Gas to solid. The structure of solid, liquid and gaseous bodies - Knowledge Hypermarket
The establishment of an ideal order in the arrangement of atoms, i.e., the formation of a solid body, is hindered by thermal movements, main feature which is, as we know, randomness, disorder. Therefore, in order for a substance to be in a solid state, its temperature must be low enough - so low that the energy of thermal motions is less than potential energy interactions of atoms.
A completely ideal crystal, in which all atoms are in equilibrium and have a minimum energy, the body can only be when absolute zero. In fact, all substances become solid at much more high temperatures. The only exception is helium, which remains liquid even at absolute zero, but this is due to some quantum effects, which we will briefly discuss below.
A substance can change from a liquid state to a solid state, as well as from a gaseous state. In both cases, such a transition is a transition from a state devoid of symmetry to a state in which symmetry exists (this, in any case, refers to the long-range order that exists in crystals, but does not exist in either liquid or gaseous substances) . Therefore, the transition to the solid state must occur abruptly, i.e., at a certain temperature, in contrast to the gas-liquid transition, which, as we know, can also occur continuously.
Consider first the transformation of liquid- solid. The process of formation of a solid body during cooling of a liquid is the process of crystal formation (crystallization), (and it occurs at a certain temperature, the temperature of crystallization or solidification. Since the energy decreases during such a transformation, it is accompanied by the release of energy in the form of latent heat of crystallization. The reverse transformation is melting - also occurs abruptly at the same temperature and is accompanied by the absorption of energy in the form
the heat of fusion equal in magnitude to the heat of crystallization.
This is clearly seen from the graph of the coolant temperature versus time shown in Fig. 179 (curve a). Section 1 of the curve a gives the course of a monotonous decrease in the temperature of the liquid due to the removal of heat from it. Horizontal section 2 shows that at a certain temperature, its decrease stops, despite the fact that heat removal continues. After a while, the temperature starts to decrease again (section 3). The temperature corresponding to section 2 is the crystallization temperature. The heat released during crystallization compensates for the removal of heat from the substance and therefore the decrease in temperature temporarily stops. After the end of the crystallization process, the temperature, now of a solid body, again begins to decrease.
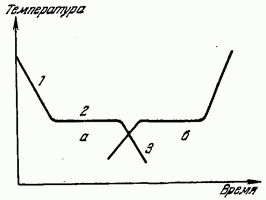
Such a course of the temperature decrease graph is typical for crystalline bodies. When cooling liquids that do not crystallize (amorphous substances), latent heat does not stand out and the cooling curve is a monotonic curve without stopping the cooling.
In the reverse process of the transition of a substance from solid state into liquid (melting) on the heating curve, there is also a stop in the increase in temperature, due to the absorption of the latent heat of melting - heat, due to which the crystal lattice is destroyed (curve in Fig. 179).
To start crystallization, the presence of a center or centers of crystallization is necessary. Such centers could be random accumulations of liquid particles stuck to each other, to which more and more particles could join, until the entire liquid turned into a solid body. However, the formation of such accumulations in the liquid itself is hampered by thermal movements, which destroy them even before they have time to acquire any noticeable dimensions. Crystallization is greatly facilitated if sufficiently large solid particles in the form of dust particles and bodies are present in the liquid from the very beginning, which become centers of crystallization.
The formation of crystallization centers in the liquid itself is facilitated, of course, with decreasing temperature. Therefore, the crystallization of a pure liquid, devoid of foreign formations,
usually begins at a temperature somewhat lower than the true crystallization temperature. Under normal conditions, in a crystallizing liquid there are many centers of crystallization, so that many crystals grow together in the liquid, and the solidified substance turns out to be polycrystalline.
Only under special conditions, which are usually difficult to provide, can a single crystal be obtained - a single crystal growing from a single crystallization center. If, in this case, the same conditions for the accumulation of particles are provided for all directions, then the crystal is obtained correctly faceted according to its symmetry properties.
The liquid-solid transition, as well as the reverse transformation, is a phase transition, since the liquid and solid states can be considered as two phases of a substance. Both phases at the crystallization (melting) temperature can come into contact with each other, being in equilibrium (ice, for example, can float in water without melting), just as a liquid and its saturated vapor can be in equilibrium.
Just as the boiling point depends on pressure, the crystallization temperature (and its equivalent melting point) also depends on pressure, usually increasing with increasing pressure. It grows because the external pressure brings the atoms together, and to destroy the crystal lattice during melting, the atoms must be moved away from each other: at more pressure this requires a greater energy of thermal motions, i.e., a higher temperature.
