Radius in chemistry. Lecture notes on general chemistry
Determination of atomic radii also involves some problems. First, an atom is not a sphere with a strictly defined surface and radius. Recall that an atom is a nucleus surrounded by a cloud of electrons. The probability of detecting an electron with distance from the nucleus gradually increases to a certain maximum, and then gradually decreases, but becomes equal to zero only for infinitely. long distance. Secondly, if we still choose some condition for determining the radius, such a radius still cannot be measured experimentally.
The experiment makes it possible to determine only internuclear distances, in other words, the bond lengths (and even then with certain reservations given in the caption to Fig. 2.21). To determine them, X-ray diffraction analysis or the electron diffraction method (based on electron diffraction) is used. The radius of an atom is assumed to be equal to half the smallest internuclear distance between identical atoms.
Van der Waals radii. For unbonded atoms, half of the smallest internuclear distance is called the van der Waals radius. This definition is illustrated in Fig. 2.22.
Rice. 2.21. Link length. Due to the fact that the molecules are constantly vibrating, the internuclear distance, or bond length, does not have a fixed value. This figure schematically depicts the linear vibration of a simple diatomic molecule. The vibrations make it impossible to define the bond length simply as the distance between the centers of two bonded atoms. A more precise definition looks like this: the bond length is the distance between the bonded atoms, measured between the centers of mass of two atoms and corresponding to the minimum bond energy. The minimum energy is shown on the Morse curve (see Fig. 2.1).
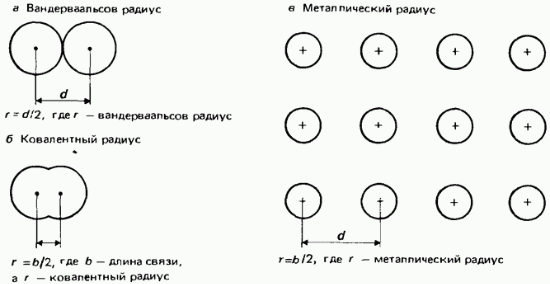
Rice. 2.22. Atomic radii, a - van der Waals radius; b - covalent radius; c - metal radius.
covalent radii. The covalent radius is defined as half the internuclear distance (bond length) between two identical atoms, bound friend with another covalent bond (Fig. 2.22, b). As an example, let's take a chlorine molecule with a bond length of 0.1988 nm. The covalent radius of chlorine is assumed to be 0.0944 nm.
Knowing the covalent radius of an atom of one element, one can calculate the covalent radius of an atom of another element. For example, the experimentally established value of the bond length is 0.1767 nm. Subtracting from this value the covalent radius of chlorine (0.0994 nm), we find that the covalent radius of carbon is 0.0773 nm. This calculation method is based on the principle of additivity, according to which atomic radii obey a simple addition law. Thus, the bond length is the sum of the covalent radii of carbon and chlorine. The principle of additivity applies only to simple covalent bonds. Double and triple covalent bonds are shorter (Table 2.7).
The length of a simple covalent bond also depends on its environment in the molecule. For example, the bond length varies from 0.1070 nm at a trisubstituted carbon atom to 0.115 nm in the compound
metal radii. The metal radius is assumed to be equal to half the internuclear distance between neighboring ions in the metal crystal lattice (Fig. 2.22, c). The term atomic radius generally refers to the covalent radius of atoms of non-metallic elements, while the term metallic radius refers to atoms of metallic elements.
Ionic radii. The ionic radius is one of the two parts of the internuclear distance between adjacent monatomic (simple) ions in a crystalline ionic compound (salt). The determination of the ionic radius is also associated with considerable problems, since interionic distances are measured experimentally, and not the ionic radii themselves. Interionic distances depend on the packing of ions in the crystal lattice. On fig. 2.23 shows three possible ways of packing ions in a crystal lattice. Unfortunately, the experimentally measured interionic distances
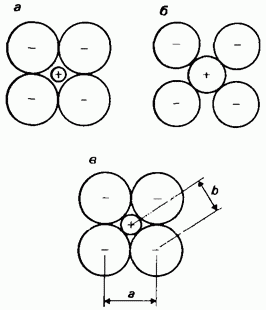
Rice. 2.23. Ionic radii, a - anions are in contact with each other, but cations are not in contact with anions; b - cations are in contact with anions, but anions are not in contact with each other; c - conditionally accepted arrangement of ions, in which cations are in contact with anions and anions are in contact with each other. The distance a is determined experimentally. It is taken as twice the radius of the anion. This makes it possible to calculate the interion distance b, which is the sum of the anion and cation radii. Knowing the interion distance b, one can calculate the radius of the cation.
do not allow us to judge which of these three methods of packaging is actually carried out in each case. The problem is to find the proportion in which the interionic distance should be divided into two parts corresponding to the radii of the two ions, in other words, to decide where one ion actually ends and where the other begins. As shown, for example, in Fig. 2.12, the maps of the electron density of salts do not allow solving this issue either. To overcome this difficulty, it is usually assumed that: 1) the interionic distance is the sum of two ionic radii, 2) the ions are spherical, and 3) neighboring spheres are in contact with each other. The last assumption corresponds to the ion packing method shown in Fig. 2.23, c. If one ionic radius is known, other ionic radii can be calculated based on the principle of additivity.
