What is the heat of fusion. Big encyclopedia of oil and gas
It is a well-known fact that a substance can be in one of the states - gaseous, liquid, solid. And it can move from one to the other. The simplest example - a piece of ice melts, turns into a liquid and then into steam. In this whole process of turning into steam, the melting stage is very interesting and one of its parameters is the specific heat of fusion.
If you remember how melting takes place, then several stages can be distinguished. Let's take lead as an example. At the first stage, lead is heated, the temperature rises to 327 (melting point). After melting has begun, nothing happens for a long time.
The temperature of the lead, in spite of the heat supplied to it, remains constant and remains so until the whole process is completed. And only after that, with continued heating, the temperature begins to rise again. Some conclusions follow from the observed picture. In a solid body, all molecules are in a certain order and are rigidly connected to neighboring molecules.
In order for them to move freely to another place, bonds with neighboring molecules must be broken, which happens during the melting process. To do this, the body must transfer a certain rate of heat, called the heat of fusion. Each substance will require a different amount of heat. The reason is due to such a property of a substance as the specific heat of fusion, which is defined as the amount of heat expended to melt one kilogram of a substance. The unit of measure is Joule/kilogram.
As already mentioned, for each material this value is different. melting lead is different from the same value for ice. And here a very curious moment arises. The specific heat of fusion of steel is on average 85 kJ/kg, and for water (ice) the same parameter is on average 335 kJ/kg. For ice, a high value of this parameter can be considered a great gift from nature.
Indeed, thanks to this, all the snow, ice does not melt instantly, but everything happens for a long time. Otherwise, the snow would melt very quickly, and the floods would be more abundant and destructive. In addition, such unique properties of water contribute to the stabilization of the climate on the planet.
There are tables with data on the specific heat of fusion of individual materials. Knowing this value, it is calculated how much heat is needed in order to melt the material, and determine how much fuel is needed for melting. If the body is heated to the melting point, then heat is needed only for melting, and if its temperature is below the melting point, then heat is needed to heat the substance to
Such calculations are extremely useful in industry for calculating production costs.
By the way, when the molten substance cools, the reverse process of melting occurs - crystallization. In this case, when the substance cools, broken bonds between molecules are restored and heat is released.
Considering the process of melting a substance and the phenomena taking place in this process, such a concept as the specific heat of fusion was defined. This indicator was compared for different substances, and it was determined how the high value of this parameter in ice has a beneficial effect on the planet's climate.
Page 3
The specific heats of ice melting and water evaporation at 273 K are 0 33 and 2 5 MJ/kg, respectively.
Air is quickly pumped out of a heat-insulated vessel containing water at 0 C, 117% of water has evaporated in a print, and due to intensive evaporation, all unevaporated water has been frozen. The specific heat of melting of ice is 0 33 MJ/kg.
What is the maximum work Lmax that can be obtained from a cyclically operating machine, the heater of which is the mass mi of 1 kg of water at initial temperature T 373 K, and the refrigerator - m2 - 1 kg of ice at a temperature T2 273 K, by the time when all the ice has melted. What will be the temperature of the water T at this moment. The specific heat of melting of ice is q 335 kJ / kg, the dependence of the specific heat of water on temperature is neglected.
Many calculations of heat transfer processes in which melting and solidification occur are carried out using the equation heat balance. As an example, consider how such an equation is compiled when determining the specific heat of melting of ice using a calorimeter.
From a vessel in which there is 100 g of water at 0 C, air is quickly pumped out; at the same time, due to intensive evaporation, the unevaporated water is frozen. How much water can be turned into ice in this way. Specific heat of vaporization at 0 C / - 597 cal / g; the specific heat of ice melting A is 80 cal/g. Consider the vessel to be thermally insulated.
From a vessel in which there is a mass of t100 g of water at a temperature of 0 C, air is quickly pumped out; at the same time, due to intensive evaporation, the unevaporated water is frozen. What mass of water can be turned into ice in this way. Specific heat of vaporization at 0 C / - 2 49 MJ / kg; specific heat of ice melting Х 0 336 MJ/kg. Vessel is considered thermally insulated.
The specific heat of vaporization of water at 0 C is 2.54 - 10e J / kg, the specific heat of ice melting is 3 35 105 J / kg.
The resulting ice is different in its physical properties from ordinary ice, which has a temperature of 0 C. Specific heat water in the temperature range from - 10 C to OS is equal to cl 4 17 103 JDkg K); the specific heat capacity of ice in this temperature range is c2 2 17 103 JDkg K); the specific heat of ice melting at 0 C is 3 32 105 J/kg.