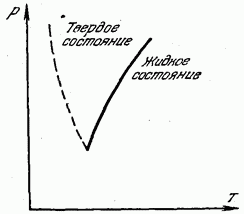
On fig. 180 shows a curve of melting (crystallization) temperature versus pressure. The solid curve divides the entire region into two parts. The area to the left of the curve corresponds to the solid state, and the area to the right of the curve corresponds to the liquid state. Any point lying on the melting curve itself corresponds to the equilibrium of the solid and liquid phases: at these pressures and temperatures, the substance in the liquid and solid states is in equilibrium, in contact with each other, and the liquid does not harden, and the solid does not melt.
Dotted line in fig. 180 shows the melting curve for those few substances (bismuth, antimony, ice, germanium) in which, during solidification, the volume does not decrease, but increases. Such
substances, of course, the melting point decreases with increasing pressure.
The change in melting point is related to the change in pressure by the Clausius-Clapeyron relation:
![]()
Here, is the melting (crystallization) temperature, and are, respectively, the molar volumes of the liquid and solid phases and the molar heat of fusion.
This formula is also valid for other phase transitions. In particular, for the case of evaporation and condensation, the Clausius-Clapeyron formula was derived in Chap. VII [see (105.6)].
From the Clapeyron-Clausius formula, it can be seen that the sign of the change in melting temperature with a change in pressure is determined by which of the two values, or more. The steepness of the curve also depends on the value of the latent heat of transition; the lower the temperature, the less the melting temperature changes with pressure. In table. 20 shows the values of the specific (i.e., per unit mass) heat of fusion for some substances.
Table 20 (see scan) Specific heat melting for some substances
The Clausius-Clapeyron equation can also be written in this form:
![]()
This equation shows how the pressure under which both equilibrium phases are located changes with temperature.
A solid can be formed not only by crystallization of a liquid, but also by the condensation of a gas (vapor) into a crystal, bypassing the liquid phase. In this case, the latent heat of transition is also released, which, however, is always greater than the latent heat of fusion. After all, the formation of a solid at a certain temperature and pressure can occur both directly from the gaseous state, and by preliminary liquefaction, In both
cases, the initial and final states are the same. It means that the energy difference of these states is the same. Meanwhile, in the second case, firstly, the latent heat of condensation is released during the transition from the gaseous to the liquid state and, secondly, the latent heat of crystallization during the transition from the liquid to the solid state. It follows that the latent heat in the direct formation of a solid from the gaseous phase must be equal to the sum of the heats of condensation and crystallization from the liquid. This applies only to heats measured at the melting point. At lower temperatures, the heat of condensation from the gas increases.
The reverse process of evaporation of a solid is usually called sublimation or sublimation. Evaporating particles of a solid form vapor above it in exactly the same way as occurs when a liquid evaporates. At certain pressures and temperatures, vapor and solid can be in equilibrium. Vapor in equilibrium with a solid is also called saturated steam. As in the case of a liquid, elasticity saturated steam above a solid depends on temperature, decreasing rapidly with decreasing temperature, so that for many solids at ordinary temperatures the saturated vapor pressure is negligible.
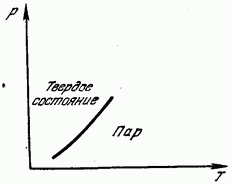
On fig. 181 shows the curve of saturated vapor pressure versus temperature. This curve is the line of equilibrium between the solid and gaseous phases. The region to the left of the curve corresponds to the solid state, to the right of it, to the gaseous state. Sublimation, as well as melting, is associated with the destruction of the lattice and requires the expenditure of the energy necessary for this. This energy manifests itself as the latent heat of sublimation (sublimation), equal, of course, to the latent heat of condensation. The heat of sublimation is therefore equal to the sum of the heats of melting and vaporization.
Most of the substances that can stay in three 
states: liquid, solid and gaseous. These states are called aggregate states.
 A substance passes from one state to another when heated or cooled, as well as when pressure changes.
A substance passes from one state to another when heated or cooled, as well as when pressure changes.
You already know that if you heat water to boiling point, it will turn into steam. That is, it will go to gaseous state. There is a theory that explains the properties of all three states.
It is called kinetic and is based on the statement that particles move in the composition of matter.
Kinetic theory.
