The structure and energy properties of solid crystals
According to the ability to conduct electricity All solid materials are usually divided into conductors, semiconductors and dielectrics. Are conductive materials classified as conductors? > 10 6 Ohm -1 cm -1; these include metals in which high conductivity is provided by a high concentration of conduction electrons. In dielectrics at room temperature, there are very few electrons, and their conductivity is low?< 10 -10 Ом -1 см -1 . В промежуточную группу попадают полупроводники, которые могут иметь концентрацию электронов, близкую к нулю (тогда они являются диэлектриками) и близкую к концентрации электронов в металле (тогда они являются проводниками).
Metals and semiconductors, in addition to having different electrical conductivity, also differ in the dependence of electrical conductivity on temperature. In metals, electrical conductivity, as a rule, decreases almost linearly with increasing temperature. In semiconductors, in which there are no defects and impurities (they are usually called intrinsic), with increasing temperature, the conductivity increases approximately according to an exponential law:
To consider the structure and energy properties crystalline solids, which include silicon and germanium (the semiconductors most widely used for the manufacture of semiconductor devices), you should first turn to the energy properties of a single atom.
An atom consists of a nucleus around which electrons move, creating an electron shell. Total negative charge electron balances positive charge nucleus, so that in the normal state the atom is electrically neutral. According to quantum theory, the electrons of an atom can only have strictly defined energy values, called allowed ones. These energy values are called energy levels. The energy levels of the electrons are separated from each other by forbidden intervals. Moving around the nucleus in certain orbits, the electrons are removed from the nucleus at different distances and, accordingly, have different energy values: the farther from the nucleus, the greater the energy of the electron and the weaker it is associated with the nucleus.
outer layer electrons electron shell called valence. They have the highest energy and are least bound to the nucleus. Graphically, the energy spectrum of electrons in a single atom can be represented as an energy diagram. An example of such a diagram is shown in Fig. 1.1, a. Energy values are plotted along the vertical, and the corresponding energy levels are shown as horizontal lines. In accordance with the Pauli principle, no more than two electrons can simultaneously be in the same energy level, having different directions of rotation around their axis (opposite spins).
If the atom is in the normal state and does not absorb energy from outside, then all the lower allowed energy levels are occupied by electrons; the transition of an electron from one level to another is impossible. Higher allowed levels remain unoccupied by electrons and are called free. The transition of an electron to a higher free energy level, i.e. to an orbit more distant from the nucleus, is possible only when it absorbs from the outside a strictly defined portion (quantum) of energy (thermal, light, electric, magnetic), equal to the difference in the energy values of the free and occupied levels by this electron. In this case, the atom goes into an excited state.
The excited state of an atom is very unstable. It lasts only a hundred millionth of a second, and the atom returns to its normal state, which is accompanied by the transition of the electron back to its previous energy level. The transition of an atom from an excited to a normal state is accompanied by the release of excess energy in the form of a quantum of electromagnetic radiation.
If the electron receives a sufficient quantum of energy, it breaks away from the atom, the atom is ionized: it splits into a free electron and a positive ion. The reverse process is the union of a free electron and positive ion into a neutral atom - is called recombination and is accompanied by the release of excess energy in the form of a radiation quantum. The released energy is equal to the energy expended earlier on the ionization of the atom.
During the formation of crystals of a solid, an interaction occurs between atoms, as a result of which the allowed energy levels of individual atoms are split into N sublevels, forming energy zones (Fig. 1.1, b). In this case, as in a single atom, there cannot be more than two electrons with opposite spins at one energy level (the Pauli principle is preserved). Since the number of sublevels (N) is large (in 1 cm 3 of a solid there are about 10 22 - 10 23 atoms), the energy distance between the sublevels is very small, and the electron is able to move from sublevel to sublevel from the bottom of the zone to the ceiling even with small external energy influences, i.e. he behaves like a free man. This, however, is true only if the upper energy levels in the zone are not occupied, i.e. area is not completely filled.
The energy levels of an individual atom, occupied by electrons at a temperature of absolute zero (T = 0 K), form filled zones in the crystal, the upper of which, occupied valence electrons, is called the valence band.
Allowed higher energy levels of an atom not occupied by electrons at
T = 0 K, form free zones in the crystal. The free band closest to the valence band is called the conduction band, since electrons that have got there can move between atoms and create an electric current. The electrons in the filled zone cannot move under the action of the field (and, accordingly, gain energy), since all energy levels are occupied, and according to the Pauli principle, an electron cannot move from an occupied state to an occupied one. Therefore, the electrons of a completely filled valence band do not participate in the creation of electrical conductivity.
Between the conduction band and the valence band there is a band gap E g (it is measured in electron volts (eV)), in which, according to the laws of quantum mechanics, electrons cannot be (just as electrons in an atom cannot have energies that do not correspond to the energies of electron shells ). The band gap is the main parameter that determines electrical properties solid body.
According to the nature of filling the zones with electrons, all bodies can be divided into two large groups:
- to first group include bodies in which a zone filled only partially is located above the completely filled zones (Fig. 1.2, a). This zone occurs when atomic level, from which it is formed, is not completely filled in the atom. The presence of a zone filled only partially is inherent in metals. Metals have no band gap;
- to second group include bodies in which free zones are located above completely filled zones (Fig. 1.2, b, c). Typical examples of such bodies are chemical elements Group IV of the periodic table: carbon in the modification of diamond, silicon, germanium and gray tin, which has the structure of diamond. This group of bodies includes many chemical compounds - metal oxides, nitrides, carbides, halides alkali metals etc.
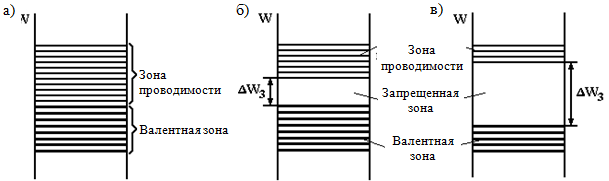
According to the width of the forbidden zone, the bodies of the second group are conditionally divided into dielectrics and semiconductors. To dielectrics include bodies with a relatively wide bandgap. For typical dielectrics, E g > 3 eV. Thus, for diamond E g = 5.2 eV; for boron nitride E g = 4.6 eV; for A1 2 O 3 E g \u003d 7 eV, etc.
To semiconductors include bodies that have a relatively narrow band gap (Fig. 1.2, b). For typical semiconductors, E g ? 1 eV, for example:
- for germanium E g = 0.72 eV;
- silicon has E g = 1.12 eV;
- for indium antimonide E g = 0.17 eV;
- for gallium arsenide E g = 1.43 eV, etc.
Energy diagrams of metals, semiconductors and dielectrics at
T = 0 K are shown in Figs. 1.2. In these diagrams, the valence band, which is filled with electrons, is shown by thicker solid lines, and the conduction band, in which there are no electrons under these conditions, by thin lines.
Answers to colloquium №2 in physics.
Energy levels of atoms and molecules. Quantum transitions in atoms and molecules. Absorption and emission of energy by atoms and molecules, absorption and emission spectra.
Electronic energy levels in an atom Electrons in the unexcited state fill the lower energy. levels, and the upper levels are free. If an atom receives energy as a result of a collision with other atoms or by absorbing a quantum of light, then it goes into an excited state and any electron of the atom goes from the lower level to one of the upper ones. After a short period of time, it returns to the lower level, emitting a quantum of light of a certain frequency.
Molecules consist of interacting atoms, intramolecular motion is more complicated than intraatomic. In a molecule, in addition to the movement of electrons relative to nuclei, oscillating motion atoms around their equilibrium position and the rotational motion of the molecule as a whole. The electronic, vibrational and rotational motion of a molecule corresponds to three types of energy levels: Eel, Ekol, Heb. The total energy of the molecule is: E=Eel+Eur+Ecol. According to quantum mechanics, the vibrational and rotational energy of molecules, as well as electronic energy, have a quantum character, i.e. change discretely. The distance between vibrational levels is much smaller than between electronic ones. Therefore, each electronic level of a molecule breaks up into a number of vibrational levels (sublevels). In turn, each vibrational level of the molecule corresponds to a number of rotational sublevels, the distance between which is even smaller than between the vibrational levels.
