected by electric and magnetic fields. However, if one or more electrons are taken away from it or one or more electrons are added to it, then it will turn into an ion, the nature of the movement of which in these fields will be determined by its mass and charge. Strictly speaking, in mass spectrometers, it is not mass that is determined, but the ratio of mass to charge. If the charge is known, then the mass of the ion is uniquely determined, and hence the mass of the neutral atom and its nucleus. Structurally, mass spectrometers can differ greatly from each other. They can use both static fields and time-varying magnetic and/or electric fields.
Consider one of the simplest options.
The mass spectrometer consists of the following main parts:
a) an ion source, where neutral atoms are converted into ions (for example, under the influence of heating or a microwave field) and accelerated by an electric field, b) areas of constant electric and magnetic fields, and in) an ion receiver that determines the coordinates of the points where the ions that cross these fields fall.
From the ion source 1 accelerated ions through the slot 2 fall into the region 3 of constant and uniform electric E and magnetic B 1 fields. Direction electric field is set by the position of the capacitor plates and is shown by arrows. The magnetic field is directed perpendicular to the plane of the figure. In region 3, the electric E and magnetic B 1 fields deflect the ions in opposite directions and the magnitudes of the electric field strength E and induction magnetic field B 1 are chosen so that the forces of their action on the ions (qE and qvB 1, respectively, where q is the charge and v is the ion velocity) compensate each other, i.e. was qЕ = qvB 1 . At the speed of the ion v = E/B 1 it moves without deviating in region 3 and passes through the second slot 4, falling into region 5 of a uniform and constant magnetic field with induction B 2 . In this field, the ion moves along the circle 6, the radius R of which is determined from the relation
Mv 2 /R = qvB 2, where M is the mass of the ion. Since v \u003d E / B 1, the mass of the ion is determined from the relation
M = qB 2 R/v = qB 1 B 2 R/E.
Thus, with a known ion charge q, its mass M is determined by the radius R circular orbit in region 5. For calculations, it is convenient to use the ratio in the system of units given in square brackets:
M[T] = 10 6 ZB 1 [T]B 2 [T]R[m]/E[V/m].
If a photographic plate is used as an ion detector 7, then this radius will be shown with high accuracy by a black dot in the place of the developed photographic plate where the ion beam hit. Modern mass spectrometers usually use electron multipliers or microchannel plates as detectors. The mass spectrometer makes it possible to determine the masses with a very high relative accuracy ΔM/M = 10 -8 - 10 -7 .
Mass spectrometer analysis of a mixture of atoms different weight allows also to determine their relative content in this mixture. In particular, the content of various isotopes of any chemical element can be established.
Mass spectrometry (mass spectroscopy, mass spectrography, mass spectral analysis, mass spectrometric analysis) is a method for studying a substance based on determining the ratio of mass to charge of ions formed during ionization of sample components of interest. One of the most powerful methods for the qualitative identification of substances, which also allows quantitative determination. We can say that mass spectrometry is the "weighing" of the molecules in the sample.
The history of mass spectrometry begins with the fundamental experiments of J. J. Thomson at the beginning of the 20th century. The ending "-metria" in the name of the method appeared after the widespread transition from the detection of charged particles using photographic plates to electrical measurements of ion currents.
Mass spectrometry is especially widely used in the analysis of organic substances, since it provides reliable identification of both relatively simple and complex molecules. The only general requirement is that the molecule be ionizable. However, to date, so many methods have been invented for ionizing sample components that mass spectrometry can be considered an almost universal method.
Almost all mass spectrometers are vacuum instruments because ions are very unstable in the presence of foreign molecules. However, there are some devices that can be conditionally classified as mass spectrometers, but which do not use a vacuum, but a stream of a special pure gas.