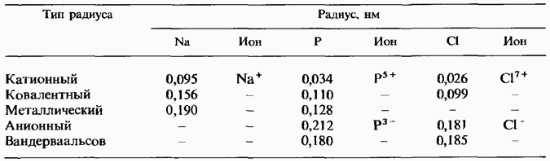
Comparison of different types of radii. In table. 2.8 shows the values of the radii of various types for the three elements of the 3rd period. It is easy to see that the largest values belong to the anion and van der Waals radii. On fig. 11.9 compares the sizes of ions and atoms for all elements of the 3rd period, with the exception of argon. The sizes of atoms are determined by their covalent radii. It should be noted that cations are smaller than atoms, and anions are big sizes than the atoms of the same elements. For each element of all types of radii, the smallest value always belongs to the cationic radius.
Table 2.8. Comparison of atomic radii of various types
Determination of the radii of atoms and ions. The application of X-rays to the study of crystals makes it possible not only to establish internal structure the latter, but also to determine the particle size,forming a crystal - atoms or ions.
Figure 46. Contacting particles in a crystal
To understand how such calculations are made, imagine that the particles that make up the crystal are spherical and in contact with each other. In this case, we can assume that the distance between the centers of two neighboring particles is equal to the sum of their radii (Fig. 46). If the particles are simple atoms and the distance between them is measured, then the radius of the atom is also determined, obviously equal to half the distance found. For example, knowing that for crystals of metallic sodium, the lattice constant d equal to 3.84 angstroms, we find that the radius r sodium atom is equal to.
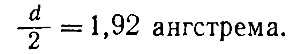
It is somewhat more difficult to determine the radii of various ions. Here it is no longer possible to simply divide the distance between the ions in half, since the sizes of the ions are not the same. But if the radius of one of the ions r 1 is known, the radius of the other r 2 is easily found by simple subtraction:
r2 = d - r1
Hence it follows that in order to calculate the radii of various ions from the constants of the crystal lattices, it is necessary to know the radius of at least one ion. Then finding the radii of all other ions will no longer present difficulties.
With the help of optical methods, it was possible to fairly accurately determine the radii of fluorine ions F - (1.33 A) and oxygen O - (1.32 A); these radii serve as initial values in calculating the radii of other ions. So, for example, the determination of the lattice constant of magnesium oxide MgO showed that it is equal to 2.1 angstroms. Subtracting from this the radius of the oxygen ion, we find the radius of the magnesium ion:
2.1 - 1.32 = 0.78 Å
The lattice constant of sodium fluoride is 2.31 Å; since the radius of the fluorine ion is 1.33 angstroms, the radius of the sodium ion should be:
2.31 -1.33 = 0.98 Å
Knowing the radius of the sodium ion and the lattice constant of sodium chloride, it is easy to calculate the radius of the chlorine ion, etc.
In this way, the radii of almost all atoms and ions are determined.
A general idea of the size of these quantities is given by the data given in table. 7.
Table 7
Radii of atoms and ions of some elements
| Element | Atom radius | Ion radius | Ion symbol |
| 1,92 | 0,98 | Na+ | |
| 2,38 | 1,33 | K+ | |
| 2,51 | 1,49 | Rb+ | |
| 2,70 | 1,65 | Cs+ | |
| 1,60 | 0,78 | Mg++ | |
| 1,97 | 1,06 | Ca++ | |
| 2,24 | 1,43 | Ba++ | |
| 0,67 | 1,33 | F- | |
| 1,07 | 1,81 | Cl- | |
| 1,19 | 1,96 | Br- | |
| 1,36 | 2,20 | J- | |
| 1,04 | 1,74 | S— |
As these data show, in metals the radii of atoms are greater than the radii of ions, in metalloids, on the contrary, the radii of ions are greater than the radii of atoms.
The relative sizes of the ions that form a crystal have a huge impact on the structure of the spatial lattice. So, for example, two very similar in their chemical nature - CsCl and NaCl nevertheless form lattices of various types, and in the first case each positive ion is surrounded by eight negative ions, and in the second - only by six. This difference is explained by the fact that the sizes of cesium ions
and sodium are not the same. A number of considerations forces us to accept that the ions should be located in the crystal so that each smaller ion, if possible, completely fills the space between the large ions surrounding it, and vice versa; in other words, the negative ions, which are almost always larger than the positive ions, must surround the positive ions as closely as possible, otherwise the system will be unstable. Since the radius of the Cs + ion is 1.65 Å, and the Na + ion is only 0.98 Å, it is obvious that more Cl - ions can be placed around the first than around the second.
The number of negative ions surrounding each positive ion in a crystal is called the coordination number of the given lattice. A study of the structure of various crystals shows that the following coordination numbers are most common: 2, 3, 4, 6, 8 and 12.
The coordination number depends on the radius ratio positive ion to the radius of the negative ion: the closer this ratio is to unity, the greater the coordination number. Considering the ions as balls located in the crystal according to the method of the closest packing, it is possible to calculate at what ratio between the radius of the positive and negative ions one or another coordination number should be obtained.
Below are the calculated theoretically largest coordination numbers for a given ratio of radii.
It is easy to verify that the coordination numbers for NaCl and CsCl, found from this table, just correspond to the actual arrangement of ions in the crystals of these substances.