Stationary energy. state: no energy is emitted or absorbed. In quantum transitions, atoms and molecules jumps from one stationary state to another, from one energy level another. This is due to the energy transitions of electrons.
When moving from higher energy levels to lower ones, an atom or molecule gives off energy, otherwise it absorbs. An atom in its ground state can only absorb energy.
The energy emitted by atoms or molecules forms the emission spectrum, and the absorbed energy forms the absorption spectrum.
At transition of an electron in an atom energy is released or absorbed in the form of a quantum of EM radiation. The atomic emission and absorption spectra are line-like.
Transition in molecules:
As a result of the fact that the electronic levels in a molecule break up into vibrational and rotational sublevels, the number of possible energy transitions increases significantly compared to atoms. Therefore, the molecular spectra become more complicated, they have a continuous character.
∆E >>∆Epol.> ∆Eur.
∆Ee ~ 1-3 EV λ~ 0.5 μV
∆Epol. ~ 10 -2 -10 -1 EP λ~1-100mkV
∆Heb. ~ 10 3 -10 -5 V λ~100-1000mkV
energy absorption of a molecule
2- radiation of the energy of an atom on a molecule
Luminescence, emission and absorption spectra, Stokes rule. The use of luminescence in biophysics and medicine.
Luminescence- radiation, which is an excess over thermal radiation at a given temperature, and having a period t (10 -15) greater than the period of the light wave. Luminescence occurs at any temperature. Luminescent substances glow without heating. It is never balanced.
According to the type of excitation, luminescence is:
1) Photoluminescence - excitation by light;
2) Electroluminescence - excitation electric field;
3) Chemiluminescence (excitation by means of a chemical reaction).
The impact of these sources leads to the excitation of atoms, molecules or ions of the luminescent substance. Radiation occurs as a result of quantum transitions of particles of matter from excited states to the ground (or less excited)
According to the duration of the glow, photoluminescence is divided into:
Fluorescence - short-term afterglow
Phosphorescence - a relatively long afterglow

The luminescence spectrum is continuous.
Basic characteristics of luminescence.
1) quantum yield:
ζ= 100% N(emission)/N(absorption),
where N(radiation) is the number of quanta that gave light,
N(absorb) – number of absorbed quanta.
2) D is the optical density of the sample.
D=lgI 0 /I λ =Esl, where E is the molecular absorption index
3) D= f(λ ) is the absorption spectrum
I λ = f(λ ) is the radiation spectrum
Stokes' rule:
The absorption spectrum of a given substance is shifted in relation to the emission spectrum towards shorter wavelengths.
The use of luminescence in biophysics and medicine:
Photoluminescence:
Detection of the initial stage of food spoilage
Sorting of pharmacological preparations
Diagnosis of certain diseases (glow of hair, scales, nails when diagnosing their damage by a fungus or lichen)
Based on photoluminescence, light sources have been created whose spectrum is more similar to daylight than that of incandescent lamps (fluorescent lamps)
Chemiluminescence - application in diagnostics
The use of special fluorescent molecules added to membrane systems from the outside. Such molecules are called fluorescent probes or labels. Changing them makes it possible to detect conformational rearrangements in proteins in membranes.
induced emission. Inverse population of levels. Optical quantum generators (lasers). Properties laser radiation and its use in medicine.
stimulated emission is the forced transition of the excited particle to the lower level. In this case, the number of transitions per second depends on the number of photons entering the substance during the same time, i.e. from the intensity of the light. In addition, forced transitions will be determined by the population of the corresponding energy states. population inversion- such a state of the medium in which the number of particles at one of the upper levels is greater than chm at the lower. The inverse population of the levels is ensured by the fact that electrons can stay at the metastable level 10 -5 times longer than at the excited level.
Vv 1960 the first quantum generator of the visible range of radiation - laser with rubies as a working substance. It creates pulsed radiation with a wavelength of 694.3 nm.
The principle of operation of the laser is similar to luminescence.
![]()
![]()
Al 2 O 3 + Cr 2 O 3 - ruby
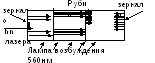
Properties of laser radiation
Laser radiation is always monochromatic
Polarization
The rays do not diverge, strictly parallel
You can get very high intensities.
Laser application:
Identification of DNA and proteins.
Ophthalmology
Treatment of trophic ulcers, malignant tumors
Lasers operating in continuous mode are used for operations on abundantly supplied organs.
X-ray radiation: characteristic and bremsstrahlung; radiation mechanism, spectra of characteristic and bremsstrahlung X-rays.
x-ray radiation is an electromagnetic wave in the range from 80 to 10 -4 nm. Long-wave X-ray radiation is overlapped by short-wave UV radiation, short-wave by long-wave γ-radiation. According to the method of excitation, X-ray radiation is divided into bremsstrahlung and characteristic
brakeRe-radiation.
The radiation that occurs when an electron decelerates in the anode material is called bremsstrahlung X-rays. When electrons slow down, only part of the energy goes to create a photon, the other part is spent on heating the anode. Since the ratio between these parts is random, when a large number of electrons decelerate, a continuous spectrum of X-ray radiation is formed.
Bremsstrahlung spectrum: (1) - at various voltages in x-ray tube)
X-ray flux is measured by the formula:
Ф=КUа-кIZ 2), where U, I - voltage and current in the X-ray tube, Z - serial number of the atomic substance of the anode, k=10 -9 V -1 - - coefficient of proportionality.
Ф=- λ min ˜ ∫Ф λ d λ
If the cathode filament temperature is increased, then the electron emission and the current in the tube will increase. This will increase the number of X-ray photons emitted every second. But its spectral composition does not change.
2) Characteristic x-ray radiation.
Increasing the voltage on the X-ray tube leads to the fact that against the background of a continuous spectrum, a line appears, which corresponds to the characteristic X-ray radiation. Electrons accelerated by a high voltage penetrate deep into the atom and knock electrons out of its inner layers. Electrons from upper levels pass to free places, as a result of which photons of characteristic radiation are emitted.
Characteristic x-ray spectrum atom does not depend on the chemical compound in which this atom is included. Spectrum:
Interaction of X-rays with matter (coherent scattering, photoelectric effect, Compton effect). The law of attenuation of the X-ray intensity. X-ray protection.