The mass spectrum is the dependence of the intensity of the ion current (amount of substance) on the ratio of mass to charge (the nature of the substance). Since the mass of any molecule is made up of the masses of its constituent atoms, the mass spectrum is always discrete, although at low resolution of the mass spectrometer, peaks of different masses may overlap or even merge. The nature of the analyte, the characteristics of the ionization method, and secondary processes in the mass spectrometer can affect the mass spectrum (see metastable ions, accelerating voltage gradient over ion production sites, inelastic scattering). Thus, ions with the same mass-to-charge ratios can end up in different parts of the spectrum and even make part of it continuous.
Most small molecules, upon ionization, acquire only one positive or negative charge. The larger the molecule, the more likely it is that during ionization it will turn into a multiply charged ion. Therefore, this effect is especially strong for extremely large molecules, such as proteins, nucleic acids and polymers. With some types of ionization (for example, electron impact), a molecule can break up into several characteristic parts, which provides additional opportunities for identifying and studying the structure of unknown substances.
Accurate determination of the mass of the analyzed molecule allows you to determine its elemental composition (see: elemental analysis). Mass spectrometry also provides important information about the isotopic composition of the analyzed molecules.
History of mass spectrometry
- 1912 - JJ Thomson creates the first mass spectrograph and obtains mass spectra of oxygen, nitrogen, carbon monoxide, carbon dioxide and phosgene molecules.
- 1913 - With the help of his mass spectrograph, J. J. Thomson discovers neon isotopes: neon-20 and neon-22.
- 1918 - Arthur Dempster builds the first mass spectrograph.
- 1919 - Francis Aston, independently of Dempster, builds his first mass spectrograph and begins isotope research. This device had a resolution of about 130.
- 1923 - Aston measures the mass defect with a mass spectrometer.
- 1932 - Kenneth Bainbridge builds a mass spectrometer with a resolution of 600 and a sensitivity of 1 part per 10,000
- 1936 - Arthur Dempster, Kenneth Tompkins Bainbridge and Josef Heinrich Elizabeth Mattauch construct a double focusing mass spectrograph. Dempster develops the spark ionization source.
- 1940 - Alfred Nir using preparative mass spectrometry isolates uranium-235.
- 1940 - Alfred Nir creates the first reliable source of electron impact using an ionization chamber.
- 1942 Lawrence launches the Calutron, an industrial uranium isotope separation facility based on a magnetic sector mass spectrometer.
- 1946 - William Stevens proposes the concept of a time-of-flight mass spectrometer.
- 1948 - Cameron and Eggers created the first mass spectrometer with a time-of-flight mass analyzer.
- 1952 - Talroze and Lyubimova first observe the signal of methonium CH5+ in an electron impact ion source at elevated methane pressure in the ionization chamber (in 1966 Munson and Field apply this discovery for analytical purposes and create an ion source with chemical ionization).
- 1953 Paul patents the quadrupole mass analyzer and ion trap.
- 1956 - McLafferty and Gohlke create the first gas chromatography-mass spectrometer.
- 1966 - Munson and Field create an ion source with chemical ionization.
- 1972 - Karataev and Mamyrin invent a time-of-flight focusing mass analyzer, which significantly improves the resolution of the analyzer.
- 1974 - First liquid chromatography-mass spectrometer created by Arpino, Baldwin and McLafferty
- 1981 - Barber, Bordoli, Sedgwick and Tylor create the Fast Atom Bombardment (FAB) ionizer.
- 1982 - First mass spectrum of a whole protein (insulin) by fast atom bombardment (FAB).
- 1983 Blanky and Bestal invent the thermal spray.
- 1984 - L. N. Gall and then Fenn publish works on the electrospray method.
- 1987 - Karas, Bachmann, Bahr and Hillenkamp invent Matrix Assisted Laser Desorption Ionization (MALDI).
- 1999 - Alexander Makarov (English) Russian invents the Orbitrap electrostatic ion trap.
