Properties and structure of gaseous, liquid and solid bodies
All non-living matter consists of particles, the behavior of which may differ. The structure of gaseous, liquid and solids has its own characteristics. Particles in solids are held together because they are very close to each other, which makes them very strong. In addition, they can keep a certain shape, since their smallest particles practically do not move, but only vibrate. Molecules in liquids are quite close to each other, but they can move freely, so they do not have their own shape. Particles in gases move very fast, and there is usually a lot of space around them, which suggests that they are easily compressed.
Properties and structure of solids
What is the structure and features of the structure of solids? They are made up of particles that are very close to each other. They cannot move and therefore their shape remains fixed. What are the properties of a solid body? It does not shrink, but if it is heated, its volume will increase with increasing temperature. This is because the particles begin to vibrate and move, which leads to a decrease in density.

One of the features of solids is that they have a fixed shape. When a solid is heated, the movement of the particles increases. Faster moving particles collide more violently, causing each particle to push its neighbors. Therefore, an increase in temperature usually leads to an increase in the strength of the body.
Crystal structure of solids
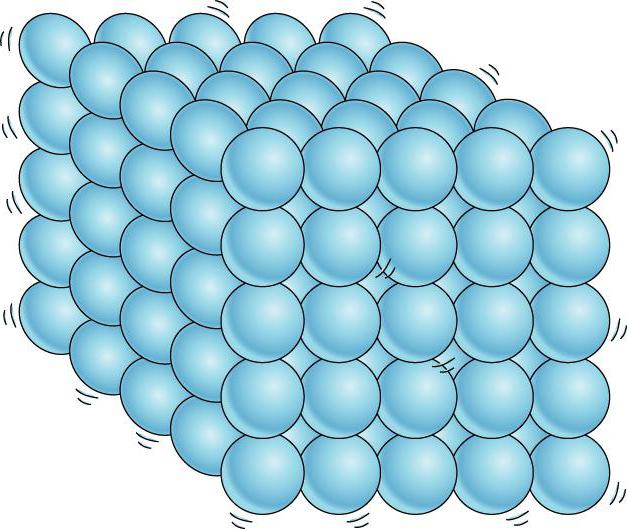
Intermolecular forces of interaction between adjacent molecules of a solid are strong enough to keep them in a fixed position. If these smallest particles are in a highly ordered configuration, then such structures are usually called crystalline. The internal ordering of particles (atoms, ions, molecules) of an element or compound is dealt with by a special science - crystallography.
The chemical structure of a solid is also of particular interest. By studying the behavior of particles, how they are made, chemists can explain and predict how certain kinds of materials will behave under certain conditions. The smallest particles of a solid body are arranged in the form of a lattice. This is the so-called regular arrangement of particles, where various chemical bonds between them.

The band theory of the structure of a solid body considers it as a set of atoms, each of which, in turn, consists of a nucleus and electrons. AT crystalline structure the nuclei of atoms are located in the nodes of the crystal lattice, which is characterized by a certain spatial periodicity.

What is the structure of a liquid?
The structure of solids and liquids is similar in that the particles of which they are composed are located on close range. The difference lies in the fact that the molecules move freely, since between them it is much weaker than in a solid.
What are the properties of a liquid? Firstly, it is fluidity, and secondly, the liquid will take the form of the container in which it is placed. If it is heated, the volume will increase. Due to the proximity of the particles to each other, the liquid cannot be compressed.

What is the structure and structure of gaseous bodies?
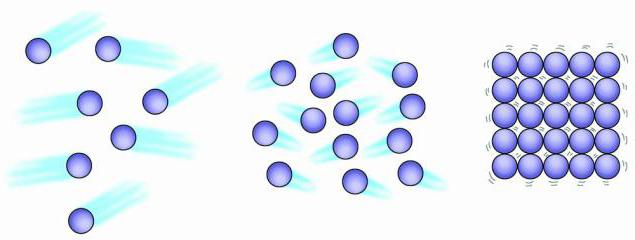
Gas particles are randomly arranged, they are so far apart that there can be no attractive force between them. What properties does a gas have and what is the structure of gaseous bodies? As a rule, the gas uniformly fills the entire space in which it was placed. It compresses easily. Particle speed gaseous body increases with increasing temperature. At the same time, there is also an increase in pressure.

The structure of gaseous, liquid and solid bodies is characterized by different distances between the smallest particles of these substances. The particles of a gas are much farther apart than in a solid or liquid state. In air, for example, the average distance between particles is about ten times the diameter of each particle. Thus, the volume of molecules occupies only about 0.1% of the total volume. The remaining 99.9% is empty space. In contrast, liquid particles fill about 70% of the total liquid volume.
Each gas particle moves freely along a straight path until it collides with another particle (gas, liquid or solid). The particles usually move fast enough that after two of them collide, they bounce off each other and continue on their way alone. These collisions change direction and speed. These properties of gas particles allow gases to expand to fill any shape or volume.