Interaction of X-ray radiation with matter
1)
Coherent scattering– scattering of long-wavelength X-rays Condition: hn hn 1 = hn 2, where n is the frequency. 2)
photoelectric effect. hn≥A and. In this case, the X-ray quantum is absorbed, and the electron is detached from the atom. The detached electron acquires kinetic energy. If it is large, then the electron can ionize neighboring atoms by collision. removal of an electron - the process of ionization hn \u003d Eion + m 0 v 2 / 2 3) Compton effect hn>>Ai. In this case, the electron breaks away from the atom and acquires some kinetic energy. The energy of the photon itself decreases. That. as a result of incoherent scattering (Compton effect), secondary scattered radiation is formed and ionization of the atoms of matter occurs. hn \u003d Eion + mv 2 / 2 + hn The law of attenuation of the X-ray intensity: Ix \u003d I 0 e -µx µ=µ p +µ p the contribution of each process term is determined by the photon energy. photoelectric effect Compton effect 6. The device of the X-ray tube and X-ray apparatus. Rigidity and intensity of radiation, their adjustment. Method for obtaining x-rays. The use of x-rays in medicine. X-ray tube device: The tube is a glass flask (with possibly high vacuum), with two electrodes: an anode and a cathode, to which a high voltage is applied. The cathode is the source of electrons. Anode is a metal rod inclined surface in order to direct the emerging x-rays at an angle to the axis of the tube. Under the action of high voltage, the electrons emitted by the hot cathode filament are accelerated to high energy. The kinetic energy acquired by an electron is equal to the work of electric forces and is proportional to the voltage between the cathode and anode. X-ray radiation arises as a result of intense deceleration of fast electrons in the anode material upon collision with its atoms (interaction with an electric field atomic nucleus and electrons). Uа-к≈100∙10 3 V λmin= hc/ eUa-k 1) Short-wavelength X-rays usually have a greater penetrating power than long-wavelength ones and are called hard, and long-wave soft. Soft radiation is more strongly absorbed by matter. Increasing the voltage on the x-ray tube, change the spectral composition of the radiation and increase the rigidity. Getting x-rays. If an inhomogeneous body is placed in the path of X-rays and a fluorescent screen is placed in front of it, then this body, absorbing or attenuating the radiation, forms a shadow on the screen. By the nature of this shadow, one can judge the shape, density, structure, and in many cases the nature of bodies. those. a significant difference in the absorption of x-ray radiation by different tissues allows you to see the image of the internal organs in the shadow projection. µ1<µ2
I 2 < application of x-ray radiation in medicine: X-ray diagnostics: 1) Fluoroscopy (the X-ray tube is located behind the patient. A fluorescent screen is located in front of him. A shadow (positive) image is observed on the screen). 2) radiography (the object is placed on a cassette in which a film with a special photographic emulsion is inserted. The x-ray tube is located above the object. The resulting radiograph gives a negative image, i.e. the opposite in contrast to the picture observed during transmission. In this method, there is a greater clarity of the image A promising variant of this method is X-ray tomography and computed tomography). 3) Fluorography 4) X-ray therapy - the use of x-rays to destroy malignant tumors. 7. The principle of X-ray tomography. X-ray tomograph. His device. What are the main differences between an x-ray tomogram and an x-ray? 1) Re absorption µ - absorption coefficient, tissue property 2) Re-study Ia \u003d I 0 e -µ x 1 Iv \u003d I 0 e -µ2 x 2 Iv \u003d I 0 e -µ1x1 e -µ2x2 \u003d I 0 e - (µ1x1 + µ2x2) The purpose of the diagnosis. Determine µ3 and ∆µ3 lnI1/I 0 = -(µ1+µ2)∆х lnI2/I 0 = -(µ3+µ4)∆х lnI3/I 0 = -(µ3+µ1)∆х lnI4/I 0 = -(µ4+µ2)∆х I 0 - set I1,I2,I3,I4 - measured (known) ∆x - set (known) find µ1µ2µ3µ4 by solving a system of 4 equations 2048∙2048= 4194304 System of Radon's theorems If I take an infinite number of images of an object, then it is possible to restore it with any accuracy. Technical solution. stage: measurements I1,I2,I3,I4… stage: building an image To each value of µ, the PC assigns its own brightness (color) 1 slice - 1 second Contrast ∆µ/µ=0.1%(by 10%) Layer thickness - 1-2mm Resolution limit 0.2mm With tomography, it is possible to obtain a layered X-ray image of the body with details less than 2 mm. This allows you to distinguish between the gray and white matter of the brain and see very small tumor cells. education 8. Types of ionizing radiation. The action of ionizing radiation on matter. Absorbed, exposure and biological (equivalent) doses, the relationship between them. Units of doses in the SI system and non-systemic units used in medicine. In the process of radiation of the phenomenon of radioactivity, 3 types of rays emitted by radioactive nuclei were discovered. Alpha decay consists in the spontaneous transformation of the nucleus with the emission of α-particles (helium nucleus). A Z X → A-4 Z -2 Y+ 4 2 During α-decay, the daughter nucleus can be formed in an excited state. In this case, the energy of the excited nucleus is most often released in the form of a -quantum. Therefore, alpha decay is accompanied by radiation. beta decay consists in the spontaneous transformation of the nucleus with the emission of electrons (or positrons). A Z X→ A Z +1 Y + 0 -1 β +ν Where ν is the designation of the antineutrino particle. An electron is formed as a result of the intranuclear transformation of a neutron into a proton. Gamma radiation has an electromagnetic nature and is a photon with a wavelength of λ≤10 -10 m Radiation of this type accompanies not only -decay, but also more complex nuclear reactions. Interaction with matter: A charged particle passing through matter loses its energy due to ionization drag. At the same time, its kinetic energy is spent on the excitation and ionization of the atoms of the medium. For a quantitative characteristic of the interaction of a charged particle with a substance, the following quantities are used: linear ionization density, i, number of pairs of ions formed per unit path of the particle: i=dn/dl linear stopping power of a substance (S) is the energy lost by a charged particle per unit path: S=dE/dl the average linear range of a charged ionizing particle (R) is the distance between the beginning and the end of the particle range in a given substance. Characteristic features of the interaction of various types of radiation with matter: Alpha radiation: As the alpha particle moves in the medium, I first increases (with a decrease in speed, the time it spends near the molecule of the medium increases, so the probability of ionization increases), and at the end of the run (x = R) it sharply decreases, which is associated with a decrease in the speed of movement. When the energy of the particle becomes less than the energy required for ionization, the formation of ions stops. Graph of the dependence of the linear ionization density on the path traversed by the alpha particle in the medium: Ionization and excitation are primary processes. Secondary: increase in the speed of molecular thermal motion, characteristic X-ray radiation, radioluminescence, chemical processes. Beta radiation. Causes ionization, excitation, X-ray radiation (when electrons decelerate), characteristic Cherenkov radiation, when the speed of an electron in a medium exceeds the speed of light in this medium. Gamma radiation causes a slight primary ionization, coherent and incoherent scattering, an ionizing photoelectric effect, the formation of electron-positron pairs, photonuclear reactions due to the interaction of a quantum with a nucleus. Absorbed dose (D)
-
a value equal to the ratio of the energy ∆E transferred to the element of the irradiated substance to the mass m of this element: D=∆E /m. In C, the unit of absorbed dose is the gray (Gy). 1 Gy corresponds to the dose of radiation at which the energy of ionizing radiation of 1 J is transferred to the irradiated substance weighing 1 kg. Non-systemic unit 1rad=10 -2 Gy Exposure dose radiation (X) characterizes the ionizing effect of X-ray and γ-radiation in the air surrounding the irradiated body. The SI unit of exposure dose is C/kg. The SI unit of exposure dose is C/kg. 1C/kg corresponds to the exposure dose of photon radiation, at which ions with a charge of 1C of each sign are formed as a result of ionization of 1kg of dry air (n.c.). The unit of exposure dose rate is 1A/kg, and the off-system unit is 1R/s. Since the radiation dose is proportional to the incident ionizing radiation, there should be a proportional relationship between the radiated and exposure doses: D=fХ, where f is a certain transition coefficient depending on the irradiated substance and photon energy. Equivalent dose - (N) used to assess the effect of ionizing radiation on biological objects; it has the same dimension as the absorbed radiation dose, but the name is different. In SI: Sievert [Sv], 1Sv=1J/kg Off-system unit: 1ber=10 -2 Sv. There is a relationship between exposure and absorbed doses: H=KD, where K is the quality factor (shows how many times the effectiveness of the biological action of a given type of radiation is greater than that of photon radiation at the same dose of radiation in tissues). 9. dose rate. Relationship between the exposure dose rate and the activity of a radioactive preparation. Dose rate is the value that determines the dose received by the object per unit of time. With a uniform effect of radiation, the dose rate N is numerically equal to the ratio of the dose D to the time t, during which the ionizing radiation acted: N=D/t. The unit of radiation dose rate is gray (Gy), which corresponds to the radiation dose at which ionizing radiation energy of 1J is transferred to an irradiated substance weighing 1kg; radiation dose rate is expressed in Gy/sec. The non-systemic unit of radiation dose is rad (1 rad=10 -2 Gy=100erg/g), its power is rad per second. The exposure dose of radiation (X) characterizes the ionizing effect of X-ray and γ-radiation in the air surrounding the irradiated body. The SI unit of exposure dose is C/kg. 1C/kg corresponds to the exposure dose of photon radiation, at which ions with a charge of 1C of each sign are formed as a result of ionization of 1kg of dry air (n.c.). The unit of exposure dose rate is 1A/kg, and the off-system unit is 1R/s. Since the radiation dose is proportional to the incident ionizing radiation, there should be a proportional relationship between the radiated and exposure doses: D=fХ, where f is a certain transition coefficient depending on the irradiated substance and photon energy. For water and human soft tissues, f=1, therefore, the radiation dose in rads is numerically equal to the corresponding exposure dose in roentgens. Relationship between the exposure dose rate and the activity of the radioactive preparation: From the source, γ-photons fly out in all directions. The number of these photons penetrating 1m 2 of the surface of a certain sphere in 1s is proportional to the activity A and inversely proportional to the surface area of the sphere (4πr 2) / The exposure dose rate (X / t) in the volume V depends on the number of photons, since it is they that cause ionization: X /t=k γ A/r 2 Where k γ - which is typical for the given radionuclide. 10. Law of radioactive decay (conclusion). Law chart. artificial radioactivity. Tagged atom method, application in medicine. radioactive decay is a statistical phenomenon. Let dN nuclei decay in a short time interval dt. This number is proportional to the time interval dt, as well as to the total number N of radioactive nuclei: dN=-λNdt, where λ is the decay constant, which is proportional to the decay probability of a radioactive nucleus t, which is different for different radioactive nuclei, decreases with time. We integrate the resulting expression and obtain lnN/N 0 =-λt. N= N 0 e - λt . This is the basic law of radioactive decay: for an equal period of time, the same fraction of the initial number of nuclei decays. Processes of radioactive decay for two substances λ1>λ2. Task number 1.