The principle of operation and the device of the mass spectrometer
Ion sources
The first thing to do in order to obtain a mass spectrum is to turn neutral molecules and atoms that make up any organic or inorganic substance into charged particles - ions. This process is called ionization and is carried out differently for organic and inorganic substances. The second necessary condition is the transfer of ions into the gas phase in the vacuum part of the mass spectrometer. Deep vacuum ensures the unhindered movement of ions inside the mass spectrometer, and in its absence, the ions will scatter and recombine (turn back into uncharged particles).
Conventionally, the methods of ionization of organic substances can be classified according to the phases in which the substances are located before ionization.
Gas phase Electron ionization (EI) Chemical ionization (CI) Electron capture (EC) Electric field ionization (FI) Liquid phase Thermal spray ionization atmospheric pressure(AP)
- electrospray (APESI)
- atmospheric pressure chemical ionization (APCI)
- atmospheric pressure photoionization (APPI)
AT inorganic chemistry to analyze the elemental composition, harsh ionization methods are used, since the binding energies of atoms in solid body much more and much more severe methods must be used in order to break these bonds and get ions.
- ionization in inductively coupled plasma (ICP)
- thermal ionization or surface ionization
- glow discharge ionization and spark ionization (see spark discharge)
- ionization during laser ablation
Historically, the first ionization methods were developed for the gas phase. Unfortunately, many organic substances cannot be evaporated, that is, converted into the gas phase, without decomposition. This means that they cannot be ionized by electron impact. But among such substances, almost everything that makes up living tissue (proteins, DNA, etc.), physiologically active substances, polymers, that is, everything that is of particular interest today. Mass spectrometry has not stood still and last years special methods have been developed for the ionization of such organic compounds. Today, two of them are mainly used - atmospheric pressure ionization and its subspecies - electrospray (ESI), atmospheric pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI), as well as matrix-assisted laser desorption ionization (MALDI). ).
Mass analyzers
The ions obtained during ionization are transferred to the mass analyzer with the help of an electric field. There begins the second stage of mass spectrometric analysis - sorting of ions by mass (more precisely, by the ratio of mass to charge, or m / z). There are the following types of mass analyzers:
Continuous Mass Analyzers
- Magnetic and electrostatic sector mass analyzer (eng. Sector instrument)
- Quadrupole mass analyzer
- Time-of-flight mass analyzer
- Ion trap
- Quadrupole ion trap
- Fourier transform ion cyclotron resonance mass analyzer
- Orbitrap
The difference between continuous and pulsed mass analyzers lies in the fact that in the first, ions enter in a continuous stream, and in the second, in portions, at certain time intervals.
The mass spectrometer can have two mass analyzers. Such a mass spectrometer is called a tandem mass spectrometer. Tandem mass spectrometers are used, as a rule, together with “soft” ionization methods, in which there is no fragmentation of ions of the analyzed molecules (molecular ions). Thus, the first mass analyzer analyzes molecular ions. Leaving the first mass analyzer, molecular ions are fragmented under the action of collisions with inert gas molecules or laser radiation, after which their fragments are analyzed in the second mass analyzer. The most common configurations of tandem mass spectrometers are quadrupole-quadrupole and quadrupole-time-of-flight.
Detectors
The last element of the simplified mass spectrometer we are describing is the charged particle detector. The first mass spectrometers used a photographic plate as a detector. Now dynode secondary electron multipliers are used, in which an ion, hitting the first dynode, knocks out a beam of electrons from it, which, in turn, hitting the next dynode, knock out even more electrons from it, etc. Another option is photomultipliers, registering the glow that occurs when bombarded by phosphor ions. In addition, microchannel multipliers, systems such as diode arrays, and collectors are used that collect all ions that have fallen into given point space (Faraday collectors).
Chromato-mass spectrometry
Mass spectrometers are used to analyze organic and inorganic compounds.
