What happens during melting. Why doesn't the temperature change when melting?
Melting is the transition of a substance from a solid to a liquid state.
When heated, the temperature of the substance increases, and the speed of thermal motion of particles increases, while increasing internal energy body.
When the temperature of a solid reaches its melting point, the crystal lattice of the solid begins to break down.
Thus, the main part of the energy of the heater, supplied to the solid body, is used to reduce the bonds between the particles of the substance, i.e., to destroy the crystal lattice.
As it falls, it forms a drop shape. In the region of weightlessness, liquids, as a rule, form spheres. On the other hand, gases tend to spread in all directions in zero gravity. In the Earth's gravitational field, gases can form layers. Their propensity to spread is also given in the gravitational field. With increasing pressure, liquids and solids hardly react with a decrease in volume if they remain in their state of aggregation. If they change their aggregate state, they can also change their volumes dramatically.
In this case, the energy of interaction between particles increases.
The molten substance has a greater store of internal energy than in the solid state.
The remaining part of the heat of fusion is spent on doing work to change the volume of the body during its melting.
When melted, the volume of the majority crystalline bodies increases (by 3-6%), and decreases during hardening.
Gases expand when the pressure is reduced, and there are various possibilities for increasing the pressure. Which one occurs, you can see. When cooled below this temperature, water expands. Ice has a lower density than water and therefore floats on water. In winter, the water does not freeze completely due to the water anomaly. This water can no longer be circulated.
It even insulates the lower layers of water from cooling too quickly. For this reason, aquatic organisms such as fish can survive the winter in a frozen lake. The phenomenon of expansion of liquids during solidification occurs only in a few other substances. Large crystals develop very significant forces during their growth, so that large stones can be blown away or fist sizes can develop on the road surface. At the boiling point, the liquid changes into gaseous state, then the liquid and gaseous phases are in a state of thermodynamic equilibrium.
But, there are substances in which, when melted, the volume decreases, and when solidified, it increases.
These include, for example, water and cast iron, silicon and some others. That is why ice floats on the surface of the water, and solid cast iron - in its own melt.
Solids called amorphous (amber, resin, glass) do not have a specific melting point.
At the boiling point, only the described equilibrium state is reached. When a liquid is heated, heat is first supplied to heat the liquid. How more temperature liquid approaches the boiling point, the more heat is evaporated to evaporate the liquid. When the boiling point is reached, the temperature does not increase for a longer time, it remains constant. The heat supplied then only serves to evaporate the liquid. At constant temperature the boiling point is reached.
In this way, substances can be recognized at their boiling points. In distillation, the boiling point is used to separate mixtures of substances. Since the boiling point of a substance decreases with decreasing pressure, distillations are also carried out under vacuum. Reducing the pressure lowers the boiling point, so there must be less energy to heat. If the external pressure and temperature are converted into a diagram, a so-called state diagram can be represented for a substance. The lines show at what pressure and at what temperature the substance changes its state.

The amount of heat required to melt a substance is equal to the product specific heat melting per mass of a given substance.
The specific heat of fusion shows how much heat is needed to completely convert 1 kg of a substance from a solid to a liquid state, taken at the melting rate.
The unit of specific heat of fusion in SI is 1J/kg.
Thermodynamic equilibrium is present on the lines themselves. In a triple point, all three aggregate states can be present at the same time. Below the triple point, a sublimation phenomenon occurs, the dividing line between the absolute zero point and triple point called the sublimation curve. The dividing line between the solid and liquid phases is called the melt curve, the dividing line between the boiling curve of the liquid and the gaseous phase. At the critical point, gas and liquid can no longer be distinguished due to their identical density.
We can say that the substance is gaseous and liquid. Technicians at a power plant working with water at a critical point speak of "excessive water". Phase boundary line can no longer be specified higher critical point. Additional Notes: The melting curve of water is different from other substances. When ice melts, the melting point is observed as pressure increases. This phenomenon is called melt water anomalies. The state diagram provided is simplified for didactic and illustrative reasons, and the proportions in the diagram are not shown correctly.

During the melting process, the temperature of the crystal remains constant. This temperature is called the melting point. Each substance has its own melting point.
The melting point for a given substance depends on atmospheric pressure.
In crystalline bodies at a melting point, one can observe the substance at the same time
in solid and liquid states.
Only a few substances show classical states of aggregation with typical temperatures during transformations. Some substances, like many organic substances, decompose when heated, even when the air is closed. There are minerals, such as calcite, that do not melt at all. In the case of quartz and many glasses, the transition from solid to liquid is fluid. As the glass tube heats up, the glass gradually becomes soft, becoming more viscous as it heats up, but never quite as liquid as water.
Crystalline sulfur becomes liquid on heating, then on further heating a viscous state appears before it becomes liquid again and then evaporates at the boiling point. All these phenomena cannot be explained by the model of classical states of aggregation.
Yes or no
If two identical polyethylene vessels are filled with water at 0 degrees C, and one vessel is placed in water at 0 degrees C and the other in crushed ice at 0 degrees C, will the water in any of these vessels freeze?