What parts of a plant cell are visible under a light school microscope?
1. Ribosomes 2. Mitochondria 3. Cell wall 4. Plasma membrane Explanation: large portions of cells are visible under a light microscope. Of the presented, such a large organelle is the cell wall (it is quite thick), and the plasma membrane is difficult to visually (with a small increase) isolate from the cell wall. The correct answer is 3. Task number 2.
A fragment of a DNA molecule, consisting of 12 nucleotides, stores information about
1. 4 amino acids in a protein molecule 2. 16 nucleotides of a tRNA molecule 3. 12 amino acids mRNA molecule 4. 24 nucleotides rRNA molecule Explanation: Three nucleotides of a DNA molecule code for one amino acid (this structure is called a triplet), so 12 nucleotides of DNA code for 4 amino acids in a protein molecule. The correct answer is 1. Task number 3.
The transition of electrons to a higher energy level occurs in the light phase in molecules
1. Chlorophyll 2. Water 3. Glucose 4. Carbon dioxide Explanation: during photosynthesis, it is the chlorophyll molecule that goes into an excited state, since it is from the pigment that the chain of biochemical reactions begins during photosynthesis. The correct answer is 1. Task number 4.
In the course of the individual development of an animal, a multicellular organism develops from a zygote by
1. Meiosis 2. Mitosis 3. Phylogeny 4. Gametogenesis Explanation: after the formation of a zygote, the zygote begins to split up precisely by mitosis (this is the process of division of somatic cells), that is, two diploid cells are formed from one diploid cell: two from one cell, 4 from two, 8 from four, etc. The correct answer is 2. Task number 5.
The peculiarity of asexual reproduction is that a new organism develops from
1. Male gamete 2. Unfertilized egg 3. Zygotes with a double set of chromosomes 4. Somatic cell with a double set of chromosomes Explanation: asexual reproduction is not associated with germ cells, therefore, from the presented answer options, asexual reproduction is possible only when the organism develops from a somatic cell with a double set of chromosomes. With vegetative reproduction, a daughter organism is obtained, identical to the parent. The correct answer is 4. Task number 6.
Determine the breeding pattern if it is known that when a round-fruited tomato, whose genotype is unknown, is crossed with a pear-shaped tomato (recessive trait), 50% of the offspring have pear-shaped fruits. 1. AA x aa 2. Aa x aa 3. Aa x Aa 4. AA x AA Explanation: 50% of the offspring will have pear-shaped fruits only if a heterozygote is crossed with a homozygote for a recessive trait - Aa x aa. The correct answer is 2. Task number 7.
Independent divergence of homologous chromosomes in meiosis is the cause of variability
1. Genomic 2. Combinative 3. Chromosomal 4. Cytoplasmic Explanation: combinative variability - the variability that occurs when the parental genes are recombined. The reasons may be violations in: crossing over in the metaphase of meiosis, divergence of chromosomes in meiosis, fusion of germ cells. The correct answer is 2. Task number 8.
N.I. Vavilov, while studying the characteristics of the inheritance of traits of cultivated plants, substantiated the law 1. Homologous series in hereditary variability 2. Independent inheritance of non-allelic genes 3. Dominance of first generation hybrids 4. Sex-linked inheritance Explanation: N.I. Vavilov formulated the law of homological series, which is as follows: due to the great similarity of their genotypes (almost identical sets of genes), closely related species have similar potential hereditary variability (similar mutations of identical genes); as the evolutionary-phylogenetic removal of the studied groups (taxa), due to the emerging genotypic differences, the parallelism of hereditary variability becomes less complete. Consequently, the basis of parallelisms in hereditary variability are mutations of homologous genes and genotype regions in representatives of different taxa, that is, truly homologous hereditary variability. However, even within the same species, outwardly similar characters can be caused by mutations in different genes; such phenotypic parallel mutations of various genes can, of course, also occur in different, but rather closely related, species. The correct answer is 1. Task number 9.
The kingdom of bacteria includes
1. Chlamydomonas 2. E. coli 3. Infusoria-shoe 4. Malarial Plasmodium Explanation: chlamydomonas, ciliate slipper and malarial plasmodium are protozoa, and Escherichia coli ( Escherichia coli - bacterium. The correct answer is 2. Task number 10.
Tuber, bulb is
1. Organs of soil respiration 2. Modified shoots 3. Generative organs 4. Rudimentary shoots Explanation: tuber and bulb (as well as, for example, rhizome) are modified shoots. The correct answer is 2. Task number 11.
Ferns, unlike flowering plants, reproduce by
1. Dispute 2. Roots 3. Rhizome 4. Budding Explanation: flowering plants reproduce by seeds, while ferns reproduce by spores. The correct answer is 1. Task number 12.
What class do arthropods have simple eyes and four pairs of walking legs?
1. Insects 2. Cephalopods 3. Shellfish 4. Arachnids Explanation: look at the picture and conclude that the correct answer is 4. Task number 13.
A high metabolic rate allows birds
1. Take care of offspring 2. Lay eggs in nests 3. Eat plant foods 4. Expend a lot of energy during the flight Explanation: a high metabolic rate is one of the adaptations for flying, so we choose to expend a lot of energy during the flight. The correct answer is 4. Task number 14.
If a person suffers from anemia, then in his blood, compared to the norm, the content of
1. Leukocytes 2. Red blood cells 3. Platelets 4. Fibrinogen Explanation: anemia (another name - anemia) is expressed in a reduced content of red blood cells - erythrocytes and / or hemoglobin. Symptoms of the disease: dizziness, increased fatigue, etc. due to insufficient supply of oxygen to cells. The correct answer is 2. Task number 15.