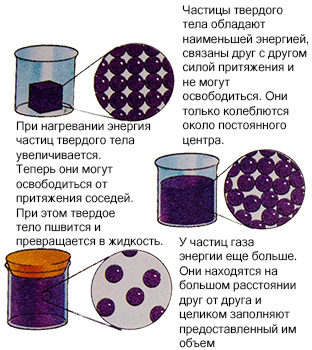 Most hypotheses in science are not accepted until they are proven, but are considered true only because they explain certain phenomena. Explain the properties of solid, liquid and gaseous bodies, based on the energy of the particles that are in their composition, gives kinetic theory. The particles of a solid body are located very closely to each other, are bound by the force of attraction and cannot be released. They only oscillate around the center. But as soon as we start heating the body, the energy of its particles will begin to grow. That's when they can break away from each other. The solid begins to melt and flow. Gas particles have even more energy and are located at an even greater distance from each other. Heating allows you to increase the energy of the particles, they move faster and the body passes into another state of aggregation.
Most hypotheses in science are not accepted until they are proven, but are considered true only because they explain certain phenomena. Explain the properties of solid, liquid and gaseous bodies, based on the energy of the particles that are in their composition, gives kinetic theory. The particles of a solid body are located very closely to each other, are bound by the force of attraction and cannot be released. They only oscillate around the center. But as soon as we start heating the body, the energy of its particles will begin to grow. That's when they can break away from each other. The solid begins to melt and flow. Gas particles have even more energy and are located at an even greater distance from each other. Heating allows you to increase the energy of the particles, they move faster and the body passes into another state of aggregation.
Brownian motion.
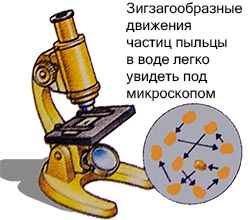 English biologist Robert Brown in 1927 examined pollen particles placed in a liquid under a microscope.
English biologist Robert Brown in 1927 examined pollen particles placed in a liquid under a microscope.
He noticed that they were moving in a zigzag pattern, but he could not explain it.
This random movement of molecules is called brownian motion. The explanation was later given by Albert Einstein.
He stated that particles placed in a liquid move due to the collision of also moving, but invisible molecules.
 State change.
State change.
As the temperature rises, the energy of the particles of the body increases and reaches the melting point.Then there is a break in the bonds between the particles and the body melts.
For example, paraffin from a candle. When heated, it flows down, while cooling, it solidifies again in a solid state.
With further heating, the boiling point is reached, and the particles are completely freed from the mutual bond and the liquid turns into vapor.
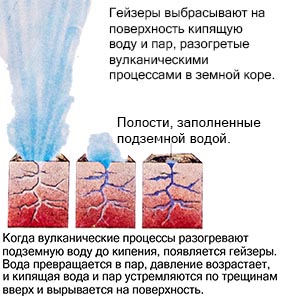 An example is a geyser, which, heated by volcanic processes, ejects boiling water and steam to the surface. But when it cools down, the reverse process occurs. The gas condenses and becomes a liquid, and the liquid, cooling further, freezes, turning into a solid.
An example is a geyser, which, heated by volcanic processes, ejects boiling water and steam to the surface. But when it cools down, the reverse process occurs. The gas condenses and becomes a liquid, and the liquid, cooling further, freezes, turning into a solid.
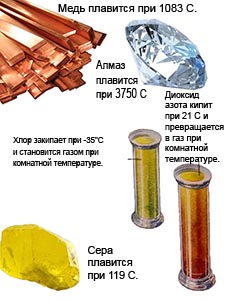
Carbon dioxide can change from a solid to a gaseous state, bypassing the liquid state.
The lack of water on Mars is explained by the negligible atmospheric pressure.
The water there immediately boils and evaporates. For different substances, the transition to another state occurs at different temperatures.
You can also change the melting and boiling point by adding some impurity to the substance or changing the pressure.
We call the pressure of the earth's atmosphere atmospheric pressure.
 |
 |
 Surface tension.
Surface tension.
You saw how water striders calmly walk along the surface of the reservoir.
They do not sink and can move around the water surface as much as they like.
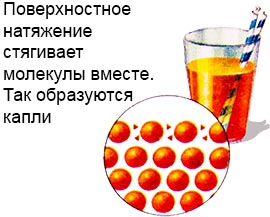 This is possible because the liquid has a layer of surface tension.