Organic substances in most cases are multicomponent mixtures of individual components. For example, it is shown that the smell of fried chicken is 400 components (ie, 400 individual organic compounds). The task of analytics is to determine how many components make up organic matter, find out what these components are (identify them) and find out how much of each compound is contained in the mixture. For this, the combination of chromatography with mass spectrometry is ideal. Gas chromatography is best suited to be combined with the ion source of a mass spectrometer with electron impact ionization or chemical ionization, since the compounds are already in the gas phase in the chromatograph column. Devices in which a mass spectrometric detector is combined with a gas chromatograph are called chromato-mass spectrometers (“Chromass”).
Many organic compounds cannot be separated into components using gas chromatography, but can be separated using liquid chromatography. Today, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) sources are used to combine liquid chromatography with mass spectrometry, and the combination of liquid chromatography with mass spectrometers is called LC / MS (English LC / MS). The most powerful systems for organic analysis demanded by modern proteomics are built on the basis of a superconducting magnet and operate on the principle of ion cyclotron resonance. They are also called FT/MS because they use the Fourier transform of the signal.
Characteristics of mass spectrometers and mass spectrometric detectors
The most important technical characteristics of mass spectrometers are sensitivity, dynamic range, resolution, scanning speed.
The most important characteristic in the analysis of organic compounds is sensitivity. In order to achieve the highest possible sensitivity while improving the signal-to-noise ratio, detection is resorted to for individual selected ions. In this case, the gain in sensitivity and selectivity is colossal, but when using low-resolution devices, another important parameter has to be sacrificed - reliability. After all, if you recorded only one peak from the entire characteristic mass spectrum, you will need a lot of work to prove that this peak corresponds to exactly the component that you are interested in. How to solve this problem? Use high resolution on dual focus instruments where you can achieve high level reliability without sacrificing sensitivity. Or use tandem mass spectrometry, where each peak corresponding to the parent ion can be confirmed by the mass spectrum of the daughter ions. So, the absolute champion in sensitivity is a high-resolution organic chromatography-mass spectrometer with double focusing.
According to the characteristics of the combination of sensitivity with the reliability of the determination of components, ion traps follow high-resolution devices. The classic new generation quadrupole instruments have improved performance due to a number of innovations, such as the use of a curved quadrupole pre-filter, which prevents neutral particles from reaching the detector and therefore reduces noise.
Applications of mass spectrometry
Development of new drugs to save people from previously incurable diseases and drug production control, genetic engineering and biochemistry, proteomics. Without mass spectrometry, control over the illegal distribution of narcotic and psychotropic drugs, forensic and clinical analysis of toxic drugs, analysis explosives.
Finding out the source of origin is very important for solving a number of issues: for example, determining the origin of explosives helps to find terrorists, drugs - to fight their distribution and block their traffic routes. The economic security of the country is more reliable if the customs services can not only confirm by analysis in doubtful cases the country of origin of the goods, but also its compliance with the declared type and quality. And the analysis of oil and oil products is needed not only to optimize oil refining processes or geologists to search for new oil fields, but also to identify those responsible for oil spills in the ocean or on land.
In the era of "chemization Agriculture» the presence of trace amounts of applied chemicals (e.g. pesticides) in food products. In trace amounts, these substances can cause irreparable harm to human health.
A number of technogenic (that is, not existing in nature, but resulting from industrial human activity) substances are supertoxicants (having a toxic, carcinogenic or harmful effect on human health in extremely low concentrations). An example is the well-known dioxin.
Existence nuclear power unthinkable without mass spectrometry. With its help, the degree of enrichment of fissile materials and their purity are determined.
Of course, medicine is not complete without mass spectrometry. Isotope mass spectrometry of carbon atoms is used for direct medical diagnosis of human infection with Helicobacter pylori and is the most reliable of all diagnostic methods. Also, mass spectrometry is used to determine the presence of doping in the blood of athletes.