The primary breakdown of complex carbohydrates in the human body occurs in
1. Oral cavity under the action of saliva enzyme 2. Gastric cavities under the action of the enzyme pepsin 3. Liver cells that store glycogen 4. Pancreatic cells that produce hormones Explanation: an enzyme produced in the stomach and oral cavity - amylase begins the breakdown of complex carbohydrates - polymers in the oral cavity. The correct answer is 1. Task number 16.
The function of destroying foreign microorganisms in human blood is performed by
1. Neurons 2. Red blood cells 3. Epithelial cells 4. Lymphocytes Explanation: lymphocytes are immunity cells, that is, they fight foreign microorganisms in human blood. The correct answer is 4. Task number 17.
The impact of twilight light is converted by the human visual analyzer into nerve impulses in
1. The lens of the eye 2. The pupil of the iris 3. Retinal sticks 4. Sclera of the eyeball Explanation: receptors that perceive light signals at dusk and darkness are retinal rods. From them, the signal is transmitted to the central nervous system. The correct answer is 3. Task number 18.
Splinting a broken limb
1. Reduces bleeding 2. Reduces limb edema 3. Prevents the penetration of microorganisms into the fracture site 4. Prevents displacement of broken bones Explanation: a splint is placed on the broken limb to prevent displacement of the bones. The correct answer is 4. Task number 19.
The creative role of natural selection is manifested in
1. Strengthening intraspecific struggle 2. Development by organisms of new habitats 3. The emergence of new mutations 4. The emergence of new species Explanation: natural selection is one of the driving forces of evolution. The goal of evolution is to create the most adapted species in given conditions, therefore the creative role of natural selection is also manifested in the creation of new species. The correct answer is 4. Task number 20.
In modern biological science, a population is considered to be
1. The totality of organisms of one kingdom 2. Individuals forming a food chain 3. Individuals of different species forming a biocenosis 4. Individuals of the same species living in the same territory Explanation: population - a group of individuals of the same species living in the same territory and interbreeding freely. The correct answer is 4. Task number 21.
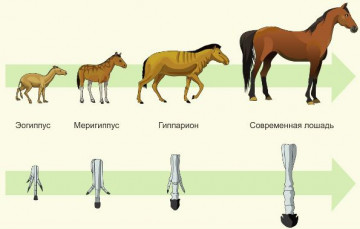
The phylogenetic series of the horse testifies to (about)
1. Reversibility of the process of evolution 2. Regular return to ancestral forms 3. Similarity of postembryonic development of organisms 4. Historical development of the modern look Explanation: Consider the phylogenetic series of the horse. As can be seen from the picture, the phylogenetic series of a species is a tracking of the stages of its historical development. The correct answer is 4. Task number 22.
Which of the following examples illustrates the competitive relationship between organisms?
1. Squirrel - woodpecker 2. Oak - white mushroom 3. Cow - bull tapeworm 4. Already - a frog Task number 23.
As a result of long-term biotic relations, predator-prey in the natural biocenosis is observed
1. Unregulated increase in the number of predators 2. Regular fluctuation in the abundance of both groups of organisms 3. Accumulation of mutant alleles in the gene pool of victims 4. Manifestation of dominant traits in a population of predators Explanation: with a long-term predator-prey relationship, a constant number of these two species is maintained, but due to changes in environmental conditions, population waves occur and they are gradually extinguished by the constancy of this system (that is, such a system is quite stable). The correct answer is 2. Task number 24.
Thanks to living matter in the biosphere, the circulation of substances
1. Open 2. Involves a lot of chemical elements 3. Increases the diversity of agrocenoses on Earth 4. Provides accumulation of inert gases in the atmosphere Explanation: thanks to living organisms (and especially microorganisms), in nature there are cycles of many elements (and complex substances - carbon dioxide, water), such as: carbon, hydrogen, oxygen, sulfur, and many others. others and even iron. The correct answer is 2. Task number 25.
Are the following statements about the forms of natural selection correct?
A. Stabilizing selection is manifested in conditions of a sudden change in the sex composition of the population.
B. Driving selection contributes to an increase in the number of individuals with an average value of the trait.
1. Only A is true 2. Only B is true 3. Both judgments are correct 4. Both judgments are wrong Explanation: stabilizing selection works only under constant environmental conditions and preserves individuals with an average value of the trait, A - incorrect. Driving selection retains individuals with deviating from the average value of the trait, B - incorrect. The correct answer is 4. Task number 26.
During spermatogenesis
1. Male sex cells are formed 2. Somatic cells are formed 3. Halving the number of chromosomes 4. Four gametes are formed 5. One egg is formed 6. Cells with a diploid set of chromosomes are formed Explanation: spermatogenesis - the process of formation of male germ cells, while four haploid germ cells are formed from the precursor (diploid) cell (that is, the chromosome set is halved). The correct answer is 1, 3, 4. Task number 27.
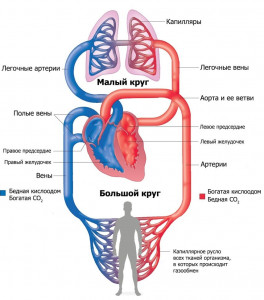
The systemic circulation in the human body
1. Begins in the left ventricle 2. Originates in the right ventricle 3. Saturated with oxygen in the alveoli of the lungs 4. Supplies organs and tissues with oxygen and nutrients 5. Ends in the right atrium 6. Brings blood to the left side of the heart Explanation: Consider the systemic circulation. As can be seen from the figure, a large circle originates in the left ventricle and carries blood (oxygen, hormones, etc.) to all cells of the body, and carbon dioxide takes it from the cells and carries it back to the heart, namely, to the right atrium. The correct answer is 1, 4, 5. Task number 28.
The processes leading to the formation of new species in nature include
1. Mitotic cell division 2. Jump-like mutation process 3. Modification variability 4. Geographic isolation 5. Asexual reproduction of individuals 6. Natural selection Explanation: various mutations lead to the formation of new species (this is preceded by their accumulation and distribution throughout the population), geographic isolation (it is in the absence of reproduction with individuals of other populations that a new species can appear) and natural selection is a directed process during which the most adapted survive an organism and, subsequently, a species that is maximally adapted to the given environmental conditions is formed. The correct answer is 2, 4, 6. Task number 29.
Establish a correspondence between the characteristics of autotrophic nutrition and its type.
Characteristic Type of autotrophic nutrition A. Oxidation energy is used 1. Photosynthesis inorganic substances 2. Chemosynthesis B. Energy source - sunlight B. Carried out in plant cells D. Oxidation of ammonia occurs D. Oxygen is released into the atmosphere Explanation: photosynthesis is the process of converting the energy of the Sun into the energy of chemical bonds, carried out by phototrophs. Chemosynthesis is the process of converting inorganic substances into organic substances using the energy of oxidation of chemical compounds. Therefore, photosynthesis refers to: the source of energy is sunlight, the process occurs in plant cells and the release of oxygen into the atmosphere. The remaining two provisions refer to chemosynthesis. The correct answer is 21121. Task number 30.
Establish a correspondence between the characteristic and the kingdom of organisms.
Characteristic Kingdom of organisms A. The cell wall contains chitin 1. Mushrooms B. Type of nutrition autotrophic 2. Plants B. Form organic substances from inorganic D. The reserve nutrient is starch. D. In natural systems they are decomposers E. The body is made up of mycelium Explanation: the composition of the cell wall of fungi includes chitin, they are heterotrophs (that is, they consume ready-made organic substances) and decomposers (decompose organic substances to inorganic ones), their body consists of mycelium. Plants are autotrophs (they form organic substances from inorganic substances, starch is the reserve substance. The correct answer is 122211. Task number 31.
Establish a correspondence between the sign of the regulation of functions in the human body and its mechanism.