This is possible because the liquid has a layer of surface tension.
The molecules of this layer are much more strongly bound than in the depth of the liquid.
This creates a kind of film on the surface of the liquid, and also forms drops.
And the weight of the water strider is simply not enough to break it.
Evaporation.
 The liquid evaporates constantly, even when not heated. This is due to the fact that the energy of the molecules at the surface layer is much greater and this allows the molecules to break away from the surface, that is, to evaporate.
The liquid evaporates constantly, even when not heated. This is due to the fact that the energy of the molecules at the surface layer is much greater and this allows the molecules to break away from the surface, that is, to evaporate.
As it evaporates, the temperature of the liquid decreases. This is especially true when a person sweats.
The water droplets on the skin evaporate and the skin cools.
Gases.
 A gas is a substance that has no fixed volume or shape. According to the kinematic theory, the energy of gas molecules is enough to break bonds and, flying apart, fill the entire volume around them.
A gas is a substance that has no fixed volume or shape. According to the kinematic theory, the energy of gas molecules is enough to break bonds and, flying apart, fill the entire volume around them.
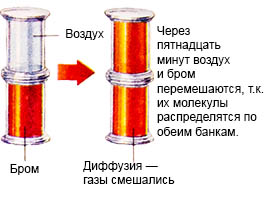
This process is called diffusion.
The amount of gas pressure depends on how strongly the gas molecules hit the walls of the vessel. If, without changing the temperature, the volume of the gas is reduced by reducing the volume of the vessel, then its pressure will increase, since the gas molecules will more often hit the walls of the vessel.Or if you pump a new portion of gas.
When heated, the gas molecules move faster and further, the gas expands and becomes less dense. If, by heating a gas, to limit its volume, then the pressure will begin to increase.
 |
 |
Volume, mass and density.
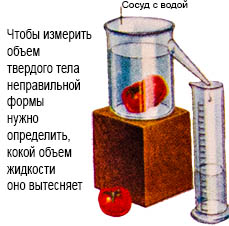
Volume is the amount of space that a liquid or solid body occupies. The volume is measured in cubic meters. To find out the volume of liquid, you need to pour it into a measuring container. The volume of a solid body correct form are recognized by the volume of liquid displaced by it from the vessel. The volume of a solid body of the correct form - according to the formula.
On one side of the scale we place the body to be weighed, on the other - a body with a known mass. The mass of a solid, liquid, or gaseous body tells us how much matter it contains. Mass is measured in kilograms and grams. It is necessary to understand the difference between mass and weight - the magnitude of the gravitational force acting on the body.
 |
 |
 By density, we can judge how tightly the particles that make up the body are “fitted”. For example, metal molecules are closer together than paper molecules.
By density, we can judge how tightly the particles that make up the body are “fitted”. For example, metal molecules are closer together than paper molecules.
This means that the density of the metal is higher. Density is calculated by dividing the mass of a body by its volume and is measured in kilograms per cubic meter (kg/m3).
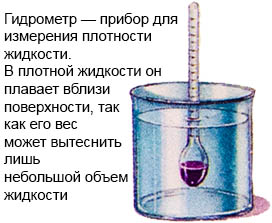
There is a device for measuring the density of a liquid - a hydrometer. In a dense liquid, it floats near the surface, since its weight can only displace a small volume of liquid.
COMBINE USEFUL WITH PLEASANT!
 This experiment shows the effect of surface tension force. Pour pure ox into a saucer and add a little powder, such as talc.
This experiment shows the effect of surface tension force. Pour pure ox into a saucer and add a little powder, such as talc.
Drip liquid soap into the middle. What's happening?
Soap destroys surface tension, i.e. attractive force between molecules. Molecules located near the walls are more tightly bound to each other, so all the powder in the saucer has gathered at the sides.
Mineral fuels in the earth's crust are concentrated in the form of accumulations of carbon (various coals and shale) and hydrocarbons (oil and gas deposits). In science and practice, the idea is firmly established that accumulations of hydrocarbons with a molecular weight of more than 60 reside in the earth's crust in liquid state, and lighter ones - in gaseous.
However, Soviet scientists Academician A. A. Trofimuk, Corresponding Member of the USSR Academy of Sciences N. V. Chersky, Doctor technical sciences F. A. Trebin, Yu. F. Makogon, candidate of technical sciences, and V. G. Vasiliev, candidate of geological and mineralogical thermodynamic conditions pass in the earth's crust into a solid state and form gas hydrate deposits, and with an incomparably higher concentration of gas per unit volume of the porous medium than in ordinary gas deposits.