For example, in the case of NaCl, the ratio of the radius of the sodium ion (0.98 Å) to the radius of the chlorine ion (1.81 Å) is 0.98:1.81 = 0.54. This ratio is in the range of 0.41-0.73; therefore, in the NaCl lattice, the coordination number must be six.
You are reading an article on the topic Determining the radii of atoms and ions
ATOMIC RADIUS- a characteristic of an atom, which makes it possible to approximately estimate the interatomic (internuclear) distances in molecules and crystals. Since atoms do not have clear boundaries, when introducing the concept of "A. R." imply that 90-98% electron atom enclosed in a sphere of this radius. A. r. have the order of 0.1 HM, however, even small differences in their values can determine the structure of crystals built from them, affect the equilibrium geometry of molecules, etc. For many others. problems, the shortest distances between atoms in molecules and condensed media can be considered as the sum of their A. R., however, such additivity is very approximate and is not satisfied in all cases. Depending on what forces act between atoms (see. Interatomic interaction), distinguish between metallic, ionic, covalent and van der Waals A. p.
metallic radii are considered equal to half the shortest distance between atoms in a crystal. the structure of the metal element, they depend on the coordinates. numbers To. If you take A. p. at K=12 per unit, then at K=8, 6 and 4 A. p. the same element resp. equal to 0.98; 0.96; 0.88. The proximity of the values of A. p. different metals - a necessary (though not sufficient) condition for mutual solubility metals substitution type. So, liquid K and Li usually do not mix and form two liquid layers, and K with Rb and Cs form a continuous series of solid solutions (A. R. Li, K, Pb and Cs are equal to 0.155; 0.236; 0.248; 0.268 HM) . Additivity A. r. allows you to approximately predict the parameters of the crystal. intermetallic gratings. connections.
Ionic radii are used for approximate estimates of internuclear distances in ionic crystals. It is assumed that the distance between the nearest cation and anion is equal to the sum of their ionic radii. The accuracy with which the indicated additivity of the A.R.
Difference A. r. ions, obtained by comparing the internuclear distances in KF and NaF, is 0.035 nm (A. R. ions in KF crystals in NaF are assumed to be the same), and for KCl and NaCl compounds it is 0.033 HM, from KBr and NaBr compounds - 0.031 HM and from compounds KI and NaI - 0.030 HM. T. o., the typical error in determining internuclear distances in ionic crystals by A. R. ~ 0.001 nm.
There are several systems of ionic A. p., differing in the values of A. p. individual ions, but leading to approximately the same internuclear distances. For the first time, work on the determination of ionic A. p. was done in the 20s. 20th century V. M. Goldschmidt (V. M. Goldschmidt), based, on the one hand, on internuclear distances in crystals, measured by X-ray methods structural analysis, and on the other hand, on the values of A. p. and , determined by the method refractometry(respectively 0.133 and 0.132 HM). Most other systems also rely on certain . methods of internuclear distances in crystals and on a certain "reference" value of A. p. def. and she. In max. widely known to Pauling's system, this reference value is A. p. (0.140HM). In the system of Belov and Bokiya, which is considered one of the largest. reliable, A. r. 0 2- is taken equal to 0.136 HM. Below are the values of the radii of certain ions:
|
in the Goldschmidt system |
in the Pauling system |
in the Goldschmidt system |
in the Pauling system |
||
For ionic crystals having the same coordinates. numbers, cf. the deviation of the sum of A. R., calculated from the A. R. given above, from the experimental values of the shortest internuclear distances in ionic crystals is 0.001-0.002 NM.
In the 70-80s. attempts were made to directly determine A. p. ions by measuring the electron density by methods x-ray structural analysis provided that the minimum of the electron density on the line connecting the nuclei is taken as the boundary of the ions. Diffraction measurements for crystals of halides of alkali metals made it possible to obtain A. r. cations Li + , Na + , K + , Rb + and Cs + equal respectively. 0.094; 0.117; 0.149; 0.163; 0.186 nm, and A. r. anions F - , Cl - , Br - , I - - equal resp. 0.116; 0.164; 0.180; 0.205HM. T. o. diffraction measurements lead to overestimated (in comparison with the traditional, given above) values of A. p. cations and to the underestimated values And. anions. A. R. found by measuring the distribution of electron density in a crystal cannot be transferred from one compound to another, and the deviations from their additivity are too large, so such A. R. cannot be used to predict internuclear distances.
The covalent radius is defined as half the length of a single chem. bonds X - X (where X is a non-metal element). For halogens, covalent A. R. is half the internuclear distance X - X in the X 2 molecule, for S and Se - half the distance X - X in X 8, for carbon - half the shortest distance C - C in a diamond crystal. Covalent A. r. F, Cl, Br, I, S, Se and C resp. equal to 0.064; 0.099; 0.114; 0.133; 0.104; 0.117 and 0.077 nm. For the H atom A. r. taken equal to 0.030 HM (although half the length of the H - H bond in the H 2 molecule is 0.037 HM). Additivity of covalent arr. allows predicting the shortest internuclear distances (bond lengths) in polyatomic molecules. So, according to this rule, the C-Cl bond length should be equal to 0.176 HM, and the experimentally obtained value for this value in the CCl 4 molecule is 0.177 HM. Below are the covalent A. r. for atoms of certain elements, calculated on the basis of the lengths of single bonds:

In molecules with double or triple chem. bonds, use reduced values of covalent A. p., because multiple bonds are shorter than single ones. Below are the covalent radii of atoms in the formation of multiple bonds:
Van der Waals radii determine the eff. sizes of noble gas atoms. In addition, van der Waals A. p. consider half of the internuclear distance between the nearest atoms of the same name that are not interconnected chemically. bond and belonging to different molecules (eg, in molecular crystals). When atoms approach each other at a distance less than the sum of their van der Waals radii, a strong interatomic repulsion occurs. Therefore, van der Waals A. p. characterize the minimum allowable contacts of atoms belonging to different molecules. Below are the values of van der Waals atomic radii for some atoms:

Van der Waals A. r. on Wednesday. 0.08 nm more covalent A. p. Ionic A. r. for a negatively charged ion (eg, Cl -) practically coincides with the van der Waals radius of an atom in the neutral state.