Sign Mechanism of regulation A. Carried out by the endocrine system 1. Nervous B. Hormones spread 2. Humoral B. Delivered to organs by blood D. The speed of exposure is very high D. Based on bioelectrical phenomena Explanation: nervous regulation - regulation with the help of the nervous system, that is, it is carried out along the processes of nerve cells (with the help of electrical impulses), the speed of such regulation is very high. Humoral (hormonal) regulation - regulation with the help of biologically active substances - hormones that are delivered to the organs by the blood and produced by the glands of the endocrine system. The correct answer is 22211. Task number 32.
Establish a correspondence between the type of organisms and the direction of evolution that is characteristic of it.
Species Direction of evolution A. Gray rat 1. Biological progress B. Snow leopard 2. Biological regression B. Amur tiger G. Creeping wheatgrass D. Przewalski's horse E. common dandelion Explanation: biological progress - an increase in the fitness of individuals, accompanied by an increase in numbers, expansion of the range and intraspecific variability. Biological regression - a decrease in the fitness of individuals, accompanied by a decrease in numbers, a decrease in the range and further extinction of the species. The reduction in numbers is typical for the snow leopard, the Amur tiger and the Przewalski horse. The correct answer is 122121. Task number 33.
Indicate the sequence of processes of geographic speciation.
1. Distribution of a trait in a population 2. The appearance of mutations in new living conditions 3. Spatial isolation of populations 4. Selection of individuals with beneficial modifications 5. Formation of a new species Explanation: speciation begins with isolation, under such conditions, mutations begin to appear, among which only useful ones are selected, then these beneficial mutations spread among all individuals of the population. Geographic speciation ends, which is logical, with the formation of a new species. The correct answer is 32415. Task number 34.
What is the purpose of using yeast mushrooms in baking bread and bakery products? What process takes place? Explanation: when baking bread, yeast is used, since yeast carries out fermentation (oxygen-free respiration), releasing carbon dioxide, which helps to raise the dough (bubbles form in the dough), which subsequently gives the dough splendor. Task number 35.
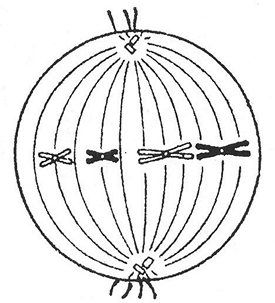
What division and what phase is shown in the figure? Indicate the set of chromosomes (n), the number of DNA molecules (s) in this period. Justify the answer. Explanation: in the figure, pairs of homologous chromosomes are lined up at the equator of the cell and are ready to diverge (that is, this is the period before anaphase 1), and before anaphase 1, metaphase 1. This means that the diploid set in the cell is 2n (since we see pairs of chromosomes), and the number of molecules DNA - 4c, since each chromosome consists of two chromatids (that is, two DNA molecules). Task number 36.
Find errors in the given text. Indicate the numbers of sentences in which errors were made, correct them.
1. Mitochondria and plastids are two-membrane organelles. 2. Photosensitive pigments are located on the inner membrane of mitochondria and plastids. 3. Unlike plastids, mitochondria contain their own circular nucleic acid molecule. 4. The process of photosynthesis takes place in chloroplasts. 5. The main function of mitochondria is the synthesis of cell lipids. Explanation: 2 - there are no pigments on the mitochondrial membrane (since they do not carry out the process of photosynthesis). 3 - plastids also contain their own nucleic acid. 5 - the main function of mitochondria is the synthesis of ATP (universal energy molecule), and not lipids. Task number 37.
How is the neurohumoral regulation of the separation of gastric juice in the human body? Explain the answer. Explanation: nervous regulation is carried out in two ways: 1. With the help of conditioned reflexes: at the sight of a lemon (or food in general) or the sound of pots in the mouth, saliva begins to be released, that is, the body prepares for eating by releasing enzymes. 2. With irritation of the receptors of the oral cavity and stomach, that is, with the help of unconditioned reflexes. Humoral regulation is carried out only when nutrients enter the blood during absorption. Hormones begin to be released and carried to the cells of the body. Task number 38.
Specify what type criteria are indicated in the text.
All individuals of the agile lizard species have the same karyotype. In males of the quick lizard, the body color is green, while in females it is brown. These animals are most numerous in the tropical and subtropical zones of the globe. Explanation: description of the karyotype - genetic criterion. Description of body color (and differences between females and males) is a morphological criterion. The description of the range of the species is a geographical criterion. Task number 39.
tRNAs with anticodons CCA, GUU, and GAA were successively involved in protein synthesis. Determine the composition of the DNA and mRNA molecules. Explanation: Let's start writing the composition of the molecules in reverse order, that is, first tRNA, then mRNA, and finally DNA. tRNA: CCA GUU GAA mRNA: GGU CAA CUU DNA: CCA GTT GAA Task number 40.
For a crested (A) green (B) female, an analyzing cross was carried out, four phenotypic classes were obtained in the offspring. The resulting crested offspring were crossed among themselves. Is it possible to get offspring without a crest in this crossing? If so, what gender will it be, what phenotype? In canaries, the presence of a crest depends on an autosomal gene, the color of plumage (green or brown) depends on a gene linked to the X chromosome. The heterogametic sex in birds is female. Explanation:
First cross: R: AaHVU x aaHvHv G: AHV, AHV, AU, AU X AHV F1: АаХВХв - crested green male ааХВХв - green male without crest AaHvU - crested brown female We cross a male and a female with a crest: R: AaHVHv x AaHvU G: AHV, AHV, AHV, AHV x AHV, AU, AHV, AU F2: we get 16 genotypes, among which only 4 phenotypes can be distinguished. Phenotypes of individuals without crest: Females: ааХВУ - green female without crest ааХвУ - brown female without crest Males: ааХВХв - green male without crest ааХвХв - brown male without crest. If the molecule of a substance is in an unexcited state, then the electrons are located at the lowest energy level. In this case, the electron shell of the molecule is in the singlet state, i.e., in such a state when all electrons are paired and the total spin moment is equal to zero. This state is called the ground singlet state, and the level at which the electrons are at this time is called the ground energy level. Let's denote it S 0 (Fig. 1). Let us consider electronic transitions in molecules using the example of electronic transitions in a tyrosine molecule. If the molecules absorb light quanta, then the electrons of the outer shells of the molecules pass to a higher energy level S 2 * (transition 1 in Fig. 1). In this case, the electron shells remain in the singlet state, although the molecule becomes excited. The value of the energy of the absorbed quantum is equal to the difference between the two energy levels between which the transition of the electron takes place: hv absorption \u003d E 2 - E 0 (8) Thus, the transition of an electron from the main singlet level to the excited singlet level will correspond to the absorption of light, which is briefly written: S 0 -> S * If a molecule can absorb light of a different wavelength, then the electron will no longer go to the S 2 * level, but to another level corresponding to the energy of the absorbed quantum. If the energy of this quantum is less, then the energy level will be located below S 2 * (in Fig. 1, this is the S 1 * level and transition 2). A molecule can have several such excited singlet levels. Each of them on the absorption spectrum will correspond to its maximum optical density. On fig. 1 shows only the electronic levels of the molecule and does not show the vibrational and rotational sublevels. If the molecules had only electronic levels, then the number of energy transitions would be limited and the molecular spectra would have a line character, and the substances would have a high absorption selectivity. In fact, due to the splitting of electronic levels into vibrational and rotational sublevels, the number of energy transitions of molecules increases significantly and the molecular spectra are continuous. In this case, the most probable electronic transitions in the absorption spectrum correspond to maxima. In addition, the interaction of molecules with a solvent, the nature of which is different for different molecules, is of great importance in the appearance of continuous spectra of molecules. Molecules cannot stay in an excited state for a long time; usually the duration of the period while the molecule is in the excited state does not exceed 10 -8 s. The electronic energy of an excited molecule can be spent as a result of several processes: it can be transferred to another molecule - energy migration; can be used to increase the vibrational (thermal) energy of the molecule. In all these cases, the electron either returns to the ground energy level S 0 or goes to some level that lies below the given excited level. Dash-dotted arrows 5 in fig. 1 depict electronic nonradiative transitions, accompanied by the waste of energy into heat. In addition to the above-mentioned processes, the luminescence of molecules can occur, which accompanies their transition to an unexcited state. The electrons then return to the ground energy level. The process of luminescence of molecules, which accompanies the transition of electrons from excited levels to the ground level, is called luminescence. Luminescence is divided into two types: fluorescence and phosphorescence (afterglow). The transition of electrons from excited levels to the ground level always begins with the transition of electrons from the upper excited levels to the lowest excited level. These are transitions In this case, the quanta are not emitted and the electronic energy of the molecule is converted into heat. This process of wasting energy occurs very quickly - in 10 -13 - 10 -12 s. The next stage of the transition of electrons is the transition from the lower excited level S 0 * to the ground level S 0 . In this case, a luminescence quantum will be emitted. Since part of the energy stored during the absorption of light was wasted into heat, the energy of the luminescence quantum will always be less than the energy of the absorbed quantum. It will be less by the amount of energy E heat spent in heat: hv lum = hv absorb - E heat (9) Therefore, the emitted light will have a lower frequency and longer wavelength than the absorbed light. This relationship is called the Stokes law: the wavelength of the light emitted during luminescence is always greater than the wavelength of the light that caused it: λ lum > λ pre. This pattern is a reflection of the second law of thermodynamics, according to which the transition of energy from one form to another is accompanied by the dissipation of energy into heat. Luminescence intensity is estimated using a special concept - quantum yield. The luminescence quantum yield φ is understood as the ratio of the number of luminescence quanta n to the number of absorbed quanta N: Since luminescence is always observed during the transition of electrons from the lower excited level to the ground level, the luminescence intensity will not depend on the level to which the electron was thrown before during the absorption of the quantum. This provision is called Vavilov's law: the quantum yield (probability) of luminescence does not depend on the wavelength of the light that caused the luminescence. Luminescence, which is observed during the transition of an electron from the lower excited singlet level to the ground S 0 * -> S 0 , is called fluorescence. Since the lifetime of molecules in an excited state is 10 -9 - 10 -8 s, fluorescence is observed only directly during the illumination of the object. However, it has long been discovered that many substances (especially at low temperatures) continue to glow intensely even after the light is turned off. This glow is due to the transition of electrons from the so-called triplet level. We have already mentioned the triplet state of electron shells, in which there are two unpaired electrons. The triplet level (T in Fig. 1) is located somewhat below the lower excited singlet level S 0 * . The triplet level is forbidden - an electron cannot get here from the ground level (transitions S 0 -> T are unlikely). An electron can enter the triplet level from an excited singlet level; then his whole path will be S 0 -> S * -> T. The electron spends part of the energy into heat and passes from the lower excited singlet level to the triplet level. During this transition, the spin of the electron is reversed, as a result of which two electrons become unpaired, and the molecule turns into a biradical. The lifetime of a molecule in the triplet state ranges from 10 -3 s to several seconds. Since in the triplet state the molecule has two unpaired electrons, it has a high chemical activity and can enter into chemical interaction. In addition, an electron can move from the triplet level to the ground level, and the energy released in this case is either dissipated into heat or emitted in the form of a luminescence quantum. The luminescence that accompanies the transition of electrons from the triplet level to the ground level T -> S 0 (transition 4 in Fig. 1) is called phosphorescence. Since the triplet level is below the excited singlet level, the wavelength of light emitted during phosphorescence is even longer than that emitted during fluorescence. If the wavelength of the light emitted during luminescence is plotted along one axis, and the luminescence intensity is plotted along the other axis, then we obtain the curve of the luminescence spectrum. Luminescence spectra are divided into fluorescence spectra and phosphorescence spectra. On fig. 1a shows the absorption and luminescence spectra of tyrosine. Level S 0 - the main singlet level of the tyrosine molecule - conditionally has an energy equal to zero. Then the transition of an electron S 0 -> S 2 * upon absorption of light requires an energy of 5.7 eV (electron volt). This transition will correspond to a maximum in the absorption spectrum at a wavelength of 217 nm. Another electronic transition during the absorption of a quantum S 0 -> S 1 * corresponds to an energy storage of 4.5 eV and in the absorption spectrum corresponds to a maximum at λ = 275 nm. Fluorescence occurs when an electron returns from the lower excited singlet level to the ground level. In this case, the emitted quantum has an energy of 4.1 eV. This energy is less than the energy of absorbed photons (5.7 and 4.5 eV). This transition corresponds to a maximum in the fluorescence spectrum at λ = 304 nm. Since the wavelength of the light emitted during fluorescence is longer than the wavelength of the absorbed light, the fluorescence spectrum is shifted to the right on the wavelength scale relative to the absorption spectrum. Phosphorescence corresponds to the transition T -> S 0 . In this case, a quantum with an energy of 3.2 eV is emitted. This transition in the phosphorescence spectrum corresponds to a maximum at λ = 387 nm. The phosphorescence spectrum is shifted even more to the right on the wavelength scale than the fluorescence spectrum. Luminescence spectra, like absorption spectra, are obtained using spectrophotometers. These spectrophotometers have a slightly different design than those discussed above. In particular, they contain a number of light filters. On the basis of luminescence data, one can judge the magnitude of energy quanta stored in a molecule. Along with data on absorbed energy quanta, this makes it possible to calculate the location of the energy levels of the molecule. Based on the luminescent method, one can judge the lifetime of molecules in an excited state - by the rate of appearance and disappearance of luminescence. Based on the intensity of luminescence, one can draw conclusions about the processes of waste of energy by a molecule. Finally, with the help of the luminescent method it is very convenient to investigate the state of matter; even its slight change (aggregation, complex formation, change in pH, etc.) affects the luminescent properties. The interpretation of the information given by the spectra is carried out using the theory of the atom. Therefore, we briefly recall some properties of atoms and their radiation. An atom is a system of a nucleus and electrons held together by electrostatic attraction. The energy of an atom (more precisely, its electron shell) can only have certain values, which are called terms, or levels. The lowest energy state is called main, the rest - excited. The scheme of energy levels of the hydrogen atom is shown in fig. 4, where each dash represents a level (the lower one represents the main one). The distances of the levels from the ground state are proportional to the energy of the atom measured from the energy of the ground state. An atom can change its energy by going from one state to another. This is usually due to a change in the motion of the outermost electron. Transitions from over high levels to lower ones can occur spontaneously, without external influence, and the difference in energy is emitted with a "portion" of light radiation, called quantum. The frequency of the emitted light is proportional to the quantum energy. Since strictly defined changes in energy correspond to different transitions, the atom emits quanta of only certain frequencies, which give rise to individual lines in the spectrum. Transitions "up", i.e., from the lower levels to the upper ones, cannot spontaneously occur; their implementation requires an external source of energy (for example, quanta of the appropriate frequency). Such a process is called takeover. The number of energy levels of an atom is infinite; they condense to a limit which corresponds to the energy at which the electron completely separates from the atom. The set of lines formed during transitions to a given level from all higher ones is called spectral series. So, hydrogen has a Lyman series corresponding to transitions to the first level, a Balmer series formed during transitions to the second level, etc. Since the first level is very far from the others, transitions to it are accompanied by a large change in energy, and the quanta of the Lyman series lie in the far ultraviolet region. For example, the line L α ;, formed during the transition from the second level to the first, has a wavelength of 1216Å 1, while the violet part of the spectrum corresponds to a wavelength of about 4000 Å. Lines of the Balmer series - H α (6563 Å), H β (4861 Å) and others, lie in the visible part of the spectrum. The rest of the hydrogen series are located in the infrared region. The energy levels of other elements are more complex. To represent them, you need to draw more than one column, as in Fig. 4, but several, with a common upper limit. 