State change
The structure of gaseous, liquid and solid bodies can change if a certain external influence is exerted on them. They can even change into each other's states under certain conditions, such as during heating or cooling.

- Evaporation. Structure and properties liquid bodies allow them, under certain conditions, to pass into a completely different physical state. For example, if you accidentally spill gasoline while refueling a car, you can quickly smell its pungent smell. How does this happen? Particles move throughout the liquid, as a result, a certain part of them reaches the surface. Their directional motion can carry these molecules off the surface and into the space above the liquid, but the attraction will pull them back. On the other hand, if a particle is moving very fast, it can break away from others by a decent distance. Thus, with an increase in the speed of particles, which usually happens when heated, the process of evaporation occurs, that is, the transformation of liquid into gas.
Behavior of bodies in different physical states
The structure of gases, liquids, solids is mainly due to the fact that all these substances are composed of atoms, molecules or ions, but the behavior of these particles can be completely different. Gas particles are chaotically distant from each other, liquid molecules are close to each other, but they are not as rigidly structured as in a solid. Gas particles vibrate and move at high speeds. The atoms and molecules of a liquid vibrate, move, and slide past each other. Particles of a solid body can also vibrate, but motion as such is not characteristic of them.

Features of the internal structure
In order to understand the behavior of matter, one must first study the features of its internal structure. What are the internal differences between granite, olive oil and helium in balloon? A simple model of the structure of matter will help answer this question.
 A model is a simplified version of a real object or substance. For example, before actual construction begins, architects first construct a model building project. Such a simplified model does not necessarily imply an exact description, but at the same time it can give a rough idea of what this or that structure will be like.
A model is a simplified version of a real object or substance. For example, before actual construction begins, architects first construct a model building project. Such a simplified model does not necessarily imply an exact description, but at the same time it can give a rough idea of what this or that structure will be like.
Simplified Models
In science, however, models are not always physical bodies. The last century has seen a significant increase in human understanding about the physical world. However, much of the accumulated knowledge and experience is based on extremely complex representations, for example in the form of mathematical, chemical and physical formulas.  In order to understand all this, you need to be quite well versed in these exact and complex sciences. Scientists have developed simplified models to visualize, explain, and predict physical phenomena. All this greatly simplifies the understanding of why some bodies have a constant shape and volume at a certain temperature, while others can change them, and so on.
In order to understand all this, you need to be quite well versed in these exact and complex sciences. Scientists have developed simplified models to visualize, explain, and predict physical phenomena. All this greatly simplifies the understanding of why some bodies have a constant shape and volume at a certain temperature, while others can change them, and so on.
All matter is made up of tiny particles. These particles are in constant motion. The volume of movement is related to temperature. An increased temperature indicates an increase in the speed of movement. The structure of gaseous, liquid and solid bodies is distinguished by the freedom of movement of their particles, as well as by how strongly the particles are attracted to each other. Physical depend on his physical condition. liquid water and ice have the same Chemical properties, but they physical properties differ significantly.
>>Physics: The structure of gaseous, liquid and solid bodies
The molecular kinetic theory makes it possible to understand why a substance can be in gaseous, liquid and solid states.
Gases. In gases, the distance between atoms or molecules is on average many times more sizes the molecules themselves ( fig.8.5). For example, at atmospheric pressure, the volume of a vessel is tens of thousands of times greater than the volume of molecules contained in it.
Gases are easily compressed, while the average distance between molecules decreases, but the shape of the molecule does not change ( fig.8.6).
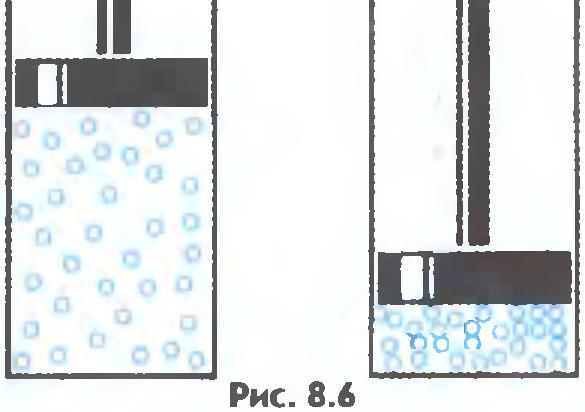
Molecules with huge speeds - hundreds of meters per second - move in space. Colliding, they bounce off each other in different directions like billiard balls. Weak forces of attraction of gas molecules are not able to keep them near each other. That's why gases can expand indefinitely. They retain neither shape nor volume.
Numerous impacts of molecules on the walls of the vessel create gas pressure.
Liquids. Molecules of a liquid are located almost close to each other ( fig.8.7), so a liquid molecule behaves differently than a gas molecule. In liquids, there is the so-called short-range order, i.e., the ordered arrangement of molecules is preserved at distances equal to several molecular diameters. A molecule oscillates about its equilibrium position by colliding with neighboring molecules. Only from time to time does it make another "jump", falling into a new position of equilibrium. In this equilibrium position, the repulsive force is equal to the attractive force, i.e., the total interaction force of the molecule is zero. Time settled life water molecules, i.e., the time of its oscillations around one specific equilibrium position at room temperature, is on average 10 -11 s. The time of one oscillation is much less (10 -12 -10 -13 s). As the temperature rises, the time of the settled life of molecules decreases.