Knowledge of van der Waals A. p. allows you to determine the shape of molecules, the conformations of molecules and their packing in molecular crystals. According to the principle of dense packing, the molecules, forming a crystal, are arranged in such a way that the "protrusions" of one molecule enter the "cavities" of another. Using this principle, one can interpret the available crystallographic data and, in some cases, predict the structure of molecular crystals.
Lit.: Boky G. B., Crystal chemistry, 3rd ed., M., 1971; Pauling L., General chemistry, trans. from English, M., 1974; Campbell J., Modern General Chemistry, trans. from English, vol. 1, M., 1975; E. Cartmell, G. V. A. Fowles, Valence and structure of molecules, trans. from English, M., 1979. V. G. Dashevsky.
The atomic radius depends on the number of nearest neighboring atoms.
Atomic radii are subdivided into the radii of metal atoms, the covalent radii of non-metallic elements, and the radii of noble gas atoms.
The atomic radius, which is determined by the totality of interactions acting in crystals, depends to some extent or the type of bond and the cn.
The atomic radii / - of the transition metals of this group - samarium, thulium and plutonium - are much larger, therefore the curve corresponding to them is located to the left of the curves for d - transition metals and exhibits characteristic breaks. Inert gases, due to the very weak molecular forces that bind their atoms in the solid state, have very large atomic radii. The location of the corresponding curve is not related to the curves for d - and / - transition metals of group VIII with a predominantly strong metallic bond.
The atomic radius increases with an increase in the principal quantum number n of this highest employed energy level. However, the average electron distribution radius for each energy level in various atoms is not the same, since it depends on the effective charge of the nucleus. The effective nuclear charge Z3d (j) is the apparent charge that acts on the considered electron. The value of 2eff is less than the true charge of the nucleus Z, because each external electron is partially shielded from the action of the nucleus by internal electrons. For the outermost electrons, the degree of shielding of the true nuclear charge by other electrons in the same atom or ion can be characterized by the shielding constant S, which is defined as the difference between the true and effective nuclear charges.
Atomic radii, like ionic ones, change for the same element, depending on the magnitude of the coordination number. In this regard, as we have indicated, it is often denoted by an index, for example, rv, to which coordination number a given radius value belongs.
The atomic radius is 156 A, the ionic radius of Ca2 is 103 A.
The atomic radius is 2 65 A, the ionic radius of Cs is 165 A. In air, it instantly oxidizes with ignition, forming peroxide and superperoxide.
Atomic radius (metal) 0 280 nm, ionic radius Fr 0 186 nm.
The atomic radii decrease in the sequence S C1 Ar, since in going from S to C1 and from C1 to Ar the charge of the nucleus increases by one. Within one period valence electrons are more strongly attracted to the nucleus with increased positive charge, so the atomic radii decrease accordingly. For isoelectronic (having the same number of electrons) atomic and ionic particles, the effective radii decrease as the charge of the nucleus (serial number of the element) increases, since in this case, too, there is a gradual increase in the attraction of electrons to the nucleus.
The atomic radii change similarly with increasing group number from I to VI and further to the zinc group. With a decrease in the length of interatomic bonds and atomic diameters, the energy of interatomic bonds increases and therefore the coefficient of thermal expansion a and the compressibility of metals k decrease.
The atomic radii and compressibility of the elements of subgroups B increase significantly in the direction IB - - VIIB, and the atomic radii of inert gases are very close in magnitude to the atomic radii of alkali metals in the corresponding periods.
Atomic radii of metals. Atomic radii are subdivided into the radii of metal atoms, the covalent radii of non-metallic elements, and the radii of noble gas atoms.
The atomic radii r d and Hz to be substituted into the equation usually differ from the radii used by Pauling; they are given in table. 4.2 in brackets. Interatomic distances calculated using these radii are given in the second column of Table. 4.1 in brackets. They often agree somewhat better with the experimental values than the values obtained from the Pauling radii, but the difference is rarely large. The Schoemaker and Stevenson scheme suffers from the disadvantage that it cannot be extended to double and triple bonds, and therefore does not have broad enough applicability to be useful in the following discussion.
Atomic radii are subdivided into the radii of metal atoms, the covalent radii of non-metallic elements, and the radii of noble gas atoms.
Atomic radii have a periodic dependence on the atomic number or nuclear charge. In general, if the periodic system of elements is presented in the most familiar - tabular form, then the atomic radii, with the same number of quantum layers, decrease from left to right, the electron shell, as it were, shrinks. From top to bottom, on the contrary, with an increase in the number of quantum layers, atomic radii increase.