INTERESTING ABOUT MELTING
Ice grains and stars.
Enter a piece pure ice into a warm room and watch it melt. It will quickly become clear that the ice, which seemed monolithic and homogeneous, breaks up into many small grains - individual crystals. In the volume of ice, they are located randomly.
Criticism of the commonly used particle model. Some school books use the particle model as an "attempt to explain" classical aggregate states. In a solid, the smallest particles, according to this idea, move little. Strong attractive forces are at work, the particles are arranged regularly and they lie relatively close to each other. The higher the temperature, the more self-propulsion increases. In the case of a liquid, the motion of the particles is so great that the particles can no longer hold their place. However, the cohesion of the particles is still provided.
Not less than interesting picture can be seen when the ice melts from the surface.
Bring a smooth piece of ice to the lamp and wait until it begins to melt. When melting touches the inner grains, very fine patterns will begin to appear there. With a strong magnifying glass, you can see that they have the shape of hexagonal snowflakes. In fact, these are melted depressions filled with water. The shape and direction of their rays correspond to the orientation of ice single crystals. These patterns are called "Tyndall stars" after the English physicist who discovered and described them in 1855.
In the case of a gas, the particles move so fast that they can no longer occupy a regular arrangement and no longer stick together. Criticism. The problem with the particle model is that it works with images from the human visible world. The microcosm, however, is an abstract and different world, like the world that we humans perceive and recognize. The particle model places too much emphasis on the visual representation of particles that "push" each other like closed blocks, like ping pong balls, or "attract" each other, like magnets.
"Tyndall stars", similar to snowflakes, are actually depressions on the surface of melted ice about 1.5 mm in size, filled with water. In their center, air bubbles are visible that have arisen due to the difference in volumes of melted ice and melted water.
DID YOU KNOW
There is a metal, the so-called Wood's alloy, which can be easily melted even
in warm water (+68 degrees Celsius).
So when stirring sugar in a glass, a metal spoon made of this alloy will melt faster than sugar!
Complex interactions of systems in the atomic field are not considered properly. Alternative A detailed study of the phenomena is accurate enough for easy understanding. Everyday life from a didactic point of view. More complex relationships are accurately described by abstract mathematical language, such as a boiling curve or a state diagram.
Gases decompose into plasma, in which electrons atomic shells fully or partially released. Free, negatively charged electrons and positively charged ions were obtained. Matter in the plasma state has completely new properties. It is generally very electrically conductive and can be easily influenced magnetic fields. Plasma states often occur in our environment, without direct perception of them. Thus, candles or thunderstorms form partial plasma states.
The most refractory substance, tantalum carbide TaCO-88, melts at a temperature of 3990°C.
In 1987, German researchers were able to supercool water to -700C while keeping it in a liquid state.
Sometimes, to make the snow on the sidewalks melt faster, they are sprinkled with salt. This is because a solution of salt in water is formed, the freezing point of which is lower than the air temperature.
The solution just flows off the sidewalk.
They are very common in the universe. Plasmas are found in stars, for example, inside our sun. Meanwhile, it is believed that the so-called "metal plasma" exists inside the planets, which are under high pressure and at a relatively low temperature. In the laboratory, plasma states can be artificially generated by strong current charges, using laser beams, or by bombarding matter with heavy ion beams. The closer the absolute zero point approaches as matter cools, the more atoms lose their individuality and function in synchrony.
Interestingly, feet get colder more on wet pavement, as the temperature of the salt-water solution is lower than that of pure snow.
If you pour tea from a teapot into two mugs: with sugar and without sugar

In severe frosts, to restore the smoothness of the ice, the rink is watered hot water.. Hot water melts a thin top layer of ice, does not freeze so quickly, has time to spread, and the ice surface is very smooth.
There is practically no interaction between atoms. quantum physicist talking about one quantum state in atoms. The combined state is called a Bose-Einstein condensate. Atoms in this state can no longer be spatially assigned. We can also say that the atoms are everywhere in the condensate at the same time. The combined fortune of Satyendra Nath Bose and Albert Einstein is already this year.
Sometimes fermion condensation is the sixth state of aggregation, or only a variant of the fifth, since the fermion condensate has not yet been defined. Whether the vacuum as a collective state can be called debatable. The resulting vacuum occurs when the various phenomena known from the gas are no longer present.
QUESTION
Ice has frozen on the bottom of the vessel. They poured water - the ice melted. Will the water level change?
Details Category: Molecular-kinetic theory Posted on 06.11.2014 13:52 Views: 8274The same substance under certain conditions can be in different states of aggregation - solid, liquid or gaseous. During the transition from one state to another, the composition of the molecules of this substance does not change. Only their location, the nature of thermal motion and the forces of intermolecular action change.
- The absence of flows, such as turbulence or suction, occurs more.
- The audio line is completely interrupted.
- Air exposure no longer occurs.
- Heat is transferred only by thermal radiation.
Among the most important physical changes we have changes of state, which are those produced by the action of heat. We can distinguish between two types of state changes depending on the influence of heat: progressive changes and regressive changes.
From solid state matter becomes liquid, and from liquid to gaseous. Such a transition is called phase transition .