It is difficult to imagine an area of human activity where there would be no place for mass spectrometry. We confine ourselves to simply listing: analytical chemistry, biochemistry, clinical chemistry, general chemistry and organic chemistry, pharmaceuticals, cosmetics, perfumery, food industry, chemical synthesis, petrochemistry and oil refining, control environment, production of polymers and plastics, medicine and toxicology, criminalistics, doping control, control drugs, control of alcoholic beverages, geochemistry, geology, hydrology, petrography, mineralogy, geochronology, archeology, nuclear industry and energy, semiconductor industry, metallurgy.
MASS SPECTROMETRY(, mass spectral analysis), a method of analysis in-va by determining the mass (more often, the ratio of mass to charge m / z) and relates. quantity obtained by ionization of the studied substance or already present in the mixture under study. The set of values m / z and relates. the magnitudes of these currents, presented in the form of a graph or table, called. mass spectrum in-va (Fig. 1).The beginning of the development of mass spectrometry was laid by the experiments of J. Thomson (1910), who studied beams of charged particles, the separation of which by mass was carried out using an electric beam. and magn. fields, and the spectrum was recorded on . The first was built by A. Dempster in 1918, and the first mass spectrograph was created by F. Aston in 1919; he also investigated isotopic. compound a large number elements. The first serial was created by A. Nir in 1940; his work marked the beginning of isotope mass spectrometry. Direct connection with gas-liquid (1959) made it possible to analyze complex mixtures of volatile compounds, and connection with liquid using thermal spray. devices (1983) - mixtures of non-volatile compounds.
Mac-spectral devices.
To separate the studied in-va according to the values \u200b\u200bof m / z, measuring these quantities and currents separated
mass spectrometers are used. Devices in which registration is carried out electrically. methods, called , and devices with registration on - mass spectrographs. Mass spectral instruments consist of an input system (inlet system), an ion source, a separating device (mass analyzer), a detector (receiver), which provide a sufficiently deep vacuum in the entire instrument system, and a control and data processing system (Fig. 2). Sometimes devices are connected to a computer.
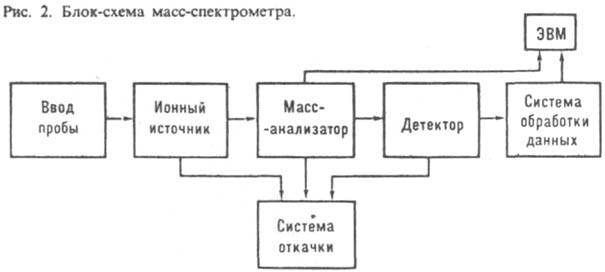
Mass spectral instruments are characterized by sensitivity, which is defined as the ratio of the number of registered to the number of entered. For abs. threshold of sensitivity take min. quantity of the examined substance (expressed in g, ), for the relative - min. mass or volume fraction of the island (expressed in%), to-rye provide registration of the output signal with a signal-to-noise ratio of 1:1.
ion source is intended for the formation of gaseous substances under investigation and the formation of an ion beam, which is sent further to the mass analyzer. max. universal method of ionization in-va - electron impact. First carried out by P. Lenard (1902). Modern sources of this type are built according to the principle of the source of A. Nir (Fig. 3).
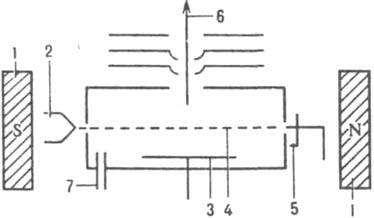
Rice. 3. Scheme of an ion source of the source type A. Nier: 1 - permanent magnet; 2 - ; 3 - pushing out; 4 - flow; 5 - trap; 6 - ion beam; 7 - input in-va.