Atomic radii are subdivided into the radii of metal atoms, covalent radii and intermolecular (van der Waals) radii, which include the radii of noble gas atoms.
Typically, atomic radii in groups increase from top to bottom.
The atomic radius of tungsten is 1 37 A, molybdenum 1 36 A, vanadium - 1 32 A and chromium-1 25 A. Experience shows that conjugated deposition is the more pronounced, the closer the atomic radius of the element is to the atomic radius of tungsten.
The atomic radii of the halogens increase in the series F c C1 Br I. In the same sequence, the boiling and melting points increase, and the color of the halogens deepens.
Scheme crystal - Repeating this operation several times oud. The atomic radius of silicon (with a coordination number of 4 and a covalent bond) is 1175 A. Due to the relatively large radius of the silicon atom, silicon has a greater metallicity than carbon. In compounds, silicon is predominantly tetravalent.
The atomic radius of boron is 0 97, and the radius of the B3 ion is estimated at 0 20 A.
The atomic radius of boron is 0 97, and the radius of the B3 ion is estimated at 0 20 A.
Iron State Diagram.| Change in the heat capacity of iron with temperature [kcalKz - atom deg. The atomic radius of Fe is 1 26 A, and the work function of an electron from the metal is 4 7 ey. As shown in fig. XIV-15, iron has four allotropic forms at normal pressure. The coefficient of thermal expansion of iron increases up to 500 C, after which it decreases to 769 C, and then increases again to 911 C. Formed at ordinary temperature under pressure of about 133 thousand atm, the e-form of iron is characterized by a hexagonal close-packed structure with rf (FeFe) 2 40 A, high density (9 1 g / cm3) and increased (approximately 25 times) electrical resistance.
The standard atomic radii of Co and Ni are 125 and 124 A, and the electron work functions characteristic of metals are 42 and 50 eV, respectively. The allotropy of these elements is much less studied than iron. For cobalt, when heated (about 450 C), the hexagonal close packing changes to a cube with centered faces, while for nickel (about 358 C), the opposite is true. What caused such an opposite behavior of both metals is not clear.
The atomic radius of Fe is 1 26 A, and the work function of an electron from the metal is 4 7 eV. As shown in fig. X1V - 15, at normal pressure iron has four allotropic forms. Of these, a, (5 and b) crystallize as a centered cube, while y crystallizes as a cube with centered faces. The coefficient of thermal expansion of iron increases to 500 C, after which it decreases to 769 C, and then increases again to 911 C.
The standard atomic radii of Co and Ni are 125 and 124 A, and the electron work functions characteristic of metals are 42 and 50 ea, respectively. The allotropy of these elements is much less studied than iron. For cobalt, when heated (about 450 C), the hexagonal close packing changes to a cube with centered faces, while for nickel (about 358 C), the opposite is true. What caused such an opposite behavior of both metals is not clear. For cobalt, one more polymorphic transformation was registered - at 1125 C.
The atomic radius of Li differs markedly from the atomic radii of its electronic counterparts, so Li forms eutectic alloys with them.
The atomic radius of vanadium is noticeably smaller than that of niobium, and when going from niobium to tantalum, the atomic radius practically does not change, despite the fact that tantalum has a new electron layer. The anomalously small value of the atomic radius of tantalum is due, as in the case of hafnium, to the effect of lanthanide contraction. Niobium and tantalum in the oxidation state 5 also have the same ionic radii, which causes a great similarity chemical properties these elements.
The atomic radii of niobium and tantalum almost coincide (Table 33), the ionic radii of the same oxidation state are also very close to each other, so their compounds are very similar in properties. Metals of subgroup VB are refractory, have good mechanical properties, strongly dependent on the content of impurities of hydrogen, carbon, oxygen and nitrogen. These impurities increase the hardness, make the metals brittle and less ductile. Vacuum electron beam melted, niobium and tantalum are very ductile and work well when cold.
The structure of the molecule - as the origin. Then in the ring associate S8 depicted in. The atomic radius of sulfur is considered equal to 0 104 nm.
Atomic radii calculated from interatomic distances in simple substances ah, nm. The atomic radii of non-metals are calculated in the same way as half the interatomic distance in molecules or crystals of simple substances.
The atomic radii of niobium and tantalum almost coincide (see Table 33), the ionic radii of the same oxidation state are also very close to each other, so their compounds are very similar in properties. Metals of subgroup VB are refractory, have good mechanical properties, strongly dependent on the content of impurities of hydrogen, carbon, oxygen and nitrogen. These impurities increase the hardness, make the metals brittle and less ductile. Vacuum electron beam melted, niobium and tantalum are very ductile and work well when cold. Under ordinary conditions, these metals are passive, as they are covered with a stable protective oxide film. At high temperature interact with oxygen, halogens, nitrogen, carbon, hydrogen, carbon dioxide and water vapor. Tantalum at 600 C and above is coated with strong, refractory, poorly conductive Ta20b oxide, which is unable to be reduced in hydrogen.
The atomic radius of vanadium is noticeably smaller than that of niobium, and when going from niobium to tantalum, the atomic radius practically does not change, despite the fact that tantalum has a new electron layer. Niobium and tantalum in oxidation state 5 also have the same ionic radii, which causes a great similarity in the chemical properties of these elements.
