Saturation in physics. Molecular physics and thermodynamics
Consider a two-phase molecular system consisting of liquid and gas components of the same substance. For example, water and steam, acetone and acetone steam, etc. If the vapor and liquid are in dynamic equilibrium, then such a vapor is called saturated.
The dynamic equilibrium of a liquid and its vapor lies in the fact that for any time interval the number of molecules evaporating from the liquid is equal to the number of molecules condensed into the liquid.
Properties saturated steam are that its pressure does not depend on the volume that it occupies, but strongly depends on the temperature at which it is located. phase transition homogeneous system from one state (for example, gas) to another (for example, liquid) obeys the Clausius-Clapeyron law.
where is the heat of evaporation at a temperature T, is the volume of one mole of vapor, is the volume of one mole of liquid, is the change in saturated vapor pressure with a change in temperature by 1 K.At low pressures and high temperatures we can assume that steam obeys the laws ideal gases, i.e. and equation (1.4) can be easily transformed into:
| , | (1.5) |
The fact that acetone (preferably ether) was used in the experiment at room temperature and reduced pressure exactly corresponds to the condition that saturated acetone vapor can be considered an ideal gas.
A slow change in the volume of the corrugated vessel is necessary for the process to proceed isothermally. This is also facilitated by the fact that acetone (or ether) evaporates easily, i.e. during evaporation and condensation, the temperature of neither the vapor nor the liquid changes significantly. If you change the volume quickly, then there will be a noticeable change in pressure. This is due to the fact that in this case the process is non-isothermal. But after some time passes, the vacuum gauge needle will return to a position that corresponds to isothermal process, because acetone, its vapor and the environment will come to a thermodynamically equilibrium state.
Saturated and unsaturated pairs.
Consider the processes occurring in a closed vessel:
- evaporation process, the rate of which gradually decreases
- condensation, the rate of which gradually increases
Over time, in a vessel closed with a lid, a state is established between the liquid and its vapor dynamic(movable) equilibrium, when the number of molecules leaving the liquid is equal to the number of molecules returning to the liquid from the vapor, that is, when the rates of evaporation and condensation are the same. Such a system is called two-phase .
A vapor in dynamic equilibrium with its liquid is calledrich.
The name "saturated" emphasizes that a given volume at a given temperature cannot contain more steam.
unsaturated steam– is a vapor that has not reached dynamic equilibrium with its liquid. At this temperature, the pressure unsaturated steam always less than the saturation vapor pressure. In the presence of unsaturated vapor above the liquid surface, the vaporization process prevails over the condensation process, and therefore the liquid in the vessel becomes less and less over time.
Consider some properties of saturated steam:
1. The concentration of saturated vapor molecules does not depend on its volume at constant temperature. If the volume of saturated vapor is reduced, then at first the concentration of its molecules will increase and more molecules will begin to pass from gas to liquid until dynamic equilibrium is again established.
2. Saturated vapor pressure at constant temperature does not depend on its volume.
p = n*k*T, because n does not depend on V, then p does not depend on V.
The volume-independent vapor pressure at which a liquid is in equilibrium with its vapor is calledsaturated steam pressure. This is the highest pressure that steam can have at a given temperature.
3. Saturated vapor pressure depends on temperature. The higher the temperature of the liquid, the more molecules will evaporate, the dynamic equilibrium will be disturbed, but the concentration of vapor molecules will increase until the equilibrium is established again, which means that the pressure of saturated vapor will also increase. With increasing temperature, the pressure saturated vapors increases.
AT atmospheric air there are always water vapors that evaporate from the surface of the seas, rivers, oceans, etc.
Air containing water vapor is calledwet .
Air humidity has a huge impact on many processes on Earth: on the development of flora and fauna, on agricultural crops. crops, livestock productivity, etc. Humidity has great importance for people's health, because it depends on the heat exchange of the human body with environment. At low humidity, rapid evaporation from the surface and drying of the nasal mucosa and larynx occur, which leads to a deterioration in the condition.
So, the humidity of the air must be able to measure. For quantification air humidity use the concepts of absolute and relative humidity.
Absolute humidity -a value showing how much water vapor is in 1 m³ of air. She is equal partial pressure steam at this temperature.
Partial pressure of steam -This is the pressure that water vapor in the air would exert if all other gases were absent.
Relative humidity -this is a measure of how far the vapor is from saturation. This is the partial pressure ratiop of water vapor contained in the air at a given temperature, to saturated steam pressure p 0 at the same temperature, expressed as a percentage:
![]()
If the air does not contain water vapor, then its absolute and relative humidity are 0.
If moist air is cooled, then the vapor in it can be brought to saturation, and then it will condense.
Examples:
dew falling in the morning

fogging of cold glass, if you breathe on it,

the formation of a drop of water on a cold water pipe,

dampness in basements.