Under the influence of the field lines of force which are directed perpendicular to the direction of motion of the ion beam, move along a circular path with a radius r = (2Vm n /zH 2) 1/2, where V is the accelerating voltage, m n is the mass, z is the charge, H is the magnetic strength. fields. with the same kinetic
energy, but with different masses or charges pass through the analyzer in dec. trajectories. Usually, the mass spectrum sweep (registration with certain values of m / z) is carried out by changing H at a constant V. energy, as well as the imperfection of focusing in directions, lead to broadening of the ion beam, which affects the resolution. For static mass analyzer R = r/(S 1 + S 2 + d ), where S 1 and S 2 - respectively. the width of the entrance and exit slots, d - broadening of the beam in the plane of the exit slit. Reducing the size of the slots to increase the resolution of the device is technically difficult to implement and, moreover, leads to very low ion currents; therefore, devices with a large trajectory radius (r = 200–300 mm) are usually designed. Resolution m. increased also when using mass analyzers with dual focusing. In such devices, the ion beam is first passed through a deflecting electric special field. forms, in which the focusing of the beam is carried out according to the energies, and then through the magnetic. a field in which they are focused in directions (Fig. 5).
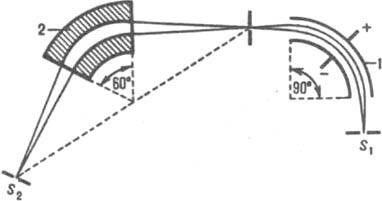
Rice. Fig. 5. Scheme of a mass analyzer with double focusing: S 1 and S 2 - source and detector slits; 1 - capacitor; 2 - magnet.
There are more than 10 types of dynamic mass analyzers: quadrupole, time-of-flight, cyclotron resonance, magnetic resonance, radio frequency, farvitron, omegatron, etc. Below are considered the most. widely used mass analyzers.
The quadrupole mass analyzer is a quadrupole capacitor (Fig. 6), a constant voltage V and an alternating high-frequency voltage V 0 cos are applied to the parallel rods w t (w - frequency, t - time); their sums for each are equal in magnitude and opposite in sign.
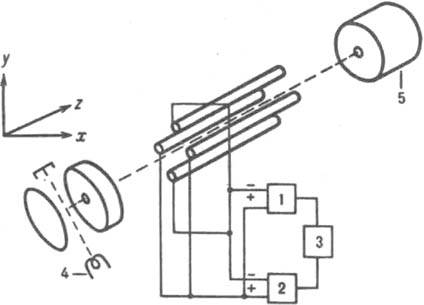
Rice. 6. Scheme of a quadrupole mass analyzer: 1 - high-frequency generator; 2 - DC voltage generator; 3 - sweep generator; 4 and 5 - source and detector.
Those emitted from the ion source move in the analyzer chamber along the z axis, parallel to the longitudinal axes of the rods, along complex volumetric helical trajectories, performing transverse vibrations along the x and y axes. At fixed values of the frequency and amplitude of the alternating voltage with certain values of m / z pass through
a quadrupole capacitor, y with other values of m / z, the amplitude of the transverse oscillations reaches such a value that they hit the rods and are discharged on them. The mass spectrum is swept by changing the DC and AC voltage or frequency. For modern quadrupole R = 8000. The first quadrupole device was built by W. Pauli and H. Steinwedel (Germany, 1953).
The time-of-flight mass analyzer is an equipotential space in which they drift, separating according to their velocities (Fig. 7). , formed in the ion source, a very short electric. pulse "injected" in the form of "ion package" through the grid into the analyzer. In the process of movement, the initial ion packet is stratified into packets consisting of packets with the same m/z values. The drift velocity of detached ion packets and, consequently, the time of their flight through an analyzer of length L is calculated from the f-le:
![]() (V - voltage). The totality of such packets entering the detector forms a mass spectrum. For modern devices R = 5000 - 10000. The first device was created by A. Cameron and D. Egters (USA, 1948), and in the USSR - by N. I. Ionov (1956).
(V - voltage). The totality of such packets entering the detector forms a mass spectrum. For modern devices R = 5000 - 10000. The first device was created by A. Cameron and D. Egters (USA, 1948), and in the USSR - by N. I. Ionov (1956).