Types of precipitates from a supersaturated solid solution. The atomic radius of aluminum is 0 143 nm, copper - 0 128 nm, zinc - 0 138 nm.
Correspondingly, the atomic radius decreases from 155 A in the case of lithium to 077 A in the case of carbon. Melting points and boiling points gradually increase; these indicators, like hardness and other such properties, reflect the strength of the bond between the atoms of a given substance; the melting point increases from 186 for lithium to 3500 for carbon, and the boiling point increases from 1336 for lithium to 4200 for carbon.
Distribution of electron density on the communication line between atoms in crystals. a - novalent-nan bond (diamond C, dotted line - electron density of the valence pair of electrons. b - ionic bond (NaCl, dotted line - region of outer electron orbits. c - metallic bond (A1, dotted line - electron density in the interatomic space. The atomic radii of gat in the structures of simple substances (elements) with metallic interatomic distances in the structures of compounds with the same type of bond are well described by the sum of atomic radii.Si - C in silicon carbide (0 189 nm) practically coincides with the sum of the indicated radii of gat (Si) 0 117 nm, rJT (C) 0 077 nm, equal to 0 194 nm.
The atomic radius of uranium is large, equal to 1 54 A, the ionic radii of - 1 03 A, U4 - 0 93 A, U5 - 0 87 A and U6 - 0 83 A.
The atomic radius of cesium is 262 A.
The atomic radii of boron, nitrogen, and silicon are 0 80, respectively; 0 74 and 1 17 A - Predict the behavior of these elements in the cases below, assuming that the atoms are solid balls, and compare the results you predicted with experimental data.
The atomic radii of vanadium, niobium and tantalum are 1 34, respectively; 1 46 and 1 46 A - Why do the atomic radii of niobium and tantalum coincide.
The atomic radii of the transition elements are smaller than the atomic radii of the non-transition elements, reflecting the greater bond strength in transition element metals.
The atomic radii of metal catalysts must lie within certain limits, since otherwise either the hydrogen atoms in cyclohexane will be too far from the catalyst atom that attracts them, or the ring of carbon atoms will not lie on the lattice. Cyclohexane dehydrogenation catalysts have atomic radii of 1236-1397 A.
The atomic radii of the elements of the copper subgroup are small: gs 128 pm; / - d g Ai 1 44 rm.
One of the most important characteristics of the chemical elements involved in the formation chemical bond, is the size of an atom (ion): with its increase, the strength of interatomic bonds decreases. The size of an atom (ion) is usually determined by the value of its radius or diameter. Since an atom (ion) does not have clear boundaries, the concept of "atomic (ionic) radius" implies that 90–98% of the electron density of an atom (ion) is contained in the sphere of this radius. Knowing the values of atomic (ionic) radii makes it possible to estimate internuclear distances in crystals (that is, the structure of these crystals), since for many problems the shortest distances between the nuclei of atoms (ions) can be considered the sum of their atomic (ionic) radii, although such additivity is approximate and is satisfied not in all cases.
Under atomic radiuschemical element(about the ionic radius, see below), involved in the formation of a chemical bond, in the general case, they agreed to understand half the equilibrium internuclear distance between the nearest atoms in the crystal lattice of an element. This concept, which is quite simple if we consider atoms (ions) as rigid spheres, actually turns out to be complex and often ambiguous. The atomic (ionic) radius of a chemical element is not a constant value, but varies depending on a number of factors, the most important of which are the type of chemical bond
and coordination number.
If the same atom (ion) in different crystals forms different types chemical bond, then it will have several radii - covalent in a crystal with a covalent bond; ionic in a crystal with an ionic bond; metallic in metal; van der Waals in a molecular crystal. The influence of the type of chemical bond can be seen in the following example. In diamond, all four chemical bonds are covalent and are formed sp 3-hybrids, so all four neighbors of a given atom are on the same and
the same distance from it d= 1.54 A˚) and the covalent radius of carbon in diamond will be
is equal to 0.77 A˚. In an arsenic crystal, the distance between atoms bound by covalent bonds ( d 1 = 2.52 A˚), much less than between atoms bound by van der Waals forces ( d 2 = 3.12 A˚), so As will have a covalent radius of 1.26 A˚ and van der Waals of 1.56 A˚ .
The atomic (ionic) radius also changes very sharply with a change in the coordination number (this can be observed during polymorphic transformations of elements). The smaller the coordination number, the lower the degree of space filling with atoms (ions) and the smaller the internuclear distances. An increase in the coordination number is always accompanied by an increase in internuclear distances.
It follows from the foregoing that the atomic (ionic) radii of different elements involved in the formation of a chemical bond can only be compared when they form crystals in which the same type of chemical bond is realized, and these elements in the formed crystals have the same coordination numbers .
Let us consider the main features of atomic and ionic radii in more detail.
Under covalent radii of elements It is customary to understand half of the equilibrium internuclear distance between the nearest atoms connected by a covalent bond.
A feature of covalent radii is their constancy in different "covalent structures" with the same coordination number Z j. In addition, covalent radii, as a rule, are additively bonded to each other, that is, the A–B distance is half the sum of the A–A and B–B distances in the presence of covalent bonds and the same coordination numbers in all three structures.
There are normal, tetrahedral, octahedral, quadratic and linear covalent radii.