The gas passes into a solid state in the earth's crust, connecting with formation water at hydrostatic pressures and relatively low temperatures - up to +25°. Experimental studies showed that at the same pressures, a gas hydrate deposit contains several times more gas than a conventional equal-volume gas deposit, because one volume of water, when it passes into a hydrate state, binds up to 220 volumes of gas, while the usual solubility of gas in water does not exceed two to four volumes , and for ice it is even lower.
The physical characteristics of a gas hydrate reservoir is very different from the physical characteristics of a conventional gas reservoir. The electrical conductivity of such a deposit is much lower than that of a conventional deposit, which makes it possible to develop new methods for interpreting geophysical characteristics in order to identify gas hydrate deposits in the earth's crust, as well as using these properties to create new processes (gas separation, storage of large volumes of gas at low pressures etc.).
The process of formation of a gas hydrate deposit can be accompanied by a significant decrease in reservoir pressure (below hydrostatic), a reduction in the size of the deposit, and in the presence of gas and water inflow, a significant increase in gas reserves in the deposit.
The zones of gas hydrate deposits are concentrated mainly in the areas of permafrost, and common area, where such deposits can be found, is more than 50% of the territory Soviet Union, about a quarter of the land of our planet and more than 90% of the oceans.
One of the researchers, the head of the Geology Department of the Ministry of the Gas Industry of the USSR, V. G. Vasiliev, said: “This is an important reserve of natural gas that will multiply the energy and chemical resources of our country. In addition, it is assumed that under the bottom of the World Ocean there are also gas deposits in state. The logic of scientists' reasoning is as follows: the ocean floor is under water pressure with a pressure of 300-500 atm, and at such a pressure, the low temperatures of the Yakutian interior are no longer needed for the formation of hydrates. Since the ocean covers most of the planet, and permafrost occupies a significant part of the land, then it can be assumed that the solid state of the gas is not an exception, but the rule.
In what way can hydrates be extracted from underground without building mines? The researchers proposed to transfer methane from a solid state to a gaseous state directly in the reservoir, and then "select" it using conventional wells. There are several ways to do this. If the pressure in the well is artificially reduced or the temperature in the reservoir is raised, this will cause the hydrate to break down, and the free gas will rise up through the pipes. But the most promising, scientists believe, is to act on water with a catalyst that accelerates the release of gas molecules from the strong grip of water molecules. The concentration of gas in the reservoir is too high, and this titanic force must be released carefully.
Is it possible today to talk about the industrial exploitation of gas hydrate deposits? Corresponding Member of the USSR Academy of Sciences N. V. Chersky answers this question: “Many hundreds of thousands of cubic meters of gas extracted from the gas hydrate formations of the Messoyakhskoye field in the Arctic,” he says, “have already arrived at the Norilsk Mining and Metallurgical Combine. The cost of gas is about the same As usual, more than 30 deposits of solid gas have already been discovered. Scientists in Moscow, Novosibirsk, and Yakutsk are working on the problems of their rational development and use."
>>Structure of solid, liquid and gaseous bodies
Submitted by readers from Internet sites
physics library, physics lessons, physics program, abstracts of physics lessons, physics textbooks, ready-made homework
Lesson content lesson summary support frame lesson presentation accelerative methods interactive technologies Practice tasks and exercises self-examination workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures graphics, tables, schemes humor, anecdotes, jokes, comics parables, sayings, crossword puzzles, quotes Add-ons abstracts articles chips for inquisitive cheat sheets textbooks basic and additional glossary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in the textbook elements of innovation in the lesson replacing obsolete knowledge with new ones Only for teachers perfect lessons calendar plan for the year guidelines discussion programs Integrated LessonsThe state of aggregation of a substance is usually called its ability to maintain its shape and volume. An additional feature is the ways in which a substance passes from one state of aggregation to another. Based on this, three states of aggregation are distinguished: solid, liquid and gas. Their visible properties are as follows:
A solid body retains both shape and volume. It can pass both into a liquid by melting, and directly into a gas by sublimation.
- Liquid - retains volume, but not shape, that is, it has fluidity. The spilled liquid tends to spread indefinitely over the surface onto which it is poured. A liquid can pass into a solid by crystallization, and into a gas by evaporation.