Rice. 7.
Scheme of the time-of-flight mass analyzer: 1 - grid; 2 - detector.
In 1973 B. A. Mamyrin designed a device with electrostatic. reflective mirror, mass reflectron.
Cyclotron resonant mass analyzer - cell in the form cuboid or a cube placed in a homogeneous magnetic field. field. , entering the cell, move in it along a spiral trajectory (cyclotron motion) with a frequency w c \u003d 1 / 2 p z. H/m, where H is the magnetic strength. fields, i.e., with the same values of m/z, have a definite cyclotron frequency. The operation of the device is based on the resonant absorption of energy when the field frequency and the cyclotron frequency coincide. The method is based on the use of a cyclotron resonant mass analyzer, which is used to determine the mass, in particular mol. formed during ionic-molecular p-tions in the gas phase; structure analysis high-mol. ; definitions of acid-base st-in-in. For lungs R = 10 8 . The first ioncyclotron resonance was built by G. Sommer, G. Thomas and J. Hipl (USA, 1950).
Detectors(receivers) are placed at the outlet of the instrument. For detection using electrometric. amplifiers that allow measuring ion currents up to 10 -
14 A, electron multipliers and scintillators. detectors with a photomultiplier, which provide counting of individual (current 10 -
19 A) and have a small time constant, and also, the advantage of which is the possibility of registering the entire mass spectrum and signal accumulation.
For the introduction of the Islands in the ion source, there is a special. system, called release system. It provides the input of strictly metered quantities of in-va, its minimum. thermal decomposition, the shortest delivery to the ionization area and automatic change of samples without violation. The input system and volatile in-in is a cold or heated glass tanks with viscous or pier. leaks, through which the gaseous
enters the ionization region. When connected to between the ion source and placed pier. separator (jet, porous or membrane), in which the carrier gas is removed and enriched with the analyzed matter.
The input system of low-volatile in-in is most often a vacuum lock, from which the ampoule with in-tion is introduced directly into the ionization. camera. The ampoule is mounted on a rod equipped with a heater, with the help of which it is created required temperature for in-va. In some cases, the ampoule is heated by the heat of the ionization. cameras. To reduce the decomposition of the island, increase the heating rate, which must exceed the thermal rate. decomposition. This is how the devices connecting the liquid source with the ion source operate. Naib. a device based on thermal spraying of the solution of the investigated substance is widespread, at which its ionization occurs. Dr. type - a belt conveyor, on a belt to-rogo in-in is delivered to the ion source through a system of locks. When the tape moves, the solvent is removed, and in the ion source, when the tape is rapidly heated, the content evaporates and ionizes. In some cases, ionization of the substance is also possible as a result of its bombardment by accelerated particles on the surface of the tape. For non-volatile inorg. conn. apply special. , called Knudsen cell. This is a high-temperature crucible with a hole of small diameter 0.1-0.3 mm, through which it flows under conditions close to equilibrium.
works in deep (10 -
5 - 10
-
6 Pa and above), which allows minimizing the loss of resolution due to the collision of the ion beam with neutral ones. The ion source and mass analyzer have different systems pumping and are interconnected by a channel of such a size, to-ry sufficient for the passage of the ion beam. This design prevents the analyzer from falling when the source rises. The source also needs a high pumping speed to reduce the memory effect ( removal of in-in, adsorbed on ext. pov-sti device). Usually, diffusion is created in devices. Turbomolecular ones are also used, providing ultrahigh (10 -
7 - 10
-
8 Pa) and pumping out at a speed of several. liters per second; these do not require the use of cold traps.
Data collection and management requires the automation of all processes with the help of a computer, which allows to carry out diff. types of studies according to a predetermined program with the conditions of analysis during the operation of the device.
Application of mass spectrometry.