The normal covalent radius of an atom corresponds to the case when an atom forms as many covalent bonds as it corresponds to its place in the periodic table: for carbon - 2, for nitrogen - 3, etc. This results in different values of normal radii depending on the multiplicity (order) bonds (single bond, double, triple). If the bond is formed when the hybrid electron clouds overlap, then they speak of tetrahedral
(Z k = 4, sp 3-hybrid orbitals), octahedral ( Z k = 6, d 2sp 3-hybrid orbitals), quadratic ( Z k = 4, dsp 2-hybrid orbitals), linear ( Z k = 2, sp-hybrid orbitals) covalent radii.
It is useful to know the following about covalent radii (the values \u200b\u200bof covalent radii for a number of elements are given in).
1. Covalent radii, unlike ionic ones, cannot be interpreted as the radii of atoms that have a spherical shape. Covalent radii are used only to calculate the internuclear distances between atoms united by covalent bonds, and do not say anything about the distances between atoms of the same type that are not covalently bonded.
2. The value of the covalent radius is determined by the multiplicity of the covalent bond. A triple bond is shorter than a double bond, which in turn is shorter than a single bond, so the covalent radius of a triple bond is smaller than the covalent radius of a double bond, which is smaller
single. It should be borne in mind that the order of the multiplicity of the relationship does not have to be an integer. It can also be fractional if the bond is resonant (benzene molecule, Mg2 Sn compound, see below). In this case, the covalent radius has an intermediate value between the values corresponding to integer orders of the bond multiplicity.
3. If the bond is of a mixed covalent-ionic nature, but with a high degree of the covalent component of the bond, then the concept of the covalent radius can be introduced, but the influence of the ionic component of the bond on its value cannot be neglected. In some cases, this effect can lead to a significant decrease in the covalent radius, sometimes down to 0.1 A˚. Unfortunately, attempts to predict the magnitude of this effect in various
cases have not yet been successful.
4. The value of the covalent radius depends on the type of hybrid orbitals that take part in the formation of a covalent bond.
Ionic radii, of course, cannot be defined as half the sum of the distances between the nuclei of the nearest ions, since, as a rule, the sizes of cations and anions differ sharply. In addition, the symmetry of the ions may differ somewhat from spherical. Nevertheless, for real ionic crystals under ionic radius It is customary to understand the radius of the ball, which approximates the ion.
Ionic radii are used for approximate estimates of internuclear distances in ionic crystals. It is assumed that the distance between the nearest cation and anion is equal to the sum of their ionic radii. The typical error in determining internuclear distances in terms of ionic radii in such crystals is ≈0.01 A˚.
There are several systems of ionic radii that differ in the values of the ionic radii of individual ions, but lead to approximately the same internuclear distances. The first work on the determination of ionic radii was carried out by V. M. Goldshmit in the 1920s. In it, the author used, on the one hand, the internuclear distances in ionic crystals measured by X-ray structural analysis, and, on the other hand, the values of the ionic radii F– and O2– determined by
refractometry method. Most other systems also rely on the internuclear distances in crystals determined by diffraction methods and on some "reference" values of the ionic radius of a particular ion. In the most widely known system
Pauling, this reference value is the ionic radius of the O2− peroxide ion, equal to
1.40 A˚. This value for O2– agrees well with theoretical calculations. In the system of G. B. Bokiya and N. V. Belov, which is considered one of the most reliable, the ionic radius O2– is taken equal to 1.36 A˚.
In the 1970s and 1980s, attempts were made to directly determine the radii of ions by measuring the electron density using X-ray structural analysis, provided that the minimum of the electron density on the line connecting the nuclei is taken as the boundary of the ions. It turned out that this direct method leads to overestimated values of the ionic radii of cations and to underestimated values of the ionic radii of anions. In addition, it turned out that the values of ionic radii determined by a direct method cannot be transferred from one compound to another, and the deviations from additivity are too large. Therefore, such ionic radii are not used to predict internuclear distances.
It is useful to know the following about ionic radii (in the tables below, the values \u200b\u200bof ionic radii according to Bokiy and Belov are given).
1. The ionic radius for ions of the same element varies depending on its charge, and for the same ion it depends on the coordination number. Depending on the coordination number, tetrahedral and octahedral ionic radii are distinguished.
2. Inside one vertical row, more precisely, within one group, periodic
system ion radii with the same charge increase with an increase in the atomic number of the element, since the number of shells occupied by electrons increases, and hence the size of the ion.
|
Radius, A˚ |
3. For positively charged ions of atoms from the same period, the ionic radii rapidly decrease with increasing charge. The rapid decrease is explained by the action of two main factors in one direction: the strong attraction of “own” electrons by the cation, the charge of which increases with increasing atomic number; an increase in the strength of interaction between the cation and the anions surrounding it with an increase in the charge of the cation.
|
Radius, A˚ |
4. For negatively charged ions of atoms from the same period, the ionic radii increase with increasing negative charge. The two factors discussed in the previous paragraph in this case act in opposite directions, and the first factor prevails (an increase in the negative charge of the anion is accompanied by an increase in its ionic radius), therefore, an increase in ionic radii with an increase in negative charge occurs much more slowly than a decrease in previous case.