The nature of molecular motion in liquids, first established by the Soviet physicist Ya.I. Frenkel, makes it possible to understand the basic properties of liquids.
Liquid molecules are located directly next to each other. With a decrease in volume, the repulsive forces become very large. This explains low compressibility of liquids.
As is known, liquids are fluid, i.e. do not retain their shape. It can be explained like this. The external force does not noticeably change the number of molecular jumps per second. But the jumps of molecules from one settled position to another occur predominantly in the direction of action external force (fig.8.8). That is why the liquid flows and takes the form of a vessel.
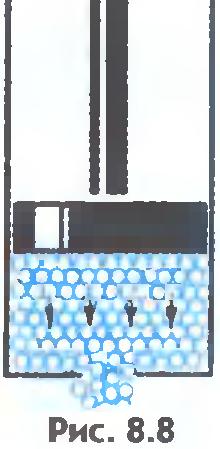
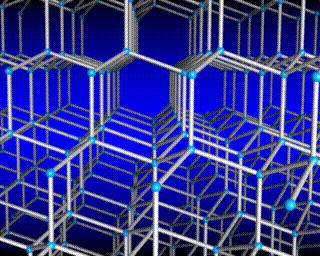
There is another important difference between liquids and solids. A liquid can be compared to a crowd of people, where individual individuals are restlessly jostling in place, and a solid body is like a slender cohort of the same individuals who, although they do not stand at attention, maintain certain distances on average between themselves. If we connect the centers of equilibrium positions of atoms or ions of a solid body, then we get the correct spatial lattice, called crystalline.
Figures 8.9 and 8.10 show the crystal lattices of table salt and diamond. The internal order in the arrangement of crystal atoms leads to regular external geometric shapes.
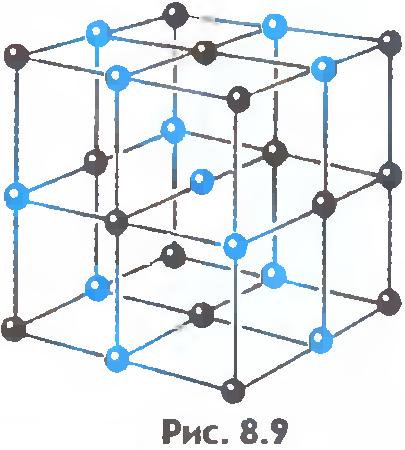
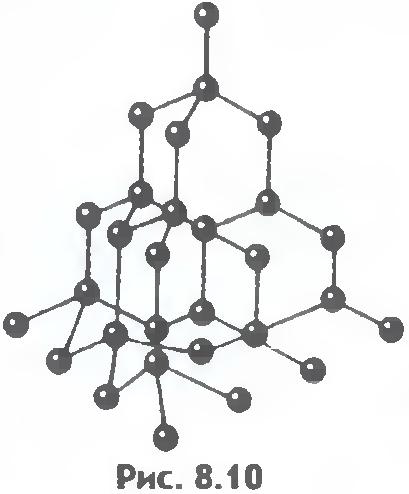
Figure 8.11 shows Yakutian diamonds.
gas distance l between molecules is much larger than the size of the molecules r0:" l>>r 0
.
For liquids and solids l≈r0. The molecules of a liquid are arranged in disorder and from time to time jump from one settled position to another.
In crystalline solids, molecules (or atoms) are arranged in a strictly ordered manner.
???
1. Gas is capable of unlimited expansion. Why does the Earth have an atmosphere?
2. What is the difference between the trajectories of motion of gas, liquid and solid molecules? Draw approximate trajectories of the molecules of substances in these states.
G.Ya.Myakishev, B.B.Bukhovtsev, N.N.Sotsky, Physics Grade 10
Lesson content lesson summary support frame lesson presentation accelerative methods interactive technologies Practice tasks and exercises self-examination workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures graphics, tables, schemes humor, anecdotes, jokes, comics parables, sayings, crossword puzzles, quotes Add-ons abstracts articles chips for inquisitive cheat sheets textbooks basic and additional glossary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in the textbook elements of innovation in the lesson replacing obsolete knowledge with new ones Only for teachers perfect lessons calendar plan for the year guidelines discussion programs Integrated LessonsIf you have corrections or suggestions for this lesson,
The molecular kinetic theory makes it possible to understand why a substance can be in gaseous, liquid and solid states.
Gas. In gases, the distance between atoms or molecules in a medium is many times greater than the size of the molecules themselves (Fig. 10). For example, when atmospheric pressure vessel volume in tens
thousand times greater than the volume of gas molecules in the vessel.
Gases are easily compressed, since when a gas is compressed, only the average distance between molecules decreases, but the molecules do not “squeeze” each other (Fig. 11).
Molecules with huge speeds - hundreds of meters per second - move in space. Colliding, they bounce off each other in different directions like billiard balls.
Weak forces of attraction of gas molecules are not able to keep them near each other. Therefore, gases can expand indefinitely. They retain neither shape nor volume.