Mass spectrometry is widely used in decomp. fields of science and technology: in and, physics, geology, biology, medicine, in industry, in paint and varnish and chemical. prom-sti, in the production of ultra-pure materials, in nuclear technology, in the village. x-ve and veterinary medicine, in food. prom-sti, in the analysis of pollution products and many others. etc. Great progress has been made in the analysis of biologically important; the possibility with a pier is shown. m. up to 15000, with a pier. m. up to 45000, etc. Mass spectrometry has found application as an express method in medicine; The principles of mass spectrometry underlie the device Naib. feels. leak detectors.
Fatherland. produced for diff. purposes, have indices: for the study of the isotopic composition - MI, for the study of chemical. composition - MX, for - MS.
Mass spectrometry in allows you to measure the exact mol. mass and calculate the elemental composition of the investigated in-va, establish a chemical. and spaces. structure, determine the isotopic composition, conduct qualities. and
quantities. analysis of complex mixtures org. connections. One of the most important tasks is to find the relationship between the nature of the mass spectrum and the structure of the studied org. . When ionizing org. a pier is formed. , in which the processes of hetero- and gomolytic further occur. rupture of ties or rupture of ties with a rearrangement and the formation of fragmentation, to-rye, in turn, may undergo further decay. Consistent decays determined from the mass spectrum, called. directions or paths of decay. Decay directions are an important characteristic of each class of compounds. The totality of all directions of decay is characteristic for each org. conn. fragmentation scheme. If the mass spectrum is simple, the fragmentation scheme is reduced to a single decay path, e.g. during the collapse of CH 3 OH + are sequentially formed CH 2 \u003d OH + and H-C \u003d O +. In the case of complex mass spectra, the fragmentation scheme corresponds to many, often overlapping, decay directions, e.g. fragmentation scheme:
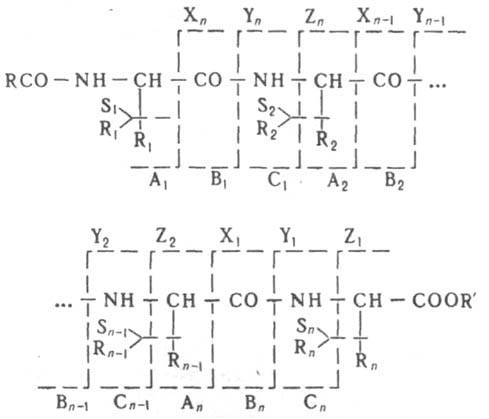
Mol. decomposes as a result of breaking the bonds of CH-CO, CO-NH, NH-CH and CH-R with the formation of fragments respectively. A n and X n , B n and Y n , C n and Z n , S n and R n (n is the number of the amino acid residue in the peptide chain), which further decompose in the same way. The total number of peaks in such a spectrum can reach several. hundreds. The number of fragments is determined by the structure of the studied, the supply of internal. energy they say. and fragmentation and the time interval between the formation and its detection. Therefore, when interpreting mass spectra, it is necessary to take into account both the measurement conditions (ionizing energy, accelerating voltage, in the ion source, temperature of the ionization chamber), and the design features of the device. At max. By standardizing the measurement conditions, it is possible to obtain fairly reproducible mass spectra. Comparison of the mass spectrum of the system under study with the spectrum available in the catalog - max. a quick and easy way, in-in in the determination of pollution, the control of human and animal food, the study of lek processes. drugs, in forensics, etc. However, only on the mass spectrum can not be unambiguous, for example. not all isomeric substances form different mass spectra.
Under the conditions of mass spectrometry, some of the excited ones decay after leaving the ion source. Such called metastable. In mass spectra, they are characterized by broadened peaks at noninteger m/z values. One of the methods for studying such - mass and kinetic. energies. The study of the decay of metastable is carried out on instruments, in which magn. the analyzer precedes the electrical one. Magn. the analyzer is tuned so that it skips the metastable