1 (Å (angstrom) - a unit of measurement of wavelengths in spectroscopy, equal to 10 -8 cm.) In most atoms, the energy levels are split into several closely spaced sublevels. Therefore, lines often consist of two or three components or more, as a result of which they are called doublets, triplets, etc., or in general - multiplets. Single lines are called singlets. In complex atoms, the level splitting can be very strong. The levels of the hydrogen atom also consist of two sublevels, but their energies are practically the same. Comparing the spectrum and the level scheme of an atom of any element, one can notice that the observed lines do not correspond to all transitions; some transitions don't happen at all. Such transitions are called prohibited. Allowed transitions correspond to strictly defined relationships between energy levels, called selection rules. It should be noted that transitions that violate the selection rules are not absolutely prohibited: under certain conditions, the corresponding lines can still be observed. The "degree" of forbiddenness is best characterized by the time that must elapse, on average, until an atom can spontaneously make a given transition. For an allowed transition, this time is usually 10 -7 - 10 -8 seconds. But in order to make a forbidden transition, the atom must be in the upper state much longer - from 10 -3 sec to several days or more, depending on the degree of inhibition. If there are no allowed "down" transitions from a certain level, then the atom under certain conditions can stay there for a relatively long time. Such levels are called metastable. For most elements and their ions, low levels close to the ground level are metastable. Forbidden lines are usually indicated by the element symbol in square brackets, with a Roman numeral indicating the degree of ionization. For example, [OH] means the forbidden line of ionized oxygen, a - the forbidden line of doubly ionized oxygen. Allowed lines do not have square brackets. In order for an atom to emit a quantum, it must be at one of the excited levels, i.e., it must be given an energy corresponding to the difference between the energies of the level under consideration and the initial one. This energy can be imparted to an atom either when it absorbs a quantum or when it collides with another particle, mainly an electron. In this case, the energy of the electron must be greater than the energy of the corresponding transition. The average particle energy is proportional to absolute temperature gas. However, even if the average energy is insufficient for excitation, there are always electrons in the gas with the required energy, but their number rapidly decreases with increasing energy. Therefore, the number of excitations of a level whose energy is noticeably higher than the average energy will be relatively small 1 . 1 (The excitation frequency depends not only on the number of electrons whose energy is greater than the excitation threshold, but also on the properties of the atom itself, more precisely, on the probability of excitation of a given level of the atom by a fast electron.) The impact of an ion or a neutral atom can also transfer the atom to an excited state, but for this the energy of the ion must be hundreds or thousands of times greater than the energy of the level, because a heavy particle always transfers only a very small part of its energy to a light electron of an atom. Therefore, excitation by heavy particles is usually of no importance under astrophysical conditions, except in cases where the level energy is exceptionally small. If an electron collides with an excited atom, the latter can transfer its energy to it. Then the atom will go to a lower state without emitting a quantum, and the colliding electron will bounce off with increased energy. Such a process is called blow of the second kind, in contrast to the process of excitation of an atom during a collision ( hits of the first kind). Impacts of the second kind are essential in dense gases, where the time interval between collisions of atoms is less than the lifetime of an atom in an excited state. In this case, the atoms collide before they have time to radiate, so that impacts of the second kind significantly attenuate the radiation. For metastable levels, from which there are no allowed `down' transitions and where, therefore, the atom stays much longer, impacts of the second kind can also be significant at low density. That is why we do not observe lines corresponding to forbidden transitions in the laboratory: the excitation usually ends with a second-order impact, and not with radiation. In a very rarefied gas, the interval between collisions can become comparable to the lifetime in metastable state, and the forbidden lines will appear. As will be seen below, it is these conditions that are realized in nebulae. If an electron acquires more energy than the limit of terms, it will break away from the atom. Such a process is called ionization. Ionization of an atom can occur both when a quantum is absorbed and when it collides with a fast electron. A free electron that is not bound to an atom can have any energy. Therefore, in contrast to transitions within an atom, where quanta of only a certain frequency can be absorbed, ionization can be produced by any quantum whose energy is greater than that required for the transition from the initial level to the ionization energy. For example, a hydrogen atom located at the first level can absorb any quantum whose wavelength is less than 912 A, and from the second level - a quantum with a wavelength less than 3646 A. If the quantum energy is greater than necessary to detach an electron, then the excess energy passes into the kinetic energy of the ejected electron. Impact ionization is possible only if the energy of the impacting electron is greater than that required for detachment. The higher the gas temperature, the more electrons has the necessary energy, the more often ionizations occur and the greater, other things being equal, the proportion of ionized atoms. A heavy particle can ionize an atom only at high energies, much higher than the ionization energy. If the gas has a very high temperature or is in a strong radiation field, then before it has time to catch the electron back, a second, third electron, etc., can be torn off from it. repeatedly ionized atoms. However, the detachment of each subsequent electron requires more and more energy, as the charge of the ion increases. Therefore, multiple ionization requires a very high temperature of the gas or source of ionizing radiation. The reverse process of ionization is called recombination. It represents the capture of an electron by an ion. An electron can be captured by an atom to any level. Therefore, an atom formed as a result of recombination can be either excited or unexcited. During recombination, an energy equal to the difference between the energies of a free and bound electron should be released, usually in the form of a quantum. If an electron with low energy recombines, then the frequency of the quantum is close to the limit of the series of the corresponding level. The greater the energy of the recombining electron, the greater the frequency of the emitted quantum. Since free electrons can have any energy, the spectrum emitted during recombinations has the form of a continuous band, starting immediately after the series limit and stretching, gradually weakening, towards higher frequencies. Such a spectrum is called continuum. The weakening of the brightness is caused by the fact that the fraction of fast electrons always decreases with increasing energy. The higher the gas temperature, the greater the average energy of recombining electrons and the slower the intensity decreases beyond the series limit. We have already said above that an atom is capable of emitting light not only during recombination, but also during transitions from one energy level to another. It turns out that a free electron, moving in the electric field of an ion, is also capable of emitting radiation. A free electron moves in the field of an ion along a hyperbolic orbit, and different orbits correspond to different energies. Just as a quantum is emitted or absorbed during the transition of an electron bound in an atom from one elliptical orbit to another, a quantum can be emitted or absorbed when an electron passes from one hyperbolic orbit to another. Such a process called a free-to-free transition. Since the energy of both orbits can have any value, emission and absorption during free-free transitions occur in a continuous spectrum. The change in energy during free-free transitions is usually a small fraction full energy electron, so that the energy of emitted photons is less than the average energy of electrons. When not very high temperature free-free gas radiation is concentrated in the region of long waves - from infrared rays to radio waves. So far we have been talking about the emission and absorption of light by individual atoms. In reality, radiation from a whole layer of gas is always observed. If the layer of gas is transparent at all frequencies, the radiation of individual atoms will simply sum up. It will consist of bright lines against the background of a less bright continuous spectrum formed during recombinations at various levels and free-to-free transitions. But if you increase the thickness of the gas layer, then part of the radiation will be absorbed by it. First of all, the quanta forming the lines will be absorbed, and therefore the lines will be less contrasted against the background of the continuous spectrum. If the homogeneous layer is practically opaque at all frequencies, then the lines will disappear. The radiation will have a continuous spectrum depending only on temperature, and the maximum intensity in this spectrum will shift towards short waves with increasing temperature of the gas layer. At the same time, the total amount of energy radiated by a unit surface will increase. The radiation of stars is complicated by the fact that the layer is not homogeneous - its temperature and density increase with depth. Therefore, there are dark lines in the spectrum of stars, and the general course of intensity in it does not coincide with the spectrum of an opaque layer of gas.

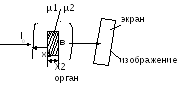

total pages: 6
where v abs. - the frequency of the absorbed light, E 2 and E 0 - the energy of the levels between which the transition is carried out.
where v lum is the frequency of the light emitted during luminescence. Page
3
total pages: 6