V 2 l of liquid nitrogen is stored in a Dewar vessel at a temperature of tt - 195 C. Determine the specific heat of vaporization of nitrogen ha, if it is known that at a temperature of t ° C, 40 g of ice will melt in the same vessel within 22 5 h. Density of liquid nitrogen p 8 - 102 kg / m3; specific heat of ice melting X l 3 3 105 J / kg. Assume that the rate of heat supply inside the vessel is proportional to the temperature difference outside and inside the vessel.
Determine the mass m of water that can be turned into ice at 0 C by the evaporation of ether, the mass of which is M 0.1 kg, and the temperature t is 20 C. Heat exchange occurs only between ether and water. The specific heat of evaporation of ether g 3 8 105 J / kg, the specific heat of fusion of ice A 3, 3 - 105 J / kg, the specific heat of water sv 4200 J / kg K, ether se 2100 J / kg K.
A piece of ice weighing 6 kg at a temperature of - 20 C was lowered into water having a temperature of 60 C; the mass of water is 10 kg. The specific heat capacity of ice is 2 1 kJ / (kg - K); specific heat of ice melting - 0 33 MJ / kg.
Determination of the specific heat of fusion of refractory bodies (bodies with high temperature melting) is a difficult task. The specific heat of fusion of such a low-melting crystal as ice can be determined using a calorimeter. Using - the law of conservation of energy, we will compose the heat balance equation (§ 209), which allows us to determine the specific heat of ice melting.
Water, subject to certain precautions, can be supercooled to a temperature of 4 - 10 C. This state of water is unstable, and with any disturbance, the water turns into ice with a temperature of t ° C. Assume that the specific heat of water does not depend on temperature and is equal to 4 2 kJ / (kg - C), and the specific heat of melting of ice g 0 / 34 MJ / kg.
A piece of ice taken at a temperature of - 8 C is lowered into a brass calorimeter of mass 300 g containing 1 kg of water at 18 C. The specific heat capacity of water is 4 19 kJ / (kg - K), ice - 2 1 kJ / (kg - K ), brass - 0 38 kJ / (kg - K); the specific heat of ice melting is 0 33 MJ/kg.
V2 l of liquid nitrogen is stored in a Dewar vessel at a temperature tl - - 195 C. The density of liquid azo p8 is 102 kg / m3; specific heat of ice melting Yal 3 3 - 105 J / kg. Assume that the rate of supply of ms inside the vessel is proportional to the temperature difference outside and inside the vessel.
As the temperature rises solids and liquids rises kinetic energy their particles: they begin to oscillate at a faster rate. At a certain temperature, which is quite definite for a given substance, the forces of attraction between the particles are no longer able to keep them in the nodes of the crystal lattice (the long-range order turns into a short-range one), and the crystal begins to melt, i.e. matter begins to liquefy.
Melting - the process of changing a substance from a solid to a liquid state.
Hardening - the process of changing a substance from a liquid to a solid state.
During the melting process, the temperature of the crystal remains constant. This temperature is called melting point. Each substance has its own melting point.
An experience: From the street (or from the freezer) they bring some snow and observe the change in its temperature with a thermometer. They see that at first the temperature of the snow rises, and then, in the process of melting, it remains constant (the thermometer readings do not change). And only after all the snow has melted, the temperature of the formed water begins to increase again.
The constancy of temperature during melting is of great practical importance, since it allows calibrating thermometers, making fuses and indicators that melt at a strictly specified temperature. Knowing the melting point various substances important from a purely domestic point of view: otherwise, who will guarantee that this pot or pan will not melt on the fire of a gas burner?
The melting point and the solidification temperature equal to it are a characteristic feature of a substance. Mercury melts and solidifies at a temperature of -39 o C, therefore mercury thermometers are not used in the Far North. Instead of mercury thermometers in these latitudes, alcohol thermometers are used (-114 o C). The most refractory metal is tungsten (3420 o C).
The amount of heat required to melt a substance is determined by the formula:
, where m is the mass of the substance, is the specific heat of fusion.
j/kg
Specific heat of fusion - the amount of heat required to melt 1 kg of a substance at its melting point. Each substance has its own. It is found in the table.
The melting point of a substance depends on pressure. For substances whose volume increases upon melting, an increase in pressure increases the melting point and vice versa. At water, the volume decreases during melting, and when the pressure increases, the ice melts at a lower temperature.
During solidification, the atoms again line up in a certain order and the heat of solidification is released. As soon as the body completely solidifies, its temperature begins to drop.
Ticket number 5.
Related information:
- A. temperature, ylgaldylyk, aua kozgalysynyn zhyldamdygy, infrakyzyl saulelen.
- Toughness - the ability of materials to resist brittle fracture at low temperatures.
- The state diagram is built in temperature-concentration coordinates.
- Van der Waals isotherms and their comparison with real isotherms. critical temperature. Internal energy of van der Waals gas.