Dew point -is the temperature at which water vapor in the air becomes saturated.
The dew point also characterizes the humidity of the air.
|
Dew point at relative air humidity in % |
||||||||||||||
Instruments used to measure air humidity hygrometers and psychrometers.
1. Condensation hygrometer.

It consists of a metal round box fixed on a stand with a polished flat surface. There are two holes in the top of the box. Through one of them, ether is poured into the box and a thermometer is inserted, and the other is connected to a rubber bulb. The action of a condensation hygrometer is based on the determination of the dew point.
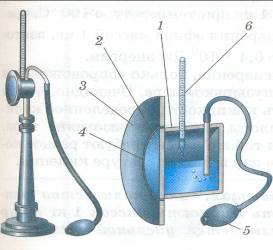
Air is blown through the ether (using a rubber bulb), while the ether quickly evaporates and cools the box. At a certain temperature, water droplets (dew) appear on the polished surface of the box. This temperature is determined by a thermometer, this will be the dew point. Absolute humidity is found in a special table by the dew point.
To find relative humidity, it is necessary to divide the saturation vapor pressure at the dew point temperature by the saturation vapor pressure at ambient temperature and multiply by 100%.
2. Hair hygrometer.

His work is based on the fact that defatted human hair lengthens with an increase in air humidity, and shortens with a decrease in humidity. The hair is wrapped around a light block, one end attached to the frame, and a weight is hung from the other. When changing the length of the hair, the pointer (arrow) attached to the block will move, moving along the scale. The scale is calibrated according to the standard instrument.
3. Psychrometer.(from the Greek "psychria" - cold).

Consists of two identical thermometers. The tank of one of them is wrapped with gauze, lowered into a vessel with water. The water wets the reservoir of the thermometer and when it evaporates, it cools. According to the temperature difference between dry and wet thermometers, the psychrometric table determines the humidity of the air.
Evaporation - this is vaporization that occurs only from the free surface of a liquid adjoining a gaseous medium or vacuum.
The uneven distribution of the kinetic energy of the thermal motion of molecules leads to the fact that at any temperature the kinetic energy of some molecules of a liquid or solid can exceed the potential energy of their connection with the rest of the molecules.
Evaporation is a process in which, from the surface of a liquid or solid body molecules fly out kinetic energy which exceeds the potential energy of interaction of molecules. Evaporation is accompanied by cooling of the liquid.
Let us consider the evaporation process from the point of view of molecular-kinetic theory. To leave the liquid, the molecules must do work by reducing their kinetic energy. Among the randomly moving molecules of a liquid in its surface layer, there will always be molecules that tend to fly out of the liquid. When such a molecule goes beyond surface layer, then a force arises that pulls the molecule back into the liquid. Therefore, only those molecules fly out of the liquid, in which the kinetic energy is greater than the work necessary to overcome the counteraction of molecular forces.
The rate of evaporation depends on:
a) the type of liquid;
b) on the area of its free surface. The larger this area, the faster the liquid evaporates.
c) the lower the vapor density of a liquid above its surface, the greater the rate of evaporation. Therefore, pumping vapors (wind) from the surface will accelerate its evaporation.
d) with increasing temperature, the rate of evaporation of the liquid increases.
vaporization is the transfer of matter from liquid state into a gaseous state.
Condensation - is the transition of a substance from a gaseous state to a liquid state.
During vaporization internal energy matter increases, and when condensed - decreases.
Heat of vaporization – is the amount of heat Q required to turn a liquid into vapor at a constant temperature.
Specific heat vaporization L is measured by the amount of heat required to turn a unit mass of liquid into steam at a constant temperature
Saturated and unsaturated steam. The evaporation of a liquid in a closed vessel at a constant temperature leads to a gradual increase in the concentration of molecules of the evaporating substance in gaseous state. Some time after the start of the evaporation process, the concentration of a substance in the gaseous state reaches a value at which the number of molecules returning to the liquid per unit time becomes equal to the number of molecules leaving the surface of the liquid in the same time. A dynamic equilibrium is established between the processes of evaporation and condensation of matter.