|
Radius, A˚ |
5. For the same element, that is, with the same initial electronic configuration, the radius of the cation is smaller than that of the anion. This is due to a decrease in the attraction of external "additional" electrons to the anion nucleus and an increase in the screening effect due to internal electrons (the cation has a lack of electrons, while the anion has an excess).
|
Radius, A˚ |
6. The sizes of ions with the same charge follow the periodicity of the periodic table. However, the value of the ionic radius is not proportional to the charge of the nucleus Z, which is due to the strong attraction of electrons by the nucleus. In addition, the lanthanides and actinides, in whose series the radii of atoms and ions with the same charge do not increase, but decrease with increasing atomic number (the so-called lanthanide contraction and actinide contraction), are an exception to the periodic dependence.11
11 Lanthanide contraction and actinide contraction are due to the fact that in lanthanides and actinides, electrons added with an increase in atomic number fill internal d and f- shells with the main quantum number less than the main quantum number of the given period. At the same time, according to quantum mechanical calculations in d and especially in f states, the electron is much closer to the nucleus than in s and p states of a given period with a large quantum number, therefore d and f-electrons are located in the inner regions of the atom, although the filling of these states with electrons ( we are talking about electronic levels in the energy space) occurs in a different way.
metal radii are considered equal to half the shortest distance between the nuclei of atoms in the crystallizing structure of a metal element. They depend on the coordination number. If we take the metallic radius of any element at Z k \u003d 12 per unit, then with Z k = 8, 6 and 4 the metallic radii of the same element will be 0.98 respectively; 0.96; 0.88. Metallic radii have the property of additivity. Knowing their values makes it possible to approximately predict the parameters of the crystal lattices of intermetallic compounds.
The atomic radii of metals are characterized by the following features (data on the values of the atomic radii of metals can be found in).
1. The metal atomic radii of transition metals are generally smaller than the metal atomic radii of non-transition metals, reflecting the greater bond strength in transition metal metals. This feature is due to the fact that the metals of transition groups and the metals closest to them in the periodic system have electronic d-shells, and electrons in d-states can take part in the formation of a chemical bond. Strengthening of the bond may be due partly to the appearance of a covalent component of the bond and partly to the van der Waals interaction of the ionic cores. In crystals of iron and tungsten, for example, electrons in d-states make a significant contribution to the binding energy.
2. Within one vertical group as we move from top to bottom, the atomic radii of metals increase, which is due to a successive increase in the number of electrons (the number of shells occupied by electrons increases).
3. Within the same period, more precisely starting from alkali metal to the middle of the transition metal group, in the direction from left to right, the atomic metal radii decrease. Increasing in the same order electric charge atomic nucleus and there is an increase in the number of electrons in the valence shell. With an increase in the number of binding electrons per atom, the metallic bond is strengthened, and at the same time, due to an increase in the charge of the nucleus, the attraction of core (internal) electrons by the nucleus increases, so the value of the metallic atomic radius decreases.
4. Transition metals of groups VII and VIII from the same period in the first approximation have almost the same metal radii. Apparently, when it comes to elements that have 5 and more d-electrons, an increase in the nuclear charge and the associated effects of attraction of core electrons, leading to a decrease in the atomic metallic radius, are compensated by the effects caused by the increasing number of electrons in the atom (ion) that do not participate in the formation of a metallic bond, and leading to an increase in the metallic radius (increasing number of states occupied by electrons).
5. The increase in radii (see paragraph 2) for transition elements, which occurs during the transition from the fourth to the fifth period, is not observed for transition elements at
transition from the fifth to the sixth period; the metallic atomic radii of the corresponding (vertical comparison) elements in these last two periods are almost the same. Apparently, this is due to the fact that the elements located between them are completed with a relatively deep f-shell, so the increase in the charge of the nucleus and the associated attraction effects turn out to be more significant than the effects associated with an increasing number of electrons (lanthanide contraction).
|
Element from 4 periods |
Radius, A˚ |
Element from period 5 |
Radius, A˚ |
Element from period 6 |
Radius, A˚ |
6. Usually, metallic radii are much larger than ionic radii, but they do not differ so significantly from the covalent radii of the same elements, although without exception they are all larger than covalent ones. The large difference in the values of the metallic atomic and ionic radii of the same elements is explained by the fact that the bond, which owes its origin to almost free conduction electrons, is not strong (hence the observed relatively large interatomic distances in the metal lattice). A significantly smaller difference in the values of the metallic and covalent radii of the same elements can be explained if we consider the metallic bond as some special "resonant" covalent bond.
Under van der Waals radius It is customary to understand half of the equilibrium internuclear distance between the nearest atoms connected by a van der Waals bond. Van der Waals radii determine the effective sizes of noble gas atoms. In addition, as follows from the definition, the van der Waals atomic radius can be considered to be half the internuclear distance between the nearest atoms of the same name, connected by a van der Waals bond and belonging to different molecules (for example, in molecular crystals). When atoms approach each other at a distance less than the sum of their van der Waals radii, a strong interatomic repulsion occurs. Therefore, van der Waals atomic radii characterize the minimum allowable contacts of atoms belonging to different molecules. Data on the values of van der Waals atomic radii for some atoms can be found in).
Knowing the van der Waals atomic radii makes it possible to determine the shape of molecules and their packing in molecular crystals. The van der Waals radii are much larger than all the radii of the same elements listed above, which is explained by the weakness of the van der Waals forces.