- Evaporation and condensation. Relative humidity and its measurement. Boiling. Boiling temperature. Specific heat of vaporization.
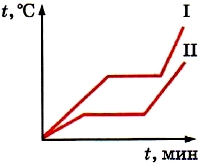
In the previous paragraph, we considered the graph of melting and solidification of ice. The graph shows that while the ice is melting, its temperature does not change (see Fig. 18). And only after all the ice has melted, the temperature of the resulting liquid begins to rise. But after all, even during the melting process, the ice receives energy from the fuel burning in the heater. And from the law of conservation of energy it follows that it cannot disappear. What is the energy consumption of the fuel during melting?
We know that in crystals the molecules (or atoms) are arranged in a strict order. However, even in crystals they are in thermal motion (oscillate). When the body is heated average speed molecular movement increases. Consequently, their average kinetic energy and temperature also increase. On the graph, this is section AB (see Fig. 18). As a result, the range of vibrations of molecules (or atoms) increases. When the body is heated to the melting temperature, the order in the arrangement of particles in crystals will be violated. Crystals lose their shape. The substance melts from solid state into liquid.
Consequently, all the energy that a crystalline body receives after it has already been heated to the melting point is spent on the destruction of the crystal. In this regard, the body temperature ceases to rise. On the graph (see Fig. 18) this is the BC section.
Experiments show that for the transformation of various crystalline substances of the same mass into a liquid at a melting point, a different amount of heat is required.
A physical quantity showing how much heat must be imparted to a crystalline body with a mass of 1 kg in order to completely transfer it to a liquid state at the melting point is called specific heat melting.
The specific heat of fusion is denoted by λ (Greek letter "lambda"). Its unit is 1 J/kg.
Determine the specific heat of fusion in the experiment. Thus, it was found that the specific heat of melting of ice is 3.4 10 5 - . This means that for the transformation of a piece of ice weighing 1 kg, taken at 0 ° C, into water of the same temperature, 3.4 10 5 J of energy is required. And in order to melt a bar of lead weighing 1 kg, taken at its melting point, it will take 2.5 10 4 J of energy.
Therefore, at the melting point, the internal energy of a substance in liquid state more internal energy the same mass of matter in the solid state.
To calculate the amount of heat Q required for melting crystalline body mass m, taken at its melting point and normal atmospheric pressure, you need to multiply the specific heat of fusion λ by the body mass m:
From this formula, it can be determined that
λ = Q / m, m = Q / λ
Experiments show that when cured crystalline substance exactly the same amount of heat is released that is absorbed when it melts. So, during the solidification of water weighing 1 kg at a temperature of 0 ° C, an amount of heat equal to 3.4 10 5 J is released. Exactly the same amount of heat is required for the melting of ice weighing 1 kg at a temperature of 0 ° C.

When a substance solidifies, everything happens in the reverse order. The speed, and hence the average kinetic energy of molecules in a cooled molten substance, decrease. Attractive forces can now keep slowly moving molecules close to each other. As a result, the arrangement of particles becomes ordered - a crystal is formed. The energy released during crystallization is spent on maintaining constant temperature. On the graph, this is the EF section (see Fig. 18).
Crystallization is facilitated if any foreign particles, such as dust particles, are present in the liquid from the very beginning. They become centers of crystallization. Under normal conditions, there are many centers of crystallization in a liquid, near which the formation of crystals occurs.
Table 4
Specific heat of fusion of certain substances (at normal atmospheric pressure)
During crystallization, energy is released and transferred to surrounding bodies.
The amount of heat released during the crystallization of a body of mass m is also determined by the formula
In this case, the internal energy of the body decreases.
Example. To prepare tea, the tourist put ice weighing 2 kg and having a temperature of 0 ° C into the pot. How much heat is needed to turn this ice into boiling water at 100°C? The energy spent on heating the kettle is not taken into account.

What amount of heat would be needed if, instead of ice, a tourist took water of the same mass at the same temperature from the hole?
Let's write down the condition of the problem and solve it.

Questions
- How to explain the process of body melting on the basis of the doctrine of the structure of matter?
- What is the fuel energy spent on during the melting of a crystalline body heated to the melting point?
- What is the specific heat of fusion?
- How to explain the process of hardening on the basis of the doctrine of the structure of matter?
- How is the amount of heat required to melt a crystalline body taken at the melting point calculated?
- How to calculate the amount of heat released during the crystallization of a body that has a melting point?
Exercise 12
Exercise
- Place two identical cans on the stove. Pour water weighing 0.5 kg into one, put several ice cubes of the same mass into the other. Note how long it takes for the water in both jars to boil. Write a short account of your experience and explain the results.
- Read the paragraph “Amorphous bodies. Melting amorphous bodies". Prepare a report on it.