Melting

At low temperatures, all substances freeze and turn into solids, the atoms and molecules of which are packed so tightly that the forces of their mutual attraction allow them to perform only oscillatory movements around the equilibrium position. Therefore, under normal conditions, solids retain volume and shape.
Progressive changes are those that occur when heat is applied. These are: progressive sublimation, fusion and evaporation. These changes are caused by the cooling of bodies, and three types are also distinguished: regressive sublimation, solidification, and condensation.
"The friction of the skis creates a coalescence of the snow, forming a layer of water that promotes gliding." "If the water hadn't evaporated, we wouldn't have rain." "The various by-products derived from the oil are achieved by separating them by boiling point." Why does the streets have a darker stripe on the pavement, each specific section? Why railway tracks is there a small gap?
The process by which a substance changes from a solid state to a liquid state is called melting . This process occurs when the temperature rises.
In the spring, when the sun warms up, snowdrifts begin to melt. The tiny ice crystals that make up snow turn into water. But, despite the fact that the air warms up and its temperature becomes above zero, the temperature of melting snow and the temperature of melt water remain equal to 0 0 C until the snow melts completely. The thing is that melting occurs gradually. During melting, the substance absorbs the heat that it receives from the outside, and for some time it is both in a solid and in a liquid state. And its temperature does not change until it all melts and becomes liquid.
Volume changes refer to the changes that a material experiences in relation to the space it occupies. For example, a body increases its volume if it increases the space it occupies and, on the other hand, a decrease in its volume means that it decreases the space it occupies.
Volume changes - two: contraction and expansion. This is the reduction in volume that a body undergoes when it cools. For example, your shoes become looser in winter; placing the inflated balloon in a pot of cold water is reduced in size. Contraction is understood because as bodies cool, the particles are closer together, their motion is reduced and, as a result, their volume is reduced.
What happens when a solid is heated? As the temperature rises, the speed of oscillations of particles inside the substance increases. Consequently, its internal energy also increases. At a certain temperature, which is called melting point , crystal cell solid body starts to break down. Molecules get more freedom. They can jump and take other positions. The substance turns into a liquid.
What happens when you put a thermometer in ice water? This is the increase in volume experienced by bodies in contact with temperature. For example, the Mercury of a thermometer expands easily and is therefore able to rise through a small capillary and indicate an increase in temperature.
This phenomenon affects not only liquids or solids, but also gases. After receiving an increase in heat, the particles separate from each other, allowing the gas to become lighter and rise. An example of this is that it allows "balloons" to be lifted and moved.
To solid begins to melt, it must be heated to the melting point. When it begins to receive heat from the outside, for some time its temperature will rise in direct proportion to the heating time. It stays that way until it starts to melt. But as soon as its temperature becomes equal to the melting point, it will stop changing and will be constant until all the substance turns into a liquid. After that, the temperature of the liquid will begin to rise again.
But if the liquid stops receiving heat, it will begin to cool. And as soon as its temperature drops to a value, equal to the temperature melting, the crystallization process begins.
Each substance has its own melting point. At normal pressure (760 mm Hg), ice begins to melt at 0 o C. The most high temperature melting among metals has tungsten - 3422°C. A simple substance carbon melts at a temperature of 3500 - 4500 ° C. And the melting point of alcohol is minus 114 o C.
Crystallization
As the temperature of a liquid decreases, its molecules become less mobile. And the attractive forces that hold the molecules in a certain strict order, characteristic of a solid body, increase.
If a liquid substance cool to a certain temperature, it will harden. Process phase transition from liquid state to the hard is called crystallization . Unlike melting, when a substance receives heat, during crystallization it gives it away, and its temperature decreases.
The temperature at which this process occurs is called crystallization temperature . For a pure substance, the melting point is equal to the crystallization temperature.
Like melting, crystallization also occurs gradually. Similarly, a liquid and a solid will have the same temperature until the entire substance has solidified.
Liquid tin melted with a soldering iron solidifies and becomes solid when we remove the soldering iron. Molten liquid metal poured into molds solidifies as the temperature drops.
We observe crystallization in nature every year, when water in reservoirs freezes at low temperatures, snowflakes fall on the ground instead of raindrops.
Graph of changes in the state of aggregation of matter
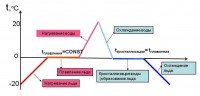
The processes of melting and crystallization are clearly visible on a graph showing how the state of aggregation of a substance changes depending on temperature.
Let's measure the temperature of a piece of ice. The thermometer shows -20 o C. Put the ice in a bucket and bring it into the room. Gradually, it will begin to melt, and its temperature will rise. When the thermometer reads 0 o C, there will be no further increase in temperature until all the ice has melted. When it all turns into water, the water in the bucket will begin to heat up until it reaches room temperature.
Let's take a bucket of water out into the cold. The water will continue to cool. When its temperature drops to 0 o C, it will begin to turn into ice. And the temperature will not change until all the water has solidified. And only after that it will again begin to gradually decrease to a value equal to the air temperature.
This chart can show the changes state of aggregation any substance.