- Gas - does not retain either shape or volume. Gas outside of any container tends to expand indefinitely in all directions. Only gravity can prevent him from doing this, thanks to which the earth's atmosphere does not dissipate into space. A gas passes into a liquid by condensation, and directly into a solid can pass through precipitation.
Phase transitions
The transition of a substance from one state of aggregation to another is called a phase transition, since the scientific synonym for the state of aggregation is the phase of matter. For example, water can exist in a solid phase (ice), liquid (ordinary water) and gaseous (steam).
The example of water also demonstrates sublimation well. Laundry hung out in the yard to dry on a frosty windless day immediately freezes through, but after a while it turns out to be dry: the ice sublimates, directly turning into water vapor.
As a rule, the phase transition from solid to liquid and gas requires heating, but the temperature of the medium does not increase: thermal energy goes to break the internal bonds in the substance. This is the so-called latent heat of phase transition. When reverse phase transitions(condensation, crystallization) this heat is released.
That is why steam burns are so dangerous. When it comes into contact with the skin, it condenses. The latent heat of evaporation/condensation of water is very high: in this respect, water is an anomalous substance; That is why life on Earth is possible. During a steam burn, the latent heat of water condensation "scalds" the burnt place very deeply, and the consequences of a steam burn are much more severe than from a flame on the same area of \u200b\u200bthe body.
Pseudophases
The fluidity of the liquid phase of a substance is determined by its viscosity, and the viscosity is determined by the nature of internal bonds, to which the next section is devoted. The viscosity of a liquid can be very high, and such a liquid can flow imperceptibly to the eye.
The classic example is glass. It is not a solid, but a very viscous liquid. Please note that glass sheets in warehouses are never stored leaning obliquely against the wall. Within a few days they will sag under their own weight and become unusable.
Other examples of pseudo-solid bodies are shoe pitch and building bitumen. If you forget the angular piece of bitumen on the roof, over the summer it will spread into a cake and stick to the base. Pseudo-solid bodies can be distinguished from real ones by the nature of melting: the real ones either retain their shape until they spread at once (solder when soldering), or float, letting out puddles and streams (ice). And very viscous liquids gradually soften, like the same pitch or bitumen.
Extremely viscous liquids, the fluidity of which is not noticeable for many years and decades, are plastics. Their high ability to retain their shape is provided by the huge molecular weight of polymers, many thousands and millions of hydrogen atoms.
The structure of the phases of matter
In the gas phase, the molecules or atoms of a substance are very far apart, many times greater than the distance between them. They interact with each other occasionally and irregularly, only during collisions. The interaction itself is elastic: they collided like hard balls, and immediately scattered.
In a liquid, molecules/atoms constantly "feel" each other due to very weak bonds of a chemical nature. These bonds break all the time and are immediately restored again, the molecules of the liquid are constantly moving relative to each other, and therefore the liquid flows. But in order to turn it into a gas, you need to break all the bonds at once, and this requires a lot of energy, which is why the liquid retains its volume.
In this regard, water differs from other substances in that its molecules in a liquid are connected by so-called hydrogen bonds, which are quite strong. Therefore, water can be a liquid at a normal temperature for life. Many substances with a molecular weight tens and hundreds of times greater than that of water, under normal conditions, are gases, like at least ordinary household gas.
In a solid, all its molecules are firmly in place due to strong chemical bonds between them, forming a crystal lattice. Crystals of the correct form require special conditions for their growth and therefore are rarely found in nature. Most solids are conglomerates of small and tiny crystals - crystallites, firmly linked by forces of mechanical and electrical nature.
If the reader has seen, for example, a cracked semi-axle of a car or a cast-iron grate, then the grains of crystallites on the scrap are visible with a simple eye. And on fragments of broken porcelain or faience dishes, they can be observed under a magnifying glass.
Plasma
Physicists also distinguish the fourth aggregate state of matter - plasma. In a plasma, electrons are detached from atomic nuclei, and it is a mixture of electrically charged particles. Plasma can be very dense. For example, one cubic centimeter of plasma from the interior of white dwarf stars weighs tens and hundreds of tons.
Plasma is isolated into a separate state of aggregation because it actively interacts with electromagnetic fields because its particles are charged. AT free space the plasma tends to expand, cooling down and turning into a gas. But under the influence of electromagnetic fields, it can retain its shape and volume outside the vessel, like a solid body. This property of plasma is used in thermonuclear power reactors - prototypes of power plants of the future.