Numerous impacts of molecules on the walls of the vessel create gas pressure.
Liquids. In liquids, the molecules are located almost close to each other (Fig. 12). Therefore, a molecule in a liquid behaves differently than in a gas. Clamped, as in a cell, by other molecules, it performs a “run in place” (oscillates around the equilibrium position, colliding with neighboring molecules). Only from time to time does it make a "jump", breaking through the "bars of the cage", but then it falls into a new "cage" formed by new neighbors. The time of "settled life" of a water molecule, i.e., the time of oscillations around one specific equilibrium position, at room temperature is on average s. The time of one oscillation is much less (s). As the temperature rises, the “sedentary life” of molecules decreases. The nature of molecular motion in liquids, first established by the Soviet physicist Ya. I. Frenkel, makes it possible to understand the basic properties of liquids.
The molecules of a liquid are located directly next to each other. Therefore, when you try to change the volume of the liquid, even by a small amount, the deformation of the molecules themselves begins (Fig. 13). And this requires a lot of power. This explains the low compressibility of liquids.
Liquids, as you know, are fluid, that is, they do not retain their shape. This is explained as follows. If the liquid does not flow, then the jumps of molecules from one "sedentary" position to another occur with the same frequency in all directions (Fig. 12). The external force does not noticeably change the number of molecular jumps per second, but the jumps of molecules from one "sedentary" position to another occur predominantly in the direction of the external force (Fig. 14). This is why the liquid flows and takes the form of a vessel
Solids. Atoms or molecules of solids, unlike liquids, oscillate around certain equilibrium positions. True, sometimes molecules change their equilibrium position, but this happens extremely rarely. That is why solids retain not only volume, but also shape.
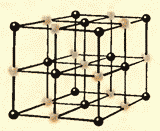
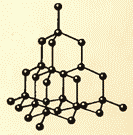
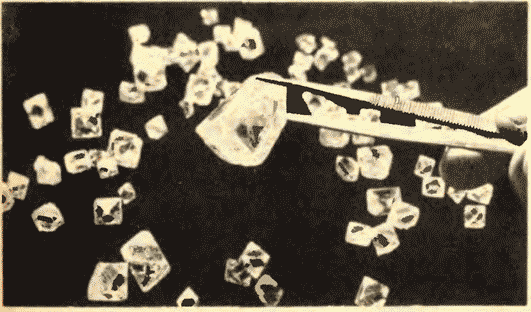
There is another important difference between liquids and solids. A liquid can be compared to a crowd, the individual members of which are uneasily pushing in place, and a solid body is like a slender cohort, the members of which, although they do not stand at attention (due to thermal motion), maintain certain intervals on average among themselves. If we connect the centers of the equilibrium positions of atoms or ions of a solid body, then we get a regular spatial lattice, called a crystalline one. Figures 15 and 16 show the crystal lattices of table salt and diamond. The internal order in the arrangement of atoms of crystals leads to geometrically correct external forms. Figure 17 shows Yakut diamonds.
A qualitative explanation of the basic properties of matter on the basis of molecular kinetic theory, as you have seen, is not particularly difficult. However, the theory that establishes quantitative relationships between experimentally measured quantities (pressure, temperature, etc.) and the properties of the molecules themselves, their number and speed of movement, is very complex. We confine ourselves to consideration of the theory of gases.
1. Provide evidence for the existence of thermal motion of molecules.
2. Why Brownian motion noticeable only for particles of small mass?
3. What is the nature of molecular forces? 4. How do the forces of interaction between molecules depend on the distance between them? 5. Why do two lead bars with smooth, clean cuts stick together when pressed against each other? 6. What is the difference between the thermal motion of the molecules of gases, liquids and